Abstract
Objective
The systematic review and meta-analysis was performed to summarize the available evidence and identify the correlates of cancer stigma.
Methods
PubMed, EMBASE, Web of Science, the Cochrane Library, and PsycINFO were electronically searched to identify eligible studies about correlates of stigma for patients with cancer. Two reviewers independently screened the literature, extracted data, and assessed the risk of bias of included studies. A meta-analysis was performed using the statistical program R.
Results
Thirty-one studies involving a total of 7114 patients were included in the systematic review and meta-analysis. The results of the meta-analysis showed that cancer stigma shared positive associations with male gender, symptoms, depression, anxiety, body image loss, self-blame, social constraint, intrusive thoughts, and ambivalence over emotional expression, and negative associations with income, NK cell subsets, QOL, self-esteem, self-efficacy, cancer screening attendance, doctor’s empathy, and medical satisfaction. The results of the descriptive analysis indicated that cancer stigma was positively associated with self-perception of aging, anger, internal attributions, stressful life events, self-perceived burden, and sleep dysfunction, while negatively associated with patient-provider communication and sleep quality.
Conclusion
Healthcare staff should pay attention to the identified correlates of cancer stigma. The results of our research can inform the design of interventions to reduce stigma and to improve clinical outcomes in people with cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is expected to be the leading cause of death and the single most important obstacle to extending life expectancy all over the world in the twenty-first century. It was estimated that there would be 18.1 million new cases and 9.6 million cancer deaths worldwide in 2018 [1]. Facing a malignant disease is one of the biggest challenges and stresses for the patient and a starting point of a life crisis which causes biological and psychological problems. This can critically have an adverse impact on their overall quality of life and recovery [2,3,4,5]. Stigma is one of the psychosocial issues of cancer from diagnosis to treatment and gradual recovery [6, 7]. The term stigma usually refers any attribute that is deeply discreditable, which causes a “normal” individual to become tainted and discounted and consequently be labeled as social disgrace [8]. It was estimated that the prevalence of stigma among cancer patients ranged from 13 to 80% [9,10,11,12]. Over 30% of cancer survivors had negative attitudes and stereotypical views toward cancer, and about 10% of the patients experienced social discrimination due to cancer [9]. Stigma is thought to cause further psychological distress, social isolation that has adverse effects on patients’ outcomes of treatment, physiological condition, mental health, and social functioning [13].
To date, although a growing body of qualitative and quantitative research has investigated the correlates of stigma for people with cancer, the identification of the correlates has still failed to reach a consensus. For example, disagreements on the relationship between age and cancer stigma were found in the studies of Rose et al. [14] and Criswell et al. [15], with the former study indicating that cancer stigma was negatively associated with age, while the latter study did not show a significant difference. In addition, the studies related to correlates of stigma for people with cancer have not yet been reviewed in a systematic manner, and there is still no meta-analysis available to help synthesize these data and provide vigorous evidence for the identification of the correlates of cancer stigma.
Therefore, we performed the first systematic review and meta-analysis to identify the correlates of cancer stigma. Our findings can provide robust evidence for policymakers and healthcare professionals to establish health prevention and promotion strategies and guide clinical practice.
Materials and methods
Protocol and registration
The protocol for this meta-analysis was registered in PROSPERO (http://www.crd.york.ac.uk/prospero/) (number: CRD42020173050).
Literature search strategy
PubMed, EMBASE, Web of Science, the Cochrane Library, and PsycINFO were electronically searched to collect cross-sectional and longitudinal studies about correlates of stigma for patients with cancer from their inception to February 2020 by using the following search terms: “cancer OR tumor OR neoplasm” “stigma OR social stigma” “correlate OR predictor OR association OR relationship OR determinant OR factor.” In addition, reference lists of the selected articles and references of the systematic reviews were screened for relevant references. The entire process was independently completed by two researchers. The searching strategies are listed in Supplementary Table S1.
Study inclusion and exclusion criteria
Studies were included if the following criteria were met: (a) patients (age ≥ 18 years) with cancer; (b) included independent quantitative measures regarding stigma and at least one other variable (such as a demographic variable); (c) reported on the statistical relationship between the two variables; (d) the Pearson correlation coefficient (r), Spearman correlation coefficient (rs), standardized regression coefficient (β), or odds ratio (OR) was given in the article; (e) cross-sectional and longitudinal study; (f) English article. Editorials, reviews, letters, and comments were excluded from this analysis.
Data abstraction and quality assessment
A data extraction form was designed for the included studies. The following data were independently extracted by two researchers: first author, year of publication, geographic location of study, cancer type, study design, stigma identification, and correlates. We also contacted the authors about unclear or missing information when necessary.
The methodological quality of included studies was independently assessed using an 11-item cross-sectional assessment provided by the Agency for Healthcare Research and Quality (AHRQ) [16]. If the article conformed to the item, we scored it “1”; an item would be scored “0” if it was answered “NO” or “UNCLEAR.” Article quality was assessed as follows: low quality (0~3), moderate quality (4~7), and high quality (8~11). Two researchers independently performed the quality assessment for included studies, and disagreements were resolved by discussion.
