Abstract
Background
To evaluate the prognostic impact of surgical intervention for initially-unresectable pancreatic ductal adenocarcinomas with long-term favorable responses to chemotherapy.
Method
Twelve patients with initially-unresectable pancreatic ductal carcinomas who underwent radical surgery after a favorable response to chemotherapy for six months or longer in principle were enrolled in this study. We retrospectively reviewed the charts of these 12 patients and performed a survival analysis.
Results
Initially, the included patients were unable to undergo resection secondary to locally-advanced disease in eight patients and metastatic disease in four patients. The length of preoperative therapy was five to 44 months (median 12). The operative procedure included resection of the area initially involved by tumor and regional major vessels. The postoperative mortality and morbidity rates were 0% for patients with locally-advanced disease and 50% in those with metastatic disease. R0 resection was achieved in nine patients (75%) and pathological CR was seen in one patient. Estimated overall five-year survival from initial therapy was 50.0%. The survival rate (0% at 5 years) of the four patients with metastatic disease as the cause of initial unresectability was significantly worse than that (100% at 5 years) of the eight patients with locally-advanced disease (P = 0.0014).
Conclusion
Surgical intervention should be considered for patients with initially-unresectable pancreatic cancers who demonstrate long-term favorable responses to chemotherapy. R0 resection may significantly contribute to cure, especially in patients with initially locally-advanced disease. Large cohort prospective studies will be necessary to demonstrate the efficacy of this strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal carcinoma has one of the poorest prognoses of all digestive organ cancers. The prognosis of patients with unresectable disease is significantly worse than that of patients with resectable tumors [1]. Gemcitabine (GEM) is a key drug in the current treatment of unresectable pancreatic carcinoma [2]. Since the introduction of GEM, however, rare long-term favorable responses to chemotherapy have been achieved in unresectable cases [3]. The survival of such patients remains only two to three years; survival greater than four years has been practically impossible [1, 4]. To improve the prognosis of unresectable pancreatic carcinoma, a novel therapeutic strategy is needed. As long-term survival can be achieved only by radical resection [5, 6], surgical interventions in initially-unresectable patients who demonstrate long-term responses to chemotherapy may be effective. As yet, such approaches have not been reported. Recently, we performed radical surgery for initially-unresectable cases of pancreatic cancer which had responded well to chemotherapy without original intent to proceed to resection. This study sought to evaluate the prognostic impact of “adjuvant surgery” for initially-unresectable pancreatic ductal adenocarcinoma in patients demonstrating long-term favorable responses to chemotherapy.
Methods
Patients included
Between January 2007 and April 2010, a total of 112 patients with pancreatic ductal carcinoma underwent radical surgery; 12 of these patients were initially unresectable, becoming resectable as a result of chemotherapy initially administered without intent to resect. Patients who underwent treatment with neoadjuvant chemotherapy with the intention of proceeding to resection were omitted from this study. Tumor histology was confirmed in all patients from the resected specimens or preoperative biopsies (another patient who experienced a complete response (CR) [7] was excluded from this study because of a lack of confirmatory pathology). Tumors were deemed unresectable when distant metastasis, such as hepatic metastasis or paraaortic lymph node metastasis, were identified, when there was involvement of the major arteries, such as the superior mesenteric, common hepatic, or celiac arteries, or when portal vein thrombosis with tumor thrombus was identified. After chemotherapy, we confirmed the absence of major vessel involvement or the disappearance or the progression-free of distant metastasis by imaging and intraoperative findings, then performed the radical operation six months or longer in principle after beginning the initial treatment. The operative procedure included resection of the area initially involved by tumor and regional major vessels. Written informed consent was obtained from all patients prior to intervention.
Assessments
Pathologic diagnosis and classification were based on the TNM classification system of the International Union Against Cancer (2009) [8]. The pathological effect of preoperative therapy was assessed by Evans’s grading system [9].
Survival analysis
The median follow-up time from initial therapy in all 12 patients was 34.7 months. Survival curves were estimated by the Kaplan–Meier method and compared using the log-rank test. P < 0.05 was considered statistically significant.
