Abstract
Postoperative cognitive dysfunction (POCD) after cardiac surgery is growing in importance as the aging of the population advances. Highly sensitive neuropsychological testing is required to detect POCD, and a well-matched control group is useful in analyzing and interpreting the results. Pathophysiology studies of cognitive change after cardiac surgery focused on the role of cardiopulmonary bypass, intraoperative microemboli, hypoperfusion, and inflammatory response as possible causes of POCD. Long-term, follow-up studies that compared patients who underwent on- or off-pump coronary artery bypass surgery failed to demonstrate a significant reduction in the incidence of POCD. Therefore, the focus of research is shifting from cardiopulmonary bypass to patient-related risk factors. There is growing evidence that patient-related risk factors such as the extent of preexisting cerebrovascular disease play an important role in the pathogenesis of both short- and long-term POCD. Establishing the degree of functionally significant vascular disease in the brain preoperatively should be an essential part of patient evaluation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
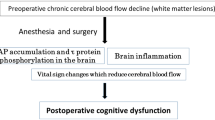
Advances in surgical techniques, perfusion systems, and perioperative management have reduced the mortality associated with cardiac surgery. However, postoperative cognitive dysfunction (POCD) remains a common outcome with potential to adversely impact quality of life. The mechanisms underlying POCD may include microemboli, hypoperfusion, and inflammatory response. Complications involving the brain are increasing substantially because older patients with advanced atherosclerotic vascular disease now undergo surgery. The objectives of this chapter are to review the manifestations and mechanisms of POCD after cardiac surgery and suggest an approach to neuroprotection during surgery.
2 Assessment of Cognitive Dysfunction
The rising number of patients of advanced age who are undergoing cardiac surgery and who have comorbid medical conditions underscores the importance of complications in overall patient outcomes. Anesthetists and surgeons have suspected for many years that some elderly patients suffer a decline in cognitive function after surgery, the so-called POCD. As noted by Shaw and colleagues in 1987, it manifests far more commonly than stroke, with 79 % of patients experiencing cognitive decline in the early period after cardiac surgery [1]. Due to the subtle nature of POCD, many physicians fail to notice when a patient’s cognition declines after surgery. In many cases, it is not detected until the patient’s relatives discover difficulties with normal activities at home or at work [2]. This condition is characterized by a decline in cognitive functions such as memory, ability to concentrate, and information processing. These changes can be detected in neuropsychological tests and present clinically as deficits in cognition and memory representing a significant change from the patient’s previous level of functioning [3].
A consensus meeting held in 1994 encouraged a more standardized and comparable methodology of assessing POCD [4]. It was recommended that neurological and neuropsychological state be tested before surgery to provide accurate baseline information. A second important recommendation was that analyses should be based on the change in performance in an individual from baseline to a specific time after surgery. The recommended core neuropsychological battery should include: (1) the Rey Auditory Verbal Learning Test to assess memory, in which patients are asked to recall as many words as possible immediately upon viewing a list of 15 words and again after 15–25 min; (2) the Trail-Making Tests A and B, in which participants connect numbered and then alternately numbered and lettered dots in order under timed conditions to assess attention and mental flexibility; and (3) the Grooved Pegboard Test, which involves inserting notched pegs into specific holes in a shallow box to test fine motor dexterity.
It is essential to consider the many pitfalls associated with repeated neuropsychological testing of surgical patients such as the practice, floor (i.e., poor initial performance that cannot decline any further), and ceiling (i.e., excellent initial performance which cannot improve) effects [5]. Other important challenges arise with obtaining a reliable assessment of preoperative performance and defining deficits in meaningful statistical analysis. Analytic criteria used commonly are percentage change from the baseline for a defined number of tests and absolute decline from baseline scores greater than a defined proportion of the standard deviation of two or more tests [6]. These statistical methods, however, do not relate cognitive decline with data from age-matched healthy controls and thus fail to account for practice effects, normal variability, and the cognitive decline that occurs in a healthy population. Therefore, contemporary studies have included control groups such as patients who have undergone percutaneous coronary intervention [7], off-pump surgery [8], and noncardiac surgery [9]. However, no generally agreed diagnostic criteria have been published, and several quite different definitions of POCD are found in the literature.
