Abstract
Purpose
A multicenter survey was conducted to explore the role of adjuvant surgery for initially unresectable pancreatic cancer with a long-term favorable response to non-surgical cancer treatments.
Methods
Clinical data including overall survival were retrospectively compared between 58 initially unresectable pancreatic cancer patients who underwent adjuvant surgery with a favorable response to non-surgical cancer treatments over 6 months after the initial treatment and 101 patients who did not undergo adjuvant surgery because of either unchanged unresectability, a poor performance status, and/or the patients’ or surgeons’ wishes.
Results
Overall mortality and morbidity were 1.7 and 47 % in the adjuvant surgery group. The survival curve in the adjuvant surgery group was significantly better than in the control group (p < 0.0001). The propensity score analysis revealed that adjuvant surgery was a significant independent prognostic variable with an adjusted hazard ratio (95 % confidence interval) of 0.569 (0.36–0.89). Subgroup analysis according to the time from initial treatment to surgical resection showed a significant favorable difference in the overall survival in patients who underwent adjuvant surgery over 240 days after the initial treatment.
Conclusion
Adjuvant surgery for initially unresectable pancreatic cancer patients can be a safe and effective treatment. The overall survival rate from the initial treatment is extremely high, especially in patients who received non-surgical anti-cancer treatment for more than 240 days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is a lethal disease, and contributes to the increasing number of cancer deaths worldwide. Only 20 % of patients can be treated by surgery, and the overall 5-year survival rate is less than 5 % [1, 2]. Irrespective of the treatment strategy adopted, prognosis in patients with unresectable pancreatic cancer continues to be disappointing, with a median survival of 8–14 months [3–7]. These patients rarely have a chance to live more than 3 years.
Medical oncologists or pancreatic surgeons have identified candidates for surgical resection in patients with initially unresectable pancreatic cancer who favorably responded to multimodal treatment. Additional surgical resection during multimodal treatment is called “adjuvant surgery” [8]. The role of adjuvant surgery has not been fully determined because the number of patients who received this type of treatment was very small in each institution. Is adjuvant surgery a safe or effective treatment option for patients with unresectable pancreatic cancer? When should a shrunken tumor be removed in the process of maintaining chemotherapy and/or radiation therapy? There is no study indicating the clinical efficacy, safety and optimal timing of adjuvant surgery. There are long-term survivors and a comparable survival rate among this subset of patients after surgical resection following multimodal treatment [8–12]. However, the duration of multimodal treatment before pancreatectomy varies from a few months to several years in previous reports [8–12]. The clinical data on initially unresectable pancreatic cancer patients with a favorable response to chemo(radio)therapy over 6 months were collected as a project study of pancreatic surgery under the supervision of the Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS), to assess the role of adjuvant surgery in the clinical setting.
Patients and methods
A multicenter survey was conducted to collect clinical data on patients who underwent adjuvant surgery for initially unresectable pancreatic cancer following a favorable response to chemo(radio)therapy over 6 months from 2001 to 2009. Detailed data on 58 patients (adjuvant surgery group) were retrospectively collected from 39 out of 150 training institutes for highly advanced surgery registered by the committee of JSHBPS in 2009. The study criterion was initially unresectable pancreatic cancer patients who underwent surgical resection following the achievement of stable disease (SD), partial response (PR), or complete response (CR) defined by Response Evaluation Criteria In Solid Tumors (RECIST version 1.1 [13]) over 6 months after initiating non-surgical anti-cancer treatments. The clinical data on 101 patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments who did not undergo surgical resection was collected as a control group from the same 39 centers. The unresectability of pancreatic cancer was based on the clinical criteria in each institute.
All patients had cytologically or pathologically proven ductal adenocarcinoma of the pancreas. The clinical variables shown in Table 1 were collected. Radiological assessment was performed according to RECIST version 1.1 [13]. The pathological parameters included residual tumor grading, Evans classification [14], and tumor staging according to TNM classification [15]. Serial data on tumor markers such as carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), DUPAN-2 or Span-1 were collected every 1–3 months during multimodal treatment. Post-operative follow-up data included serial data on tumor markers, adjuvant chemotherapy, the date and the primary site of disease recurrence, the date and cause of death, and the last follow-up date. The observation period was defined as the time from the initial treatment to the date of death for censored patients or the last follow-up date for non-censored patients. This study was performed in accordance with the precepts of the Helsinki Declaration, and was approved by the local ethics committee.