Results
Patients
The ages of the 12 patients, six men and six women, ranged from 43 to 68 years with a median of 63 years. Tumors were initially unresectable due to major artery involvement in seven patients, hepatic metastasis in three patients, paraaortic lymph node metastases in one patient, and tumor thrombus with extensive secondary portal vein thrombosis in one patient.
Preoperative therapy
Intravenous chemotherapy was administered to seven patients, transarterial chemotherapy was given to three patients, and CRT was given to two patients. The duration of preoperative treatment was five to 44 months (median 12). The chemotherapeutic regimens consisted of GEM and S-1 in seven patients, GEM and 5-Fluorouracil in three patients, and GEM alone in two patients.
Perioperative results
Five patients underwent distal pancreatectomy with en bloc celiac axis resection (DP-CAR) [10], while four underwent subtotal stomach-preserving pancreatoduodenectomy. Two additional patients underwent distal pancreatectomy, and one patient underwent total pancreatectomy with celiac axis resection. Combined resection was performed in nine cases, portal vein in six cases, portal vein and gastroduodenal artery in one case, adrenal gland in one case, and liver in one case (liver metastasis had disappeared in other two cases and not resected). The postoperative mortality rate was 0%, with a morbidity rate of 50%. Hospital stay ranged from 12 to 45 days (median 27) (Table 1).
Pathological findings in surgical specimen
The tumor was primarily located in the pancreatic body in six patients, in the pancreatic head in four patients, and in the pancreatic tail in two patients. Maximal tumor diameter was 45 mm. The pathological stages were CR in one patient, IIA in four patients, IIB in one patient, III in four patients, and IV in two patients in accordance with UICC classification. The tumor viability of resected liver specimen was Grade I by Evans’s grading system. The R0 resection rate was 75% (9/12).
Postoperative chemotherapy
The chemotherapeutic regimens consisted of S-1 alone in seven patients, GEM alone in three patients, and GEM and S-1 in two patients (Table 2). Only two patients were able to remain chemotherapy free (Patient No. 7 and 10).
Survival analysis
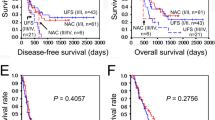
Estimated overall five-year survival from initial treatment was 50.0% (Fig. 1). The survival from the initial treatment for the four patients (0% at five years) with metastatic disease was significantly worse than that seen for the eight patients (100% at five years) with locally-advanced disease (P = 0.0014) (Fig. 2a). The survival from the time of the operation and from the initial treatment was similar (P = 0.0124) (Fig. 2b).
a Overall survival beginning at the initial treatment in patients with locally advanced disease (T-factor) or metastatic disease (M-factor) as the reason for initial unresectability (P = 0.0014). b Overall survival beginning at the time of operation in patients with locally-advanced disease (T-factor) or metastatic disease (M-factor) as the reason for initial unresectability (P = 0.0124)
Discussion
The prognosis of metastatic or locally-advanced pancreatic ductal carcinoma is extremely poor. Five-year survival is 2% or less; survival for more than five years is unlikely with standard therapies, such as chemotherapy or supportive care [1, 11]. In line with several reports suggesting that induction chemotherapy could improve outcomes [12, 13], several studies have demonstrated that long-term survival could be achieved with chemotherapy or CRT followed by radical surgery [14–17]. These reports, however, employed neoadjuvant chemotherapy with the intention of proceeding to radical surgery. To our knowledge, this is the first report in which patients with unresectable tumors were initially treated with chemotherapy, then returned to the surgeon who was then able to perform radical surgery. To distinguish between adjuvant surgery and neoadjuvant chemotherapy, our study clearly defined entry criteria as an interval greater than or equal to six months since beginning treatment.
In this study, two of the 12 patients with initially-unresectable tumors achieved long-term survival greater than five years with the combination of chemotherapy and subsequent surgical intervention. Pathological CR was achieved only in one patient. The survival of patients with unresectable disease treated with chemo(radio)therapy alone was typically no greater than two to three years, making survival greater than four years an almost impossible goal [1]. Thus, surgical intervention may be the only treatment able to result in longer-term survivals, even in patients with continuing favorable long-term responses to chemotherapy.