3 Preoperative Cognitive Status
From clinical psychiatry it is known well that depression is associated with cognitive deterioration. Depression is not unusual before surgery, but no clear association has been established between depression and POCD [10]. However, there is evidence that a considerable proportion of cardiac surgery patients may have significantly lower cognitive performance before surgery [11–13]. As is commonly known, aging is associated with structural cerebral changes, including vascular disease of the brain and impaired cognition [14]. In a study from Japan by Goto and colleagues in which cerebral magnetic resonance images (MRI) were obtained before cardiac artery bypass graft (CABG) in 421 patients, 30 % had small cerebral infarcts and 20 % had multiple cerebral infarcts (Table 54.1) [15]. Thus, one-half of this cohort had evidence of ischemic brain abnormalities before surgery.
Chronic cerebral infarcts, and even new deficits on diffusion-weighted MRI, have been identified in 4.5 % of patients, probably due to recent cardiac catheterization (Fig. 54.1) [16]. In addition, patients with such existing abnormalities had lower baseline cognitive performance and showed a worse postoperative neuropsychological test performance than those with normal preoperative findings. These limitations should be taken into account in choosing methods of analyzing and interpreting results.
Diffusion-weighted MRI of a 67-year-old man with no demonstrable neurologic deficits before off-pump CABG. (a) Preoperative MRI scan revealed small diffusion abnormality on the left posterior limb of the internal capsule. (b) Another scan performed 5 days after surgery demonstrated that size of diffusion restriction lesion had increased; the patient had dysarthria and right hemiparesis
4 Short-Term Cognitive Changes
While short-term cognitive change after cardiac surgery typically refers to changes observed in cognitive performance, cognitive decline in the immediate perioperative period could be related to adverse effects of anesthetic drugs, narcotics for pain control, and other clinical issues. Therefore, some investigators have chosen to defer follow-up testing until at least 3–4 weeks after surgery. The incidences reported, however, have varied enormously. In the brief period after cardiac surgery, the incidence of POCD varied from 22 to 79 % among different studies, depending on how the deficit was defined, the test methods applied, the composition of the target population, and study design [1, 17]. There are also reports of POCD after noncardiac surgery performed while the patient was under general anesthesia, suggesting that even if short-term cognitive change does occur after cardiac surgery, it is not specific to the use of cardiopulmonary bypass [8].
4.1 Microemboli
The pathophysiology of short-term cognitive change after cardiac surgery remains poorly understood. The focus of most investigations has been on neurological injury secondary to microemboli, hypoperfusion, and systemic inflammatory response. The cardiopulmonary bypass circuit and the surgical field in cardiac surgery are sources of a variety of embolic particles such as thrombi, fat, and gas bubbles. In addition, emboli can be generated from disrupted aortic atherosclerotic plaques by aortic manipulation and cannulation. Some earlier studies have reported an association between embolic count and short-term cognitive outcome [18, 19], but other contemporary studies have not replicated these findings [20, 21]. Application of diffusion-weighted MRI indicates that about 50 % of patients who undergo cardiac surgery develop new discrete lesions suggestive of microembolic infarcts [22]. A number of studies have found associations between short-term cognitive change and new ischemic lesions on diffusion-weighted MRI [23, 24]. In contrast, no such correlation has been found by other studies in patients after cardiac surgery [25]. It has been hypothesized that the cognitive manifestations of microemboli may depend as much on patient-related risk factors such as the degree of preexisting cerebrovascular disease as on the quantity and size of the embolic load.
4.2 Hypoperfusion
Elderly patients and those with comorbid disease such as hypertension and diabetes may be vulnerable to the effects of hypoperfusion because they have altered autoregulation of cerebral blood flow. Certain regions of the brain, including the hippocampus, periventricular white matter areas, and watershed areas, may be particularly susceptible to the effects of hypoperfusion. However, the evidence that deranged cerebral hemodynamics are associated with neurological injury is weak and sometimes conflicting. Some evidence suggests that maintaining perfusion pressure at more physiological levels during cardiopulmonary bypass (80–90 mmHg) is associated with lower short-term POCD [26]. However, a study using single-photon positron-emission computed tomography failed to show a significant association between neuropsychological test performance and postoperative global or regional blood flow [27]. As discussed above, emboli and hypoperfusion may act synergistically, in that decreased flow during surgery may fail to wash out embolic materials from the brain, particularly in the watershed areas [28].