Statistical analysis
All statistical analyses were performed using the meta-package in statistical program R based on the random effects model. To investigate the association between stigma and its correlates, we calculated the pooled z values using a Pearson correlation coefficient transformed by the Fisher z-transformation. If study values for the Pearson correlation coefficient (r) were not available, the Pearson correlation coefficient (r) was calculated from the existing Spearman correlation coefficient (rs), standardized regression coefficient (β), or odds ratio (OR). The Spearman correlation coefficient (rs) was converted to Pearson correlation coefficient (r) using the following formula: r = 2 × sin (rs × π/6) [17]. The standardized regression coefficient (β) and odds ratio (OR) were converted to Pearson correlation coefficient (r) in accordance with the reports of previous studies [18,19,20]. Heterogeneity across studies was measured using the I2 statistics. Studies with an I2 value of < 25%, ~ 50%, ~ 75%, and ~ 100% were considered to have mild, moderate, large, and extreme heterogeneities, respectively. Z value was estimated using a fixed effects model for studies with no statistically significant heterogeneity (P > 0.1 and I2 < 50%). The random effects model was employed for other studies. Subgroup and sensitivity analyses were adopted to investigate the source of the heterogeneity. The results were presented in a descriptive manner when data could not be synthesized or correlates were identified in only one study. Potential publication bias was investigated using a funnel plot and Egger’s test if sufficient studies (> 10) were included, a two-tailed value of P < 0.05 was considered significant for Egger’s test.
Results
Search process
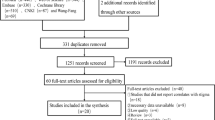
Overall, 2592 studies were identified from our initial search, and 1706 studies remained after removing duplicates, of which 1667 studied were excluded after screening via the titles and abstracts because they were not related to the topic. Then, 39 studies went forward for further review, of which 8 studies were excluded due to not focus on stigma and necessary data unavailable. Finally, 31 studies met all inclusion criteria. Supplementary Figure 1 shows the flow chart for the selection process.
Characteristics of the included studies
The thirty-one studies with a total of 7114 participants enrolled in the systematic review and meta-analysis were published between 2009 and 2020, of which twenty-nine [12, 14, 15, 21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] were cross-sectional studies and two [47, 48] were longitudinal studies. The sample sizes of all included studies ranged from 45 to 1918, and the diagnosis of participants included lung cancer, breast cancer, head and neck cancer, cervical cancer, prostate cancer, colorectal cancer, and so on. Regarding the geographic location of the study, fifteen studies [15, 21,22,23,24, 28, 30, 32, 36, 40, 41, 43,44,45, 48] took place in the USA; three in China [38, 39, 46]; two each in the UK [33, 42], India [29, 35], Canada [26, 31], Australia [14, 27], and Belgium [34, 47]; and one each in African [25], Iran [37], and Turkey [12]. The measures of stigma included Cataldo Lung Cancer Stigma Scale (CLCSS), Social Impact Scale (SIS), Cancer Responsibility and Regret Scale (CRRS), Cancer Stigma Scale (CASS), a questionnaire for measuring attitudes toward cancer (cancer stigma)—patients version, and so on. In addition, the most frequently reported correlate is depression. Characteristics of the included studies are listed in Supplementary Table 1.
Quality assessment of the studies
Among the included studies, 14 articles [14, 22,23,24, 26, 28, 30, 36, 38, 39, 42, 46,47,48] scored in the 8 to 11 range and 14 articles [12, 15, 21, 25, 31, 32, 34, 35, 37, 40, 41, 43,44,45] scored in the 4 to 7 range. However, 3 articles [27, 29, 33] with only abstracts were scored 3. In general, we deemed that the quality of these articles was sufficient to be involved in the systematic review and meta-analysis. Detailed results of the quality assessment are summarized in Supplementary Table 2.
Synthesis of results
Twenty-two variables were ultimately analyzed quantitatively, including demographic variables (age, gender, education, income, and marital status), disease-related variables (symptom severity, natural killer cell subset), and psychosocial variables (depression, anxiety, quality of life, body image loss, self-esteem, self-blame, self-efficacy, posttraumatic growth, social constraint, intrusive thoughts, cancer screening attendance, subjective well-being, doctor’s empathy, ambivalence over emotional expression, and medical satisfaction). The results of the meta-analysis for each correlate of stigma are described below. The overall results are summarized in Supplementary Table 3.
Demographic variables
Age
Nine studies [14, 15, 22, 24, 28, 32, 33, 36, 46] reported the relationship between age and cancer stigma. Nevertheless, a high level of heterogeneity was detected among the included studies (I2 = 97%, P< 0.01) and no significant difference was observed between the combined estimates (z value, 0.05, 95% CI, − 0.25, 0.34) (Supplementary Figure 2). In the sensitivity analysis, studies were detected by omitting one study in each turn from the meta-analysis; the pooled z value of the correlation between age and cancer stigma was not altered after omission. This indicated good reliability of the result (Supplementary Figure 3).