Statistical analysis
Continuous variables were expressed as median values and range. All parameters were compared between the adjuvant surgery and control groups. Statistical analyses, including the Mann–Whitney U test for continuous variables, and chi-squared statistics or Fisher’s exact test for categorical variables, were performed using SAS software version 9.2 (SAS Institute, Cary, NC, USA). The primary outcome variable was overall survival, defined as the time from non-surgical anti-cancer treatments to death or the last follow-up date. Comparisons of the overall survival between the two groups were made using the log-rank test. In addition, profound factors identified by the univariate analysis were further examined by multivariate Cox proportional-hazard models to determine independent significant factors for survival.
A propensity score methodology was used to provide adjustments since a propensity score can calculate the conditional probability of receiving a treatment given all potential confounders measured. The propensity score analysis required calculation of the conditional probabilities for the adjuvant surgery group using a multivariate logistic regression to generate a propensity score [16]. The selection of variables for calculating the propensity score was based on the potential association with the overall survival results (sex, age, radiation therapy or not, tumor marker decrease or not during non-surgical anti-cancer treatment, PR/CR vs SD, tumor size, amount of gemcitabine administration, reason for unresectability). Model discrimination was assessed with C-statistics, and model calibration was assessed with Hosmer–Lemeshow statistics. The propensity score was subdivided into quartiles as shown in Table A (Electronic Supplementary Material). The treatment effect was separately estimated within each quartile, and quartile estimates were combined to give an overall estimate of adjuvant surgery. A survival analysis using Cox proportional-hazard models was used. The hazard ratio and 95 % confidence intervals were calculated for all estimates. A 2-tailed p value less than 0.05 was considered to be statistically significant.
Results
Clinical background in the adjuvant surgery and control groups
Tables 1 and 2 show that the reason for the initially unresectable pancreatic cancer was 41 locally advanced tumor and 17 distant organ metastases in the adjuvant surgery group. Fifty-three patients received gemcitabine-based chemotherapy, and 32 patients had S-1 chemotherapy. The radiological response of SD, PR, or CR was found in 7, 39, and 12 patients, respectively. The median duration between the initial therapy and the detection of PR/CR was 150 days (21–739). The median duration between the detection of PR/CR and surgical resection was 127 days (8–1335). Forty-six of 52 patients with available value of any tumor marker showed a decrease in the level of tumor marker before surgical resection, and only four patients had an increase, relative to the pre-initial treatment level.
The control group included 43 patients judged to have unresectable disease on laparotomy (18 locally unresectable, 13 peritoneal dissemination, 10 liver metastasis, and 2 distant lymph node metastasis), and 58 patients who did not undergo surgical resection because of either unchanged unresectability, a poor performance status, and/or the patients’ or surgeons’ wishes. Thirty-seven of 58 patients had SD on RECIST, and 21 patients had PR (8 distant organ metastases and 13 locally advanced tumors; Table 1).
There were significant differences in the age, presence of peritoneal metastasis, tumor size, concomitant use of radiotherapy, and frequency of PR/CR between the adjuvant surgery and control groups (p < 0.05).
Surgical background and post-operative complications in the adjuvant surgery group
The median time from initial therapy to surgical resection was 274 days (182–1418). Concomitant resections of other organs were performed in 40 patients (69 %; Table 2). As shown in Table 2, 23 patients underwent portal vein resection. The superior mesenteric artery, celiac axis and common hepatic artery were concomitantly resected in 1, 10, and 2 patients, respectively. There were 11 adrenal resections, 5 liver resections, 2 liver biopsies, and 2 colon resections. Post-operative mortality and morbidity are summarized in Table 3. There was no incidence of aspiration pneumonia, myocardial infarction, cerebral infarction, or pulmonary thrombosis.