4.3 Systemic Inflammatory Response
Cardiac surgery is associated with a profound systemic inflammatory response, especially when cardiopulmonary bypass is used. It is known that a severe systemic inflammatory response can break down the blood–brain barrier, leading to a range of clinical consequences, including delirium and sepsis-mediated encephalopathy, with symptoms ranging from subtle cognitive deficit to coma. However, there are sparse data to support the inflammatory response alone as the causative factor. Several groups have measured biomarkers of neuronal injury such as neuron-specific enolase and S100β after cardiac surgery with cardiopulmonary bypass and have found elevated plasma levels but with varying correlations between these markers and cognitive function [29, 30]. Preclinical studies suggest that S100β may be involved in neuronal and glial growth, proliferation, and activation, thus facilitating its role as a marker to study inflammation and brain injury. Unfortunately, it is important to note that serum S100β concentrations appear to be influenced by age, sex, on-pump or off-pump surgery, the use of cardiotomy suction or a cell saver, and the assay used [31–33]. Therefore, it is currently not possible to link these findings with pathophysiological processes.
5 Anesthesia
A number of studies have reported that clinically available opioids can be neurotoxic in rats [34, 35]. Fentanyl is associated with delirium [36], but there seems to be no clear relationship between dosage and the incidence of POCD at 3 or 12 months after CABG surgery [37]. Animal studies suggest that exposure to some halogenated anesthetics increases the production of the Alzheimer’s amyloid peptide and vulnerability to neurodegeneration [38, 39], but these results are not always supported by clinical data [40]. Inflammation and stress responses might also contribute to cognitive decline induced by anesthesia [41]. Animal research and clinical trials are needed to establish whether anesthetic agents cause cognitive changes or if they affect aging-related cognitive decline.
6 Long-Term Cognitive Changes
Several studies suggest that, in addition to the immediate effects of POCD, long-term cognitive outcomes also are affected. A study by Newman and colleagues published in 2001 found that 5 years after on-pump CABG 42 % of patients available for follow-up had cognitive performance lower than at baseline [42]. This high percentage of patients with decline suggests that cardiopulmonary bypass accelerates cerebral aging and that the harmful effects of cardiopulmonary bypass become more apparent in the long term. In contrast, a 5-year follow-up by van Dijk et al. published in 2007 failed to show a difference in the frequency of POCD between patients who underwent surgery with or without cardiopulmonary bypass [43]. Interestingly, approximately 50 % of patients in both groups suffered cognitive decline, suggesting that late cognitive changes are related to factors other than cardiopulmonary bypass. Interpreting this study of cognitive outcomes after CABG has been difficult because of the lack of comparison groups, either with or without coronary artery disease. In 2009, Selnes and colleagues reported that, compared to those with no vascular disease risk factors, patients with coronary artery disease had lower baseline cognitive performance and greater decline during 6 years of follow-up [44]. Thus, vascular disease may impact cognitive performance.
Other possible causes of late cognitive decline in elderly patients include progression of subcortical small vessel disease, development of silent cerebral infarcts, and Alzheimer’s disease during the follow-up period. There is evidence from several epidemiological studies that cerebrovascular disease may be associated with accelerated cognitive decline, even without cardiac surgery. In a recent systematic review of 105 studies, silent cerebral infarcts defined on MRI were detected in 20 % of healthy elderly people [14]. Silent infarcts are associated with subtle deficits in physical and cognitive function. Moreover, the presence of silent infarcts more than doubles the risk of subsequent stroke and dementia. Given that many candidates for CABG have MRI evidence of cerebral infarct even before surgery [15], it is likely that the late cognitive decline reported previously in the literature is related to the progression of underlying cerebrovascular disease.