Gender (male)
Four studies [22, 32, 36, 42] that included a total of 2418 patients provided eligible data for demonstrating the correlation between gender and cancer stigma. The pooled results showed that higher cancer stigma scores were associated with being male (z value, 0.12, 95% CI, 0.08, 0.16) (Supplementary Figure 4).
Education
Six studies [12, 15, 32, 35,36,37] reported the correlation between education and cancer stigma. However, prominent heterogeneity was detected among the eligible studies (I2 = 90%, P< 0.01) and no significant difference was observed in the study effect size estimate (z value, − 0.06, 95% CI, − 0.24, 0.12) (Supplementary Figure 5). In the sensitivity analysis, studies were detected by omitting one study in each turn from the meta-analysis; the pooled z value of the correlation between education and cancer stigma was not altered after omission (Supplementary Figure 6). This indicated good reliability of the result.
Income
Two studies [12, 36] provided extractable data to analyze the correlation between income and cancer stigma. The random effects model showed that increased cancer stigma was associated with lower income (z value, − 0.19, 95% CI, − 0.32, − 0.06) (Supplementary Figure 7).
Marital status (married)
Three studies [32, 35, 36] reported the relationship between marital status and cancer stigma. Pooled results were obtained using a random effects model, and no significant difference was observed in the study effect size estimate (z value, 0.10, 95% CI, − 0.01, 0.20) (Supplementary Figure 8). In the sensitivity analysis, studies were detected by omitting one study in each turn from the meta-analysis, when we removed a report [36] that contributed to the final result; the pooled z value of the correlation between education and cancer stigma was not altered after omission (z value, 0.10, 95% CI, − 0.01, 0.20, I2=20%) (Supplementary Figure 9). This indicated good reliability of the result.
Disease-related variables
Symptom severity
Two studies [15, 24] reported the correlation between symptom severity and cancer stigma. Pooled results obtained using a random effects model suggested that increased cancer stigma was associated with severe symptoms (z value, 0.39, 95% CI, 0.14, 0.65) (Supplementary Figure 10).
Natural killer cell subset
Two studies [38, 39] provided available data on the association between NK cell subset and cancer stigma. Pooled results obtained using a random effects model revealed that higher levels of cancer stigma were associated with lower NK cell subsets (z value, − 0.81, 95% CI, − 1.00, − 0.62) (Supplementary Figure 11).
Psychosocial variables
Depression
Fourteen studies [12, 15, 21,22,23,24,25,26, 28, 29, 31, 33, 35, 40] that included a total of 2652 patients provided eligible data for demonstrating the correlation between depression and cancer stigma. The random effects model was applied since a high level of heterogeneity was observed in the analysis (I2 = 88%, P < 0.01). The results indicated that increased cancer stigma scores were associated with increased depression (z value, 0.43, 95% CI, 0.32, 0.54) (Supplementary Figure 12). In the sensitivity analysis, studies were detected by omitting one study in each turn from the meta-analysis; the pooled z value of the correlation between depression and cancer stigma was not altered after omission (Supplementary Figure 13). The results of the subgroup analysis showed that the differences in sample size might be the source of heterogeneity (Supplementary Figure 14). In addition, the results of funnel plots were asymmetrical, indicating the presence of potential bias (Supplementary Figure 15). Moreover, Egger’s test (P < 0.0001) (Supplementary Figure 16) revealed that obvious publication bias existed among the included studies for the meta-analysis of the correlation between depression and cancer stigma.
Anxiety
Five studies [21, 24, 28, 35, 39] provided available data on the correlation between anxiety and cancer stigma. Pooled results obtained using a random effects model revealed that higher levels of cancer stigma were associated with higher levels of anxiety (z value, 0.45, 95% CI, 0.23, 0.68) (Supplementary Figure 17). In the sensitivity analysis, studies were detected by omitting one study in each turn from the meta-analysis, when we removed a report [39] that contributed to the final result; the pooled z value of the correlation between anxiety and cancer stigma was not altered after omission (z value, 0.35, 95% CI, 0.27, 0.43, I2 = 40%) (Supplementary Figure 18). This indicated good reliability of the result.
Quality of life
Six studies [22, 28, 33, 41, 43, 47] included a total of 889 patients provided data to analyze the correlation between QOL and cancer stigma. The fixed effects model showed increased cancer stigma was associated with a lower level of QOL (z value, − 0.59, 95% CI, − 0.65, − 0.52) (Supplementary Figure 19). In the sensitivity analysis, studies were detected by omitting one study in each turn from the meta-analysis, when we removed a report [22] that contributed to the final result; the pooled z value of the correlation between QOL and cancer stigma was not altered after omission (z value, − 0.54, 95% CI, − 0.61, − 0.46, I2 = 0%) (Supplementary Figure 20). This indicated good reliability of the result.