Pathological findings in the adjuvant surgery group
Five of the 13 patients with liver metastases underwent surgical resection for metastatic lesions and two patients underwent liver biopsies. No liver tumors were found during surgery in the residual 6 patients with liver metastases. One patient had peritoneal metastasis diagnosed on computed tomography scan which was not found during surgical resection of the primary tumor. A pathological evaluation was done in 55 patients according to the Evans classification, and showed Grade I (n = 17), IIa (16), IIb (10), III (5), and IV (7). Pathological CR was found in 7 patients who had 5 locally advanced tumors, 1 para-aortic lymph node metastasis, and 1 liver metastasis. The 17 patients with distant organ metastases underwent R0 (n = 12), R1 (n = 4), and R2 (n = 1) resection, and 41 patients with locally advanced tumor had R0 (n = 36) and R1 (n = 5).
Survival analysis in the adjuvant surgery and control groups
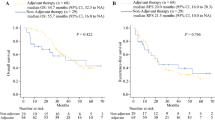
The median observation period was 51 months (20–122) in the control group. The overall survival rates at 1, 3, and 5 years in the control group were 88, 18, and 10 %, respectively, and the median survival time was 20.8 months. The median observation and post-operative observation periods in the adjuvant surgery group were 54 months (26–125) and 41 months (18–117), respectively. The overall survival rates at 1, 3, and 5 years were 95, 53, and 34 %, respectively, and the median survival time was 39.7 months. The overall survival rates after surgical resection at 1, 3, and 5 years were 76, 33, and 29 %, respectively, and the median survival time was 25 months. Figure 1 demonstrates that the survival curve in the adjuvant surgery group was significantly better than that in the control group (p < 0.0001). Five-year survival was observed in 9 patients in the adjuvant surgery group, and 4 patients in the control group. A multivariate analysis showed only a longer period of initial treatments to be a significant independent factor associated with survival in the adjuvant surgery group (Table 4). The disease-free survival rates at 1, 3, and 5 years were 54, 30, and 30 %, respectively. The primary site of recurrence was detected in a distant organ (n = 21; liver 11, lung 4, peritoneum 6, and liver and peritoneum 1) and in the loco-regional area (n = 15). One patient had an unknown site of recurrence. Twenty-one patients did not have any recurrence of disease. There was no significant difference in the primary site of recurrence and disease-free survival curve associated with the reason for unresectability.
Comparison of the overall survival curves between the adjuvant surgery (solid line) and control groups (broken line). The overall survival rates at 1, 3, and 5 years were 95, 53, and 34 % in the adjuvant surgery group, and 88, 18, and 10 % in the control group, respectively, and the median survival time was 39.7 months in the adjuvant surgery group and 20.8 months in the control group. The survival curve in the adjuvant surgery group was significantly better than that in the control group (p < 0.0001)
Univariate and multivariate Cox proportion-hazard model analyses for overall survival in all patients
Table 5 shows metastatic disease, an increase in tumor marker, dose of gemcitabine <28 g, and stable disease on RECIST each increased the risk of death relative to those without the respective risk characteristics (hazard ratio range 1.209–1.800, all p < 0.05). Data were further stratified by known clinical predictors of survival, and adjuvant surgery was protective and statistically significant among each risk group. A multivariate analysis using clinical predictors obtained by univariate analysis showed that the adjuvant surgery group, a decrease of tumor markers during non-surgical anti-cancer treatments, dose of gemcitabine (≤28 g), and RECIST evaluation (PR/CR) were significant favorable factors for survival (Table 6).
Cox proportion-hazard model analysis stratified over the propensity score
Propensity scores were calculated using multivariate logistic regression with calculation of the conditional probabilities for the adjuvant surgery group to adjust for the significant differences in the clinical backgrounds between two groups. A Cox proportional-hazard model analysis stratified over the propensity score was performed to account for the non-randomized provision of adjuvant surgery. Table 7 demonstrates that the adjuvant surgery group was a significant independent prognostic variable with an adjusted hazard ratio (95 % confidence interval) of 0.569 (0.36–0.89).
Optimal timing of adjuvant surgery in this study
Figure 2a shows that the longer the duration of the initial treatment prior to surgical resection, the longer the survival time. Figure 2b shows comparisons of the survival curves of adjuvant surgery according to the time from the initial treatment to surgical resection; group A, over 365 days after the initial treatment (n = 12); group B, between 241 and 365 days (n = 26); group C, between 180 and 240 days after initial treatment (n = 20); control group (group D, n = 101). Although there was no difference in the survival curves between groups C and D (p = 0.795), significant differences were found in the survival curve between groups B and C or D (p < 0.0001), and between groups A and B, C, or D (p < 0.005). The overall survival rate in group A + B was statistically better than in group C (p < 0.0001). There was no difference in the primary site of recurrence (60 % distant organ metastasis and 40 % loco-regional recurrence) between groups A + B and C.