7 Cognitive Recovery After Surgery
Although several previous observations are of empirical importance to the phenomenon of cognitive decline, the studies that provided them did not identify which factors influenced recovery from POCD after cardiac surgery. A 2013 study by Fontes and colleagues reported that 45 % of patients undergoing cardiac surgery who experienced cognitive decline at 6 weeks returned to baseline cognitive function by 1 year [45]. The authors suggested that heightened instrumental activities of daily living performance at 6 weeks after surgery is associated with likelihood of cognitive recovery at 1 year. One hypothesis is that interventions that encourage better performance on instrumental activities immediately after surgery improve cognitive performance.
8 Neuroprotective Strategies
Because adverse neurological events after cardiac surgery represent a wide range of injuries, differentiating the individual causes of types of injuries becomes difficult (e.g., stroke, delirium, and POCD). Additionally, there is growing evidence that patient-related risk factors such as the extent of preexisting cerebrovascular disease have a greater impact on both short- and long-term cognitive declines than do procedural variables. Therefore, it is important to assess those risk factors that indicate a predisposition toward POCD such as cerebrovascular disease and then adapt the surgical approach to high-risk patients (Table 54.2). It will become more important to reduce late cognitive decline by controlling modifiable patient-related risk factors such as hypertension, diabetes, hyperlipidemia, and smoking.
References
Shaw PJ, Bates D, Cartlidge NE, French JM, Heaviside D, Julian DG, Shaw DA (1987) Neurologic and neuropsychological morbidity following major surgery: comparison of coronary artery bypass and peripheral vascular surgery. Stroke 18:700–707
Krenk L, Rasmussen LS, Kehlet H (2010) New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesthesiol Scand 54:951–956
Rasmussen LS (1998) Defining postoperative cognitive dysfunction. Eur J Anaesthesiol 15:761–764
Murkin JM, Newman SP, Stump DA, Blumenthal JA (1995) Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg 59:1289–1295
Rasmussen LS, Larsen K, Houx P, Skovgaard LT, Hanning CD, Moller JT, ISPOCD Group (2001) The international study of postoperative cognitive dysfunction. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand 45:275–289
Rudolph JL, Schreiber KA, Culley DJ, McGlinchey RE, Crosby G, Levitsky S, Marcantonio ER (2010) Measurement of post-operative cognitive dysfunction after cardiac surgery: a systematic review. Acta Anaesthesiol Scand 54:663–677
Sweet JJ, Finnin E, Wolfe PL, Beaumont JL, Hahn E, Marymont J, Sanborn T, Rosengart TK (2008) Absence of cognitive decline one year after coronary bypass surgery: comparison to nonsurgical and healthy controls. Ann Thorac Surg 85:1571–1578
Hernandez F Jr, Brown JR, Likosky DS, Clough RA, Hess AL, Roth RM, Ross CS, Whited CM, O’Connor GT, Klemperer JD (2007) Neurocognitive outcomes of off-pump versus on-pump coronary artery bypass: a prospective randomized controlled trial. Ann Thorac Surg 84:1897–1903
Müllges W, Berg D, Schmidtke A, Weinacker B, Toyka KV (2000) Early natural course of transient encephalopathy after coronary artery bypass grafting. Crit Care Med 28:1808–1811
Johnson T, Monk T, Rasmussen LS, Abildstrom H, Houx P, Korttila K, Kuipers HM, Hanning CD, Siersma VD, Kristensen D, Canet J, Ibañaz MT, Moller JT (2002) ISPOCD2 Investigators. Postoperative cognitive dysfunction in middle-aged patients. Anesthesiology 96:1351–1357
Vingerhoets G, Van Nooten G, Jannes C (1997) Neuropsychological impairment in candidates for cardiac surgery. J Int Neuropsychol Soc 3:480–484
Millar K, Asbury AJ, Murray GD (2001) Pre-existing cognitive impairment as a factor influencing outcome after cardiac surgery. Br J Anaesth 86:63–67
Maekawa K, Goto T, Baba T, Yoshitake A, Katahira K, Yamamoto T (2011) Impaired cognition preceding cardiac surgery is related to cerebral ischemic lesions. J Anesth 25:330–336