Body image loss
Two studies [12, 26] reported the correlation between body image loss and cancer stigma. Pooled results obtained using a random effects model revealed that higher levels of cancer stigma were associated with severe body image loss (z value, 0.64, 95% CI, 0.29, 0.99) (Supplementary Figure 21).
Self-esteem
Three studies [21, 29, 46] reported the relationship between self-esteem and cancer stigma. Pooled results obtained using a random effects model revealed that increased cancer stigma was associated with poorer self-esteem (z value, − 0.41, 95% CI, − 0.73, − 0.10) (Supplementary Figure 22). In the sensitivity analysis, studies were detected by omitting one study in each turn from the meta-analysis, when we removed a report [46] that contributed to the final result; the pooled z value of the correlation between self-esteem and cancer stigma was not altered after omission (z value, − 0.26, 95% CI, − 0.37, − 0.15, I2 = 0%) (Supplementary Figure 23). This indicated good reliability of the result.
Self-blame
Three studies [21, 26, 36] reported the correlation between self-blame and cancer stigma. Pooled results obtained using a random effects model revealed that higher levels of cancer stigma were associated with higher levels of self-blame (z value, 0.19, 95% CI, 0.07, 0.31) (Supplementary Figure 24). In the sensitivity analysis, studies were detected by omitting one study in each turn from the meta-analysis, when we removed a report [21] that contributed to the final result; the pooled z value of the correlation between self-blame and cancer stigma was not altered after omission (z value, 0.13, 95% CI, 0.03, 0.23, I2 = 0%) (Supplementary Figure 25). This indicated good reliability of the result.
Self-efficacy
Three studies [38, 39, 46] reported the correlation between self-efficacy and cancer stigma. Pooled results obtained using a random effects model revealed that increased cancer stigma was associated with poorer self-efficacy (z value, − 0.74, 95% CI, − 1.12, − 0.37) (Supplementary Figure 26). In the sensitivity analysis, studies were detected by omitting one study in each turn from the meta-analysis, when we removed a report [38] that contributed to the final result; the pooled z value of the correlation between self-efficacy and cancer stigma was not altered after omission (z value, − 0.58, 95% CI, − 0.83, − 0.32, I2 = 86.2%) (Supplementary Figure 27). This indicated good reliability of the result.
Posttraumatic growth
Three studies [26, 30, 43] reported the correlation between posttraumatic growth and cancer stigma. However, obvious heterogeneity was detected among the included studies (I2 = 92%, P< 0.01) and no significant difference was found in the study effect size estimate (z value, 0.02, 95% CI, − 0.29, 0.33) (Supplementary Figure 28). In the sensitivity analysis, studies were detected by omitting one study in each turn from the meta-analysis, when we removed a report [43] that contributed to the final result; the pooled z value of the correlation between posttraumatic growth and cancer stigma was not altered after omission (z value, 0.17, 95% CI, − 0.04, 0.39, I2 = 74.3%) (Supplementary Figure 29). This indicated good reliability of the result.
Social constraint
Two studies [27, 48] provide available data on the association between social constraint and cancer stigma. The fixed effects model showed increased cancer stigma were associated with more social constraint (z value, 0.25, 95% CI, 0.09, 0.42) (Supplementary Figure 30).
Intrusive thoughts
Five studies [15, 26, 40, 41, 43] reported the correlation between intrusive thoughts and cancer stigma. Pooled results obtained using a random effects model revealed that increased cancer stigma was associated with greater frequency of intrusive thoughts (z value, 0.57, 95% CI, 0.33, 0.82) (Supplementary Figure 31). In the sensitivity analysis, studies were detected by omitting one study in each turn from the meta-analysis, when we removed a report [15] that contributed to the final result; the pooled z value of the correlation between intrusive thoughts and cancer stigma was not altered after omission (z value, 0.68, 95% CI, 0.54, 0.81, I2 = 62.6%) (Supplementary Figure 32). This indicated good reliability of the result.
Cancer screening attendance
The study conducted by Vrinten [42] provided extractable data on the association between cancer screening attendance and stigma of the cervical, breast, and colorectal cancer, respectively. Pooled results obtained using a fixed effects model indicated that higher cancer stigma was associated with not being screened (z value, 0.16, 95% CI, 0.10, 0.21) (Supplementary Figure 33).
Subjective well-being
Two studies [26, 31] reported the relationship between subjective well-being and cancer stigma. Nevertheless, obvious heterogeneity was detected among the eligible studies (I2 = 80%, P = 0.02) and no significant difference was observed between the combined estimates (z value, − 0.18, 95% CI, − 0.36, 0.01) (Supplementary Figure 34).