Survival time and curves according to time from initial treatment to surgical resection. a Survival time in each patient. Group A, 12 patients who underwent adjuvant surgery more than 365 days after initial treatment; Group B, 26 patients who underwent adjuvant surgery between 241 and 365 days; Group C, 20 patients who underwent adjuvant surgery between 180 and 240 days. b Comparisons of the survival curves of adjuvant surgery more than 365 days after the initial treatment [n = 12, group A, median survival time (MST) not reached], between 241 and 365 days (n = 26, group B, MST 43 months), between 180 and 240 days after initial treatment (n = 20, group C, MST 17 months), and the control group (n = 101, group D, MST 20 months). Although there was no difference in the survival curves between groups C and D (p = 0.795), significant differences were found in the survival curve between groups B and C or D (p < 0.0001), and between groups A and B, C, or D (p < 0.005). The overall survival rate in group A + B was significantly better than in group C (p < 0.0001). The dose of gemcitabine and S-1, and the tumor diameter, in group A + B were significantly greater than those in group C (p < 0.05) but there were no significant differences in other clinical parameters
Discussion
A multicenter survey organized by JSHBPS collected 159 initially unresectable pancreatic cancer patients with favorable response to non-surgical anti-cancer treatments over 6 months after the initial treatment between 2001 and 2009. Fifty-eight patients underwent “adjuvant surgery”, and the residual 101 patients who did not undergo adjuvant surgery served as a control group. The first clinical question of this survey was whether the addition of adjuvant surgery is safe treatment. The surgical mortality and morbidity in this study were 1.7 and 47 %, respectively, which was similar to the previous reports in initially resectable pancreatic cancer patients [17, 18], in spite of a more extensive/aggressive surgical approach (69 % of combined organ or vascular resection rate in this study). The second clinical question of this survey was whether additional adjuvant surgery is an effective treatment. Surprisingly, the overall survival rates at 1, 3, and 5 years from the initial treatment were 95, 53, and 34 %, respectively, in this highly selected group of patients, under a median observation period of 54 months (26–125), which was significantly better than those (88, 18, and 10 %) in the control group. The unadjusted and propensity-score adjusted stratified multivariate analyses showed adjuvant surgery to be a significant independent factor for overall survival. Furthermore, favorable survival rates were observed among all risk-stratified subgroups with the addition of adjuvant surgery.
Appropriate surgical management for the patients with initially unresectable pancreatic cancer is less clear. There are some reports from several groups on the use of chemo(radio)therapy to downstage unresectable pancreatic cancer to resectable disease [19–23]. They reported that the median survival time after surgery in these patients with unresectable tumor at presentation is 23.6 months [11, 19–24]. These results appear to be at least comparable to those reported with surgery alone or surgery plus postoperative adjuvant treatment in resectable patients [12]. The Memorial Sloan–Kettering Cancer Center (MSKCC) group reported that 36 patients who were able to undergo surgical resection following treatment of initial stage III pancreatic cancer experienced survival similar to those who were initially resectable as a matched control [24]. The current study found that the longer the median time from the initial therapy to surgical resection, the longer the median post-operative follow-up, and the higher the frequency of concomitant vascular resection, relative to the results from the MSKCC group. A major difference from the previous reports in this study is the investigation of the clinical safety and efficacy of adjuvant surgery in this highly selected group of patients in comparison to patients who did not undergo adjuvant surgery.
This study definitively selected patients at the initial detection of progressive disease during multimodal treatment over 6 months, and at the detection of occult distant organ metastasis during surgical exploration. Moreover, any patients with a poor functional status were also excluded in the process of non-surgical anticancer treatments. Therefore, 58 patients in the adjuvant surgery group were regard as “super-responders” to non-surgical anticancer treatments. This retrospective patient selection is one of the limitations of this study. The other limitation is that the criteria used to select patients who were eligible for surgical exploration during non-surgical anticancer treatments differed among institutions. The 58 patients in the adjuvant surgery group were collected from 39 hospitals over 8 years, and thus the average number was 1.2 cases per hospital. Moreover, it should be noted that a significantly higher rate of peritoneal metastasis was found in the control group.