Vermeer SE, Longstreth WT Jr, Koudstaal PJ (2007) Silent brain infarcts: a systematic review. Lancet Neurol 6:611–619
Goto T, Baba T, Honma K, Shibata Y, Arai Y, Uozumi H, Okuda T (2001) Magnetic resonance imaging findings and postoperative neurologic dysfunction in elderly patients undergoing coronary artery bypass grafting. Ann Thorac Surg 72:137–142
Maekawa K, Goto T, Baba T, Yoshitake A, Morishita S, Koshiji T (2008) Abnormalities in the brain before elective cardiac surgery detected by diffusion-weighted magnetic resonance imaging. Ann Thorac Surg 86:1563–1569
van Dijk D, Keizer AM, Diephuis JC, Durand C, Vos LJ, Hijman R (2000) Neurocognitive dysfunction after coronary artery bypass surgery: a systematic review. J Thorac Cardiovasc Surg 120:632–639
Pugsley W, Klinger L, Paschalis C, Treasure T, Harrison M, Newman S (1994) The impact of microemboli during cardiopulmonary bypass on neuropsychological functioning. Stroke 25:1393–1399
Stump DA, Kon NA, Rogers AT, Hammon JW (1996) Emboli and neuropsychological outcome following cardiopulmonary bypass. Echocardiography 13:555–558
Neville MJ, Butterworth J, James RL, Hammon JW, Stump DA (2001) Similar neurobehavioral outcome after valve or coronary artery operations despite differing carotid embolic counts. J Thorac Cardiovasc Surg 121:125–136
Rodriguez RA, Rubens FD, Wozny D, Nathan HJ (2010) Cerebral emboli detected by transcranial Doppler during cardiopulmonary bypass are not correlated with postoperative cognitive deficits. Stroke 41:2229–2235
Knipp SC, Matatko N, Wilhelm H, Schlamann M, Thielmann M, Lösch C, Diener HC, Jakob H (2008) Cognitive outcomes three years after coronary artery bypass surgery: relation to diffusion-weighted magnetic resonance imaging. Ann Thorac Surg 85:872–879
Barber PA, Hach S, Tippett LJ, Ross L, Merry AF, Milsom P (2008) Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke 39:1427–1433
Restrepo L, Wityk RJ, Grega MA, Borowicz L Jr, Barker PB, Jacobs MA, Beauchamp NJ, Hillis AE, McKhann GM (2002) Diffusion- and perfusion-weighted magnetic resonance imaging of the brain before and after coronary artery bypass grafting surgery. Stroke 33:2909–2915
Cook DJ, Huston J 3rd, Trenerry MR, Brown RD Jr, Zehr KJ, Sundt TM 3rd (2007) Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg 83:1389–1395
Siepe M, Pfeiffer T, Gieringer A, Zemann S, Benk C, Schlensak C, Beyersdorf F (2011) Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur J Cardiothorac Surg 40:200–207
Abildstrom H, Høgh P, Sperling B, Moller JT, Yndgaard S, Rasmussen LS (2002) Cerebral blood flow and cognitive dysfunction after coronary surgery. Ann Thorac Surg 73:1174–1178
Caplan LR, Hennerici M (1998) Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 55:1475–1482
Rasmussen LS, Christiansen M, Rasmussen H, Kristensen PA, Moller JT (2000) Do blood concentrations of neurone specific enolase and S-100 beta protein reflect cognitive dysfunction after abdominal surgery? ISPOCD Group. Br J Anaesth 84:242–244
Kofke WA, Konitzer P, Meng QC, Guo J, Cheung A (2004) The effect of apolipoprotein E genotype on neuron specific enolase and S-100beta levels after cardiac surgery. Anesth Analg 99:1323–1325
Ramlawi B, Rudolph JL, Mieno S, Khabbaz K, Sodha NR, Boodhwani M, Levkoff SE, Marcantonio ER, Sellke FW (2006) Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Ann Surg 244:593–601
Whitaker DC, Green AJ, Stygall J, Harrison MJ, Newman SP (2007) Evaluation of an alternative S100b assay for use in cardiac surgery: relationship with microemboli and neuropsychological outcome. Perfusion 22:267–272
Anderson RE, Hansson LO, Vaage J (1999) Release of S100B during coronary artery bypass grafting is reduced by off-pump surgery. Ann Thorac Surg 67:1721–1725
Kofke WA, Garman RH, Stiller RL, Rose ME, Garman R (1996) Opioid neurotoxicity: fentanyl dose–response effects in rats. Anesth Analg 83:1298–1306