Doctor’s empathy
Two studies [38, 39] reported the correlation between doctor’s empathy and cancer patient’s stigma. Pooled results obtained using a random effects model showed that higher cancer patient’s stigma was associated with less doctor’s empathy (z value, − 0.94, 95% CI, − 1.25, − 0.62) (Supplementary Figure 35)
Ambivalence over emotional expression
Three studies [40, 41, 44] provided available data on the correlation between ambivalence over emotional expression and cancer stigma. Pooled results obtained using a fixed effects model showed that increased cancer stigma was associated with more severe ambivalence over emotional expression (z value, 0.43, 95% CI, 0.32, 0.35) (Supplementary Figure 36).
Medical satisfaction
Two studies [15, 36] reported the relationship between medical satisfaction and cancer stigma. Pooled results obtained using a random effects model showed that increased cancer stigma was associated with reduced medical satisfaction (z value, − 0.33, 95% CI, − 0.61, − 0.06) (Supplementary Figure 37).
Descriptive analysis
Since the following correlates were identified in only one study, the results were presented in a descriptive manner. Cancer stigma was positively associated with self-perception of aging (r = 0.367, P < 0.001) [34], anger (r = 0.34, P < 0.01) [21], internal attributions (r = 0.29, P < 0.01) [21], stressful life events (r = 0.36, P < 0.01) [26], self-perceived burden (r = 0.41, P < 0.01) [45], and sleep dysfunction (r = 0.24, P < 0.05) [44]. while negatively associated with patient-provider communication (r = − 0.18, P < 0.05) [32] and sleep quality (r = − 0.18, P < 0.05) [44].
Discussion
Correlates of cancer stigma
In our meta-analysis, a higher level of cancer stigma was associated with male gender, low income, severe symptoms, lower NK cell subsets, increased depression, increased anxiety, poorer QOL, severe body image loss, poorer self-esteem, more self-blame, poorer self-efficacy, more social constraint, greater frequency of intrusive thoughts, poorer cancer screening attendance, less doctor’s empathy, more severe ambivalence over emotional expression, and reduced medical satisfaction. No significant differences were found in age, education, marital status, posttraumatic growth, or subjective well-being.
Regarding the demographic variables, our study found that cancer stigma was positively associated with male (z value, 0.12, 95% CI, 0.08, 0.16), which was consistent with the results of a previous study [42], However, among the studies included in this study, Cataldo et al. [22], Shen et al. [32], and Weiss et al. [36] found that there was no significant correlation between cancer stigma and gender, which might be because of the small sample size, while our result was based on a total of 2418 participants. The results of meta-analysis also indicated that there was a negative association between cancer stigma and income (z value, − 0.19, 95% CI, − 0.32, − 0.06), which was also in accordance with some previous studies [12, 36]. Nevertheless, due to limited eligible data for analysis, our result was based on only two studies; thus, more studies are needed to draw a definite conclusion.
In terms of disease-related variables, our meta-analysis revealed that cancer stigma was positively associated with symptoms severity (z value, 0.39, 95% CI, 0.14, 0.65) and negatively associated with NK cell subsets (z value, − 0.81, 95% CI, − 1.00, − 0.62), which were consistent with the results of previous studies [15, 24, 38, 39]. These indicate a higher level of cancer stigma was associated with severe symptoms and weakened immunity. However, because of the obvious heterogeneity and limited available data for analysis, more research should be performed to confirm our conclusion.
Consistent with previous studies [12, 15, 21,22,23,24,25,26, 28, 29, 31, 33, 35, 40], increased depression was associated with a higher level of cancer stigma (z value, 0.43, 95% CI, 0.32, 0.54). However, significant heterogeneity was observed among the included studies, which might be caused by differences in sample size. The present meta-analysis also revealed a significant positive correlation between cancer stigma and anxiety (z value, 0.45, 95% CI, 0.23, 0.68), which was in accordance with previous studies [21, 24, 28, 35, 39], and there was obvious heterogeneity among included studies. Moreover, a lower level of QOL was associated with a higher level of cancer stigma (z value, − 0.59, 95% CI, − 0.65, − 0.52), which was also consistent with previous studies [22, 28, 33, 41, 43, 47]. Moreover, the results of meta-analysis revealed that cancer stigma was positively associated with social constraint (z value, 0.57, 95% CI, 0.33, 0.82) and ambivalence over emotional expression (z value, 0.43, 95% CI, 0.32, 0.35), while negatively associated with cancer screening attendance (z value, 0.16, 95% CI, 0.10, 0.21), and there was no heterogeneity among included studies. Nevertheless, since there were limited studies included for analysis, further research should be conducted to validate our result. Furthermore, our meta-analysis indicated that a higher level of cancer stigma was associated with severe body image loss (z value, 0.64, 95% CI, 0.29, 0.99), poorer self-esteem (z value, − 0.41, 95% CI, − 0.73, − 0.10), more self-blame (z value, 0.19, 95% CI, 0.07, 0.31), poor self-efficacy (z value, − 0.74, 95% CI, − 1.12, − 0.37), greater frequency of intrusive thoughts (z value, 0.57, 95% CI, 0.33, 0.82), less doctor’s empathy (z value, − 0.94, 95% CI, − 1.25, − 0.62), and reduced medical satisfaction (z value, − 0.33, 95% CI, − 0.61, − 0.06). However, moderate to extreme heterogeneity and limited extractable data for analysis should be taken into consideration.