Donahue et al. [25] reported that patients with initially unresectable pancreaticobiliary malignant tumors should be selected for surgery on the basis of lack of disease progression, good functional status, and a decrease in the CA19-9 level rather than of evidence that vessel involvement has disappeared on computed tomography or magnetic resonance imaging. The third clinical question is the optimal time for adjuvant surgery in this patient population. When should the shrunken tumor be removed in the process of maintaining chemotherapy and/or radiation therapy? The sub-group analysis according to the time from the initial treatment to surgical resection showed significant favorable differences in the overall survival rates in patients who were able to undergo adjuvant surgery more than 240 days after initial treatment. Therefore, the recommended optimal time for adjuvant surgery is at least 240 days after the initial treatment. A longer duration of non-surgical anti-cancer treatment may be associated with better patient selection, greater doses of chemotherapy, a higher rate of PR/CR, and lower levels of tumor markers, thus resulting in a better prognosis of patients, since a certain period of observation time allows for the identification of progressive disease or poor surgical candidates. The primary findings of this study indicate the importance of finding the appropriate non-surgical anticancer treatments for effective tumor downsizing over at least 240 days after the initial treatment.
The adjuvant surgery group underwent major pancreatic resection with concomitant other organ and/or vascular resection in 69 % of patients. It is technically possible to perform extensive resections with vein and/or arterial reconstruction, but concomitant arterial resection remains controversial because it is associated with a high morbidity [26–28]. Laurence et al. [28] reported that an increased risk of perioperative death appears to be associated with resection performed in patients with initially designated unresectable tumors prior to neoadjuvant chemoradiation therapy. Nakao et al. [29] reported that pancreatectomy with portal vein resection can be performed safely, and long-term survival is observed in selected patients. The current study found no significant difference in overall survival or morbidity and mortality between those receiving concomitant resection or not. Therefore, the results from this study demonstrated that concomitant resections of other organs and vessels were safely performed with special caution.
In conclusion, adjuvant surgery for initially unresectable pancreatic cancer patients with a long-term favorable response to non-surgical anticancer treatments is considered to be a safe and effective treatment. The overall survival rate from the initial treatment was extremely high, especially in patients who received non-surgical anti-cancer treatment for more than 240 days. Adjuvant surgery can occupy an important position in multimodal therapy for patients with initially unresectable pancreatic cancer.
References
Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–65.
Conlon KC, Klimstra DS, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma: clinicopathological analysis of 5-year survivors. Ann Surg. 1996;223:273–9.
Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751–5.
Gastrointestinal Tumor Study Group. A multi-institutional comparative trial of radiation therapy alone and in combination with 5-fluorouracil for locally unresectable pancreatic carcinoma. Ann Surg. 1979;189:205–8.
Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–9.
Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–12.
Morganti AG, Massaccesi M, La Torre G, Caravatta L, Piscopo A, Tambaro R, et al. A systematic review of resectability and survival after concurrent chemoradiation in primarily unresectable pancreatic cancer. Ann Surg Oncol. 2010;17:194–205.
Kato K, Kondo S, Hirano S, Tanaka E, Shichinohe T, Tsuchikawa T, et al. Adjuvant surgical therapy for patients with initially-unresectable pancreatic cancer with long-term favorable responses to chemotherapy. J Hepatobiliary Pancreat Sci. 2011;18:712–6.
Ammori JB, Colletti LM, Zalupski MM, Eckhauser FE, Greenson JK, Dimick J, et al. Surgical resection following radiation therapy with concurrent gemcitabine in patients with previously unresectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2003;7:766–72.
Aristu J, Cañón R, Pardo F, Martínez-Monge R, Martin-Algarra S, Manuel Ordoñez J, et al. Surgical resection after preoperative chemoradiotherapy benefits selected patients with unresectable pancreatic cancer. Am J Clin Oncol 2003;26:30–6.
Sa Cunha A, Rault A, Laurent C, Adhoute X, Vendrely V, Béllannée G, et al. Surgical resection after radiochemotherapy in patients with unresectable adenocarcinoma of the pancreas. J Am Coll Surg 2005;201:359–65.
Gillen S, Schuster T, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Evans DB, Rich TA, Byrd DR. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–9.
