Abstract
Objectives
Oxaliplatin accumulates in dorsal root ganglia, causing an axonal neuronopathy. Symptoms include numbness, pain and gait disturbance which may persist and impact on quality of life (QOL). Despite widespread use of this drug, its late effects and patient satisfaction outcomes have not been widely reported. Furthermore, there has been limited qualitative research published in this area. The objectives of this study were to establish the incidence and clinical impact of chronic peripheral neuropathy.
Methods
We conducted a cross-sectional observational study of patients who started oxaliplatin treatment at least 2 years prior to study commencement. Patients were assessed in three ways: clinical assessment encompassing neurological examination and nerve conduction studies to calculate a total neuropathy score (TNS); self-reported assessment via validated questionnaires; and assessment by recorded interview. The clinical and questionnaire-based assessments were analysed quantitatively and the interview data used for qualitative assessment.
Results
Twenty-five patients consented to participate. The mean starting dose of oxaliplatin given was 92 mg/m2. The cumulative dose received ranged from 375 to 2,400 mg, with a mean cumulative dose of 1,515 mg. Oxaliplatin was ceased due to neuropathy in six patients (24 %), after a mean of 9 cycles of treatment. Modified TNS ranged from 1 to 15 with a mean of 9.5. There was a statistically significant correlation between cumulative oxaliplatin dose and TNS. Quality of life and functional impact questionnaires showed mildly lower physical quality of life, higher pain scores and functional impairment secondary to sensory deficit. Qualitative analysis demonstrated variable bio-psycho-social effects of chronic neuropathy but, importantly, highlighted that many patients felt they had been insufficiently warned of the risk of neuropathy. Despite this, the majority was satisfied with their decision to receive the drug.
Conclusion
Many patients objectively demonstrated mild to moderate oxaliplatin neuropathy >2 years post-treatment. The majority of patients did not recall being warned of the risks of chronic peripheral neuropathy. Many of those who recall being warned did not feel sufficient emphasis was placed on the issue. Despite a varying burden of neuropathic symptoms, the majority of patients were highly satisfied with their decision to receive oxaliplatin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Oxaliplatin is a third-generation organoplatinum compound. It is most frequently used in the treatment of colorectal cancer, both in the adjuvant and metastatic setting, but also has activity in upper gastrointestinal malignancies [1]. Oxaliplatin induces two clinically distinct forms of peripheral neuropathy. An acute neuropathy may occur soon after oxaliplatin administration. The chronic form of oxaliplatin neuropathy is a symmetric, axonal, distal sensory neuropathy without motor involvement [2]. Higher cumulative doses of oxaliplatin are associated with chronic peripheral nerve damage [3]. The mechanism of chronic oxaliplatin neuropathy may be decreased cellular metabolism and axoplasmatic transport secondary to the accumulation of oxaliplatin in cells of the dorsal root ganglia [4].
Diagnosis of oxaliplatin-induced chronic peripheral neuropathy (CPN) is based on clinical assessment, using toxicity grading scales. Its incidence is related to treatment schedule, dose per cycle, cumulative dose, time of infusion and pre-existing neuropathy [5]. CPN is a dose limiting toxicity and when significant, results in oxaliplatin dose modification and, more frequently, drug discontinuation. With increasing cumulative dose, severe CPN develops in 20–50 % of patients [6]. The clinical syndrome is characterized by distal paraesthesia and numbness, which can result in significant functional impairment. Given the widespread use of this drug in the adjuvant setting, and the improved survival for patients with stage II and III colon cancer, the persistent disability induced by CPN can have a significant impact on long-term quality of life. There is limited data on the longer term outcomes for patients with CPN. Some studies suggest reversibility of CPN post-treatment cessation [6–8] whilst others report longstanding toxicity [9–11]. The present study focuses on the incidence and severity of CPN >2 years post-treatment and seeks to examine the biopsychosocial impact of CPN on survivorship.
Methods
Study design
This is a cross-sectional observational study. Patients treated at the Department of Medical Oncology, Flinders Medical Centre, were identified from pharmacy records. Patients who received at least one dose of oxaliplatin ≥2 years prior to study commencement and were able to provide informed consent were deemed eligible. Those who consented to the study were assessed in three ways: clinical assessment, self-reported assessment via questionnaires and assessment by interview. The clinical and questionnaire-based assessments were analysed quantitatively and the interview data used for qualitative assessment.
Clinical assessments
Case records were reviewed for demographic information, stage at time of treatment and co-morbidities including diabetes and pre-existing neuropathy. Treatment details were identified, including dose of oxaliplatin per cycle, cumulative dose, details of dose modifications and treatment cessation.
Neuropathy grade as per the National Cancer Institute Common Toxicity Criteria (NCI CTC) [12] was ascertained retrospectively from medical records for the period during and immediately post-oxaliplatin therapy. Current neuropathy (at least 2 years since starting oxaliplatin) was also determined according to the NCI CTC. Current neuropathy severity was objectively evaluated by a single neurologist, using the total neuropathy score (TNS), which uses symptoms, examination findings and nerve conduction studies (NCS) to evaluate neuropathy [13]. Sensory NCS were performed on the left sural nerve, and motor NCS on the left common peroneal nerve, recording extensor digitorum brevis (EDB). Quantitative vibration testing was not performed; thus, a modified TNS has been reported in this study.
Questionnaires
The World Health Organization Quality of Life (WHOQOL BREF) scale [