Patients with locally-advanced disease as the initial cause of unresectability had good responses to the surgical intervention, with no deaths occurring in the study period. In contrast, patients with metastatic disease had poor responses, and all patients died due to recurrent disease. Locally-advanced disease may be a good indication for adjuvant surgery after sustained favorable responses to chemotherapy, even in patients with initially-unresectable disease.
In conclusion, surgical intervention should be considered for unresectable pancreatic cancers after a long-term favorable response to chemotherapy. In particular, patients with locally advanced disease as the reason for unresectability may be able to achieve R0 resection, which may contribute to cure of the disease or at least a delay in disease progression. Further studies using larger cohorts and/or prospective trials will be required to confirm the conclusion of this study.
References
Japan Pancreas Society. Pancreatic Cancer Registry Report 2007: Trend of survival of the patients with histologically confirmed unresectable invasive pancreatic cancer of JPS Stage IVa, IVb and unknown Stage. Suizo [Internet] 2007;22:e45-46. http://www.jstage.jst.go.jp/article/suizo/22/1/e45/_pdf/-char/ja/.
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13.
Goulart BH, Clark JW, Lauwers GY, Ryan DP, Grenon N, Muzikansky A, et al. Long term survivors with metastatic pancreatic adenocarcinoma treated with gemcitabine: a retrospective analysis. J Hematol Oncol. 2009;2:13.
Tanaka T, Ikeda M, Okusaka T, Ueno H, Morizane C, Hagihara A, et al. Prognostic factors in Japanese patients with advanced pancreatic cancer treated with single-agent gemcitabine as first-line therapy. Jpn J Clin Oncol. 2008;38:755–61.
Adham M, Jaeck D, Le Borgne J, Oussoultzouglou E, Chenard-Neu MP, Mosnier JF, et al. Long-term survival (5–20 years) after pancreatectomy for pancreatic ductal adenocarcinoma: a series of 30 patients collected from 3 institutions. Pancreas. 2008;37:349–51.
Shimada K, Sakamoto Y, Nara S, Esaki M, Kosuge T, Hiraoka N. Analysis of 5-year survivors after a macroscopic curative pancreatectomy for invasive ductal adenocarcinoma. World J Surg. 2010;34:1908–15.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Sobin LH, Gospodarowicz MK, Wittekind CH, editors. International Union Against Cancer TNM Classification of malignant tumours. 7th ed. New York: John Wiley & Sons, Inc.;2009.
Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–9.
Hirano S, Kondo S, Hara T, Ambo Y, Tanaka E, Shichinohe T, et al. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg. 2007;246:46–51.
David M, Lepage C, Jouve JL, Jooste V, Chauvenet M, Faivre J, et al. Management and prognosis of pancreatic cancer over a 30-year period. Br J Cancer. 2009;101:215–8.
Huguet F, Girard N, Guerche CS, Hennequin C, Mornex F, Azria D. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27:2269–77.
Krishnan S, Rana V, Janjan NA, Varadhachary GR, Abbruzzese JL, Das P, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55.
Morganti AG, Massaccesi M, La Torre G, Caravatta L, Piscopo A, Tambaro R, et al. A systematic review of resectability and survival after concurrent chemoradiation in primarily unresectable pancreatic cancer. Ann Surg Oncol. 2010;17:194–205.
Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS med. 2010;7:e1000267.
Turrini O, Viret F, Moureau-Zabotto L, Guiramand J, Moutardier V, Lelong B, et al. Neoadjuvant chemoradiation and pancreaticoduodenectomy for initially locally advanced head pancreatic adenocarcinoma. Eur J Surg Oncol. 2009;35:1306–11.
Allendorf JD, Lauerman M, Bill A, DiGiorgi M, Goetz N, Vakiani E, et al. Neoadjuvant chemotherapy and radiation for patients with locally unresectable pancreatic adenocarcinoma: feasibility, efficacy, and survival. J Gastrointest Surg. 2008;12:91–100.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kato, K., Kondo, S., Hirano, S. et al. Adjuvant surgical therapy for patients with initially-unresectable pancreatic cancer with long-term favorable responses to chemotherapy. J Hepatobiliary Pancreat Sci 18, 712–716 (2011). https://doi.org/10.1007/s00534-011-0391-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-011-0391-8