Sobin L, Gospodarowicz MK, Wittekind C, eds. UICC TNM Classification of Malignant Tumors, 7th ed. New York: Wiley-Liss; 2009.
Newgard CD, Hedges JR, Arthur M, Mullins RJ. Advanced statisics: the propensity score––a method for estimating treatment effect in observational research. Acad Emerg Med. 2004;11:953–61.
White RR, Hurwitz HI, Morse MA, Lee C, Anscher MS, Paulson EK, et al. Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas. Ann Surg Oncol. 2001;8:758–65.
Marti JL, Hochster HS, Hiotis SP, Donahue B, Ryan T, Newman E. Phase I/II trial of induction chemotherapy followed by concurrent chemoradiotherapy and surgery for locoregionally advanced pancreatic cancer. Ann Surg Oncol. 2008;15:3521–31.
Adhoute X, Smith D, Vendrely V, Rault A, Sa Cunha A, Legoux JL, et al. Subsequent resection of locally advanced pancreatic carcinoma after chemoradiotherapy. Gastroenterol Clin Biol. 2006;30:224–30.
Wilkowski R, Thoma M, Schauer R, Wagner A, Heinemann V. Effect of chemoradiotherapy with gemcitabine and cisplatin on locoregional control in patients with primary inoperable pancreatic cancer. World J Surg. 2004;28:1011–8.
Massucco P, Capussotti L, Magnino A, Sperti E, Gatti M, Muratore A, et al. Pancreatic resections after chemoradiotherapy for locally advanced ductal adenocarcinoma: analysis of perioperative outcome and survival. Ann Surg Oncol. 2006;13:1201–8.
Wanebo HJ, Glicksman AS, Vezeridis MP, Clark J, Tibbetts L, Koness RJ, et al. Preoperative chemotherapy, radiotherapy, and surgical resection of locally advanced pancreatic cancer. Arch Surg. 2000;135:81–7.
Andriulli A, Festa V, Botteri E, Valvano MR, Koch M, Bassi C, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012;19:1644–62.
Bickenbach KA, Gonen M, Tang LH, O’Reilly E, Goodman K, Brennan MF, et al. Downstaging in pancreatic cancer: a matched analysis of patients resected following systemic treatment of initially locally unresectable disease. Ann Surg Oncol. 2012;19:1663–9.
Donahue TR, Isacoff WH, Hines OJ, Tomlinson JS, Farrell JJ, Bhat YM, et al. Downstaging chemotherapy and alteration in the classic computed tomography/magnetic resonance imaging signs of vascular involvement in patients with pancreaticobiliary malignant tumors. Influence on patient selection for surgery. Arch Surg. 2011;146:836–43.
Bockhorn M, Burdelski C, Bogoevski D, Sgourakis G, Yekebas EF, Izbicki JR. Arterial en bloc resection for pancreatic carcinoma. Br J Surg. 2011;98:86–92.
Amano H, Miura F, Toyota N, Wada K, Katoh K, Hayano K, et al. Is pancreatectomy with arterial reconstruction a safe and useful procedure for locally advanced pancreatic cancer? J Hepatobiliary Pancreat Surg. 2009;16:850–7.
Laurence JM, Tran PD, Morarji K, Eslick GD, Lam VWT, Sandroussi C. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15:2059–69.
Nakao A, Kanzaki A, Fujii T, Kodera Y, Yamada S, Sugimoto H, et al. Correlation between radiographic classification and pathologic grade of portal vein wall invasion in pancreatic head cancer. Ann Surg. 2012;255:103–8.
Acknowledgments
We would like to express our deep condolences over the passing of Professor Satoshi Kondo. Professor Kondo made a great contribution as the leader of this study by conceiving the ideas of the clinical study. Regrettably, he passed away without seeing the completion of the research. A professional writing service was used in the English review of this article, and a professional service was also used in a statistical analysis of this study. JHBPS funded the use of these services. Finally, we greatly thank the institutions which provided the detailed patient data (Supplemental Table). Funding: the Japanese Society of Hepato-Biliary-Pancreatic Surgery.
Conflicts of interest
The authors have no commercial affiliations that might pose any conflicts of interest in connection with this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Satoi, S., Yamaue, H., Kato, K. et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 20, 590–600 (2013). https://doi.org/10.1007/s00534-013-0616-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-013-0616-0