Clinical implication
Findings from our research can be applied to interventions to reduce the stigma of cancer and to improve clinical outcomes. Because the male gender is associated with higher cancer stigma, medical staff should pay more attention to the occurrence of stigma in male patients with cancer. Moreover, policymakers should implement improvements in medical insurance to help low-income patients. Additionally, interventions that target symptoms and some psychosocial factors that included body image loss, self-efficacy, self-esteem, social constraint, self-blame, doctor’s empathy, ambivalence over emotional expression, medical satisfaction, self-perception of aging, internal attribution, stressful life events, self-perceived burden, and patient-provider communication may be useful to reduce cancer stigma and consequently may reduce psychological distress and improve physical functioning and quality of life.
Limitations
There are several limitations in our study that warrant mention. First, the studies included in the meta-analysis had different characteristics such as differences in sample size, the different cultural backgrounds of the patients, and diverse measurement tools applied, which can lead to heterogeneity and affect the reliability of the results. Second, some of our results show high levels of heterogeneity, and it is difficult to determine the possible sources of heterogeneity. Third, publication bias was detected among the included studies for the meta-analysis of the correlation between depression and cancer stigma. Fourth, some research data needed to be recalculated, and data conversion may be bias. At last, the analysis of some correlates is limited by the number of studies, which may have an impact on results and lead to potential bias. Hence, further research should be conducted to confirm these results.
Conclusions
This systematic review and meta-analysis identified the correlates of stigma for cancer patients. Further research should be conducted to validate our conclusion. The results of our research can inform the design of interventions to reduce stigma and to improve clinical outcomes in people with cancer.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 68(6):394–424. https://doi.org/10.3322/caac.21492
Meacham E, Orem J, Nakigudde G, Zujewski JA, Rao D (2016) Exploring stigma as a barrier to cancer service engagement with breast cancer survivors in Kampala, Uganda. Psychooncology 25(10):1206–1211. https://doi.org/10.1002/pon.4215
Carter-Harris L, Hermann CP, Schreiber J, Weaver MT, Rawl SM (2014) Lung cancer stigma predicts timing of medical help-seeking behavior. Oncol Nurs Forum 41(3):E203–E210. https://doi.org/10.1188/14.Onf.E203-e210
Quinn DM, Chaudoir SR (2009) Living with a concealable stigmatized identity: the impact of anticipated stigma, centrality, salience, and cultural stigma on psychological distress and health. J Pers Soc Psychol 97(4):634–651. https://doi.org/10.1037/a0015815
Cvetkovic J, Nenadovic M (2016) Depression in breast cancer patients. Psychiatry Res 240:343–347. https://doi.org/10.1016/j.psychres.2016.04.048
Knapp S, Marziliano A, Moyer A (2014) Identity threat and stigma in cancer patients. Health Psychol Open 1(1):2055102914552281. https://doi.org/10.1177/2055102914552281
Hamann HA, Pujol JL (2018) Introduction to the special section: the experience and impact of lung cancer stigma. J Thorac Oncol 13(8):1053–1054. https://doi.org/10.1016/j.jtho.2018.06.006
Yuan JM, Zhang JE, Zheng MC, Bu XQ (2018) Stigma and its influencing factors among Chinese patients with stoma. Psychooncology 27(6):1565–1571. https://doi.org/10.1002/pon.4695
Cho J, Choi EK, Kim SY, Shin DW, Cho BL, Kim CH, Koh DH, Guallar E, Bardwell WA, Park JH (2013) Association between cancer stigma and depression among cancer survivors: a nationwide survey in Korea. Psychooncology 22(10):2372–2378. https://doi.org/10.1002/pon.3302
Ernst J, Mehnert A, Dietz A, Hornemann B, Esser P (2017) Perceived stigmatization and its impact on quality of life - results from a large register-based study including breast, colon, prostate and lung cancer patients. BMC cancer 17(1):741. https://doi.org/10.1186/s12885-017-3742-2
Phelan SM, Griffin JM, Jackson GL, Zafar SY, Hellerstedt W, Stahre M, Nelson D, Zullig LL, Burgess DJ, van Ryn M (2013) Stigma, perceived blame, self-blame, and depressive symptoms in men with colorectal cancer. Psychooncology 22(1):65–73. https://doi.org/10.1002/pon.2048
Yilmaz M, Dissiz G, Usluoglu AK, Iriz S, Demir F, Alacacioglu A (2020) Cancer-related stigma and depression in cancer patients in a middle-income country. Asia Pac J Oncol Nurs 7(1):95–102. https://doi.org/10.4103/apjon.apjon_45_19