Kofke WA, Attaallah AF, Kuwabara H, Garman RH, Sinz EH, Barbaccia J, Gupta N, Hogg JP (2002) The neuropathologic effects in rats and neurometabolic effects in humans of large-dose remifentanil. Anesth Analg 94:1229–1236
Burkhart CS, Dell-Kuster S, Gamberini M, Moeckli A, Grapow M, Filipovic M, Seeberger MD, Monsch AU, Strebel SP, Steiner LA (2010) Modifiable and nonmodifiable risk factors for postoperative delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 24:555–559
Silbert BS, Scott DA, Evered LA, Lewis MS, Kalpokas M, Maruff P, Myles PS, Jamrozik K (2006) A comparison of the effect of high- and low-dose fentanyl on the incidence of postoperative cognitive dysfunction after coronary artery bypass surgery in the elderly. Anesthesiology 104:1137–1145
Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE (2006) The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology 104:988–994
Tang J, Eckenhoff MF, Eckenhoff RG (2010) Anesthesia and the old brain. Anesth Analg 110:421–426
Avidan MS, Searleman AC, Storandt M, Barnett K, Vannucci A, Saager L, Xiong C, Grant EA, Kaiser D, Morris JC, Evers AS (2009) Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology 111:964–970
Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z (2013) Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology 118:502–515
Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA (2001) Neurological outcome research group and the cardiothoracic anesthesiology research endeavors investigators. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 344:395–402
van Dijk D, Spoor M, Hijman R, Nathoe HM, Borst C, Jansen EW, Grobbee DE, de Jaegere PP, Kalkman CJ (2007) Octopus Study Group. Cognitive and cardiac outcomes 5 years after off-pump vs on-pump coronary artery bypass graft surgery. JAMA 297:701–708
Selnes OA, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, McKhann GM (2009) Do management strategies for coronary artery disease influence 6-year cognitive outcomes? Ann Thorac Surg 88:445–454
Fontes MT, Swift RC, Phillips-Bute B, Podgoreanu MV, Stafford-Smith M, Newman MF, Mathew JP (2013) Neurologic outcome research group of the duke heart center. Predictors of cognitive recovery after cardiac surgery. Anesth Analg 116:435–442
Katz ES, Tunick PA, Rusinek H, Ribakove G, Spencer FC, Kronzon I (1992) Protruding aortic atheromas predict stroke in elderly patients undergoing cardiopulmonary bypass: experience with intraoperative transesophageal echocardiography. J Am Coll Cardiol 20:70–77
Ura M, Sakata R, Nakayama Y, Goto T (2000) Ultrasonographic demonstration of manipulation-related aortic injuries after cardiac surgery. J Am Coll Cardiol 35:1303–1310
Murkin JM, Martzke JS, Buchan AM, Bentley C, Wong CJ (1995) A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. II. Neurologic and cognitive outcomes. J Thorac Cardiovasc Surg 110:349–362
Grigore AM, Grocott HP, Mathew JP, Phillips-Bute B, Stanley TO, Butler A, Landolfo KP, Reves JG, Blumenthal JA, Newman MF (2002) Neurologic outcome research group of the duke heart center. The rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgery. Anesth Analg 94:4–10
Puskas F, Grocott HP, White WD, Mathew JP, Newman MF, Bar-Yosef S (2007) Intraoperative hyperglycemia and cognitive decline after CABG. Ann Thorac Surg 84:1467–1473
Djaiani G, Fedorko L, Borger MA, Green R, Carroll J, Marcon M, Karski J (2007) Continuous-flow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation 116:1888–1895
Acknowledgments
The author would like to thank Tomoko Goto, MD, for her valuable comments and suggestions and Jon Moon, PhD, for his editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Maekawa, K. (2015). Postoperative Cognitive Dysfunction After Cardiac Surgery and Neuroprotection. In: Uchino, H., Ushijima, K., Ikeda, Y. (eds) Neuroanesthesia and Cerebrospinal Protection. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54490-6_54
Download citation
DOI: https://doi.org/10.1007/978-4-431-54490-6_54
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54489-0
Online ISBN: 978-4-431-54490-6
eBook Packages: MedicineMedicine (R0)