Webb LA, McDonnell KK, Adams SA, Davis RE, Felder TM (2019) Exploring stigma among lung cancer survivors: a scoping literature review. Oncol Nurs Forum 46(4):402–418. https://doi.org/10.1188/19.Onf.402-418
Rose S, Kelly B, Boyes A, Cox M, Palazzi K, Paul C (2018) Impact of perceived stigma in people newly diagnosed with lung cancer: a cross-sectional analysis. Oncol Nurs Forum 45(6):737–747. https://doi.org/10.1188/18.Onf.737-747
Criswell KR, Owen JE, Thornton AA, Stanton AL (2016) Personal responsibility, regret, and medical stigma among individuals living with lung cancer. J Behav Med 39(2):241–253. https://doi.org/10.1007/s10865-015-9686-6
Kim MJ, Moon S, Oh BC, Jung D, Ji K, Choi K, Park YJ (2018) Association between perfluoroalkyl substances exposure and thyroid function in adults: a meta-analysis. PloS one 13(5):e0197244. https://doi.org/10.1371/journal.pone.0197244
Zhao X, Wang H, Li J, Shan Z, Teng W, Teng X (2015) The correlation between polybrominated diphenyl ethers (PBDEs) and thyroid hormones in the general population: a meta-analysis. PloS one 10(5):e0126989. https://doi.org/10.1371/journal.pone.0126989
Peterson RA, Brown SP (2005) On the use of beta coefficients in meta-analysis. J Appl Psychol 90(1):175–181. https://doi.org/10.1037/0021-9010.90.1.175
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Publishers, Hillsdale
Rosenthal R (1994) Parametric measures of effect size. In: The handbook of research synthesis. Russell Sage Foundation, New York, pp 231–244
Else-Quest NM, LoConte NK, Schiller JH, Hyde JS (2009) Perceived stigma, self-blame, and adjustment among lung, breast and prostate cancer patients. Psychology & health 24(8):949–964. https://doi.org/10.1080/08870440802074664
Cataldo JK, Jahan TM, Pongquan VL (2012) Lung cancer stigma, depression, and quality of life among ever and never smokers. European journal of oncology nursing : the official journal of European Oncology Nursing Society 16(3):264–269. https://doi.org/10.1016/j.ejon.2011.06.008
Gonzalez BD, Jacobsen PB (2012) Depression in lung cancer patients: the role of perceived stigma. Psychooncology 21(3):239–246. https://doi.org/10.1002/pon.1882
Cataldo JK, Brodsky JL (2013) Lung cancer stigma, anxiety, depression and symptom severity. Oncology 85(1):33–40. https://doi.org/10.1159/000350834
Hamilton JB, Deal AM, Moore AD, Best NC, Galbraith KV, Muss H (2013) Psychosocial predictors of depression among older African American patients with cancer. Oncol Nurs Forum 40(4):394–402. https://doi.org/10.1188/13.Onf.394-402
Lebel S, Castonguay M, Mackness G, Irish J, Bezjak A, Devins GM (2013) The psychosocial impact of stigma in people with head and neck or lung cancer. Psychooncology 22(1):140–152. https://doi.org/10.1002/pon.2063
Anderson NH, Schofield P, Aranda S, Chambers S, Krishnasamy M, Ball D, Michael M, Kenny P, Lipson-Smith R, Stubbins I, Ugalde A (2014) Social constraint amongst people with advanced cancer: a correlate with stigmatisation and anxiety. Asia-Pacific Journal of Clinical Oncology 10:128–264. https://doi.org/10.1111/ajco.12332
Brown Johnson CG, Brodsky JL, Cataldo JK (2014) Lung cancer stigma, anxiety, depression, and quality of life. Journal of psychosocial oncology 32(1):59–73. https://doi.org/10.1080/07347332.2013.855963
Ahmed R, Tripathy L, Datta SS, Agrawal S, Chatterjee S (2015) Correlates of body image difficulties following breast cancer surgery. European Journal of Surgical Oncology 41(6):S38. https://doi.org/10.1016/j.ejso.2015.03.079
Shen MJ, Coups EJ, Li Y, Holland JC, Hamann HA, Ostroff JS (2015) The role of posttraumatic growth and timing of quitting smoking as moderators of the relationship between stigma and psychological distress among lung cancer survivors who are former smokers. Psychooncology 24(6):683–690. https://doi.org/10.1002/pon.3711
Lebel S, Payne AYM, Mah K, Irish J, Rodin G, Devins GM (2016) Do stigma and its psychosocial impact differ between Asian-born Chinese immigrants and Western-born Caucasians with head and neck cancer? Psychology Health & Medicine 21(5):583–592. https://doi.org/10.1080/13548506.2016.1139138
Shen MJ, Hamann HA, Thomas AJ, Ostroff JS (2016) Association between patient-provider communication and lung cancer stigma. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 24(5):2093–2099. https://doi.org/10.1007/s00520-015-3014-0
Maguire R, Lewis L, McPhelim J, Cataldo J, Milroy R, Woods K, Perham M (2017) The relationship between lung cancer stigma and patient reported outcomes. Journal of Thoracic Oncology 12(1):S300
Schroyen S, Marquet M, Jerusalem G, Dardenne B, Van den Akker M, Buntinx F, Adam S, Missotten P (2017) The link between self-perceptions of aging, cancer view and physical and mental health of older people with cancer: a cross-sectional study. J Geriatr Oncol 8(1):64–68. https://doi.org/10.1016/j.jgo.2016.09.001
Tripathi L, Datta SS, Agrawal SK, Chatterjee S, Ahmed R (2017) Stigma perceived by women following surgery for breast cancer. Indian J Med Paediatr Oncol 38(2):146–152. https://doi.org/10.4103/ijmpo.ijmpo_74_16
Weiss J, Yang H, Weiss S, Rigney M, Copeland A, King JC, Deal AM (2017) Stigma, self-blame, and satisfaction with care among patients with lung cancer. Journal of psychosocial oncology 35(2):166–179. https://doi.org/10.1080/07347332.2016.1228095
Hasan Shiri F, Mohtashami J, Nasiri M, Manoochehri H, Rohani C (2018) Stigma and related factors in Iranian people with cancer. Asian Pacific journal of cancer prevention : APJCP 19(8):2285–2290. https://doi.org/10.22034/apjcp.2018.19.8.2285
Yang N, Cao Y, Li X, Li S, Yan H, Geng Q (2018) Mediating effects of patients’ stigma and self-efficacy on relationships between doctors’ empathy abilities and patients’ cellular immunity in male breast cancer patients. Medical science monitor : international medical journal of experimental and clinical research 24:3978–3986. https://doi.org/10.12659/msm.910794
Yang NX, Xiao H, Wang W, Li SY, Yan H, Wang YF (2018) Effects of doctors’ empathy abilities on the cellular immunity of patients with advanced prostate cancer treated by orchiectomy: the mediating role of patients’ stigma, self-efficacy, and anxiety. Patient preference and adherence 12:1305–1314. https://doi.org/10.2147/ppa.S166460
Tsai W, Lu Q (2019) Ambivalence over emotional expression and intrusive thoughts as moderators of the link between self-stigma and depressive symptoms among Chinese American breast cancer survivors. J Behav Med 42(3):452–460. https://doi.org/10.1007/s10865-018-9996-6
Tsai W, Wu IHC, Lu Q (2019) Acculturation and quality of life among Chinese American breast cancer survivors: the mediating role of self-stigma, ambivalence over emotion expression, and intrusive thoughts. Psychooncology 28(5):1063–1070. https://doi.org/10.1002/pon.5053
Vrinten C, Gallagher A, Waller J, Marlow LAV (2019) Cancer stigma and cancer screening attendance: a population based survey in England. BMC cancer 19(1):566. https://doi.org/10.1186/s12885-019-5787-x
Wong CCY, Pan-Weisz BM, Pan-Weisz TM, Yeung NCY, Mak WWS, Lu Q (2019) Self-stigma predicts lower quality of life in Chinese American breast cancer survivors: exploring the mediating role of intrusive thoughts and posttraumatic growth. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation 28(10):2753–2760. https://doi.org/10.1007/s11136-019-02213-w
Wu IHC, Tsai W, McNeill LH, Lu Q (2019) The associations of self-stigma, social constraints, and sleep among Chinese American breast cancer survivors. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 28:3935–3944. https://doi.org/10.1007/s00520-019-05233-x
Yeung NCY, Lu Q, Mak WWS (2019) Self-perceived burden mediates the relationship between self-stigma and quality of life among Chinese American breast cancer survivors. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 27(9):3337–3345. https://doi.org/10.1007/s00520-018-4630-2
Liu XH, Zhong JD, Zhang JE, Cheng Y, Bu XQ (2020) Stigma and its correlates in people living with lung cancer: a cross-sectional study from China. Psychooncology 29(2):287–293. https://doi.org/10.1002/pon.5245
Schroyen S, Missotten P, Jerusalem G, Van den Akker M, Buntinx F, Adam S (2017) Association between self-perception of aging, view of cancer and health of older patients in oncology: a one-year longitudinal study. BMC cancer 17(1):614. https://doi.org/10.1186/s12885-017-3607-8
Williamson TJ, Choi AK, Kim JC, Garon EB, Shapiro JR, Irwin MR, Goldman JW, Bornyazan K, Carroll JM, Stanton AL (2018) A longitudinal investigation of internalized stigma, constrained disclosure, and quality of life across 12 weeks in lung cancer patients on active oncologic treatment. J Thorac Oncol 13(9):1284–1293. https://doi.org/10.1016/j.jtho.2018.06.018
Author information
Authors and Affiliations
Contributions
All four authors conceived and designed the review, collected, and interpreted the data and drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24400 kb)
Rights and permissions
About this article
Cite this article
Huang, Z., Yu, T., Wu, S. et al. Correlates of stigma for patients with cancer: a systematic review and meta-analysis. Support Care Cancer 29, 1195–1203 (2021). https://doi.org/10.1007/s00520-020-05780-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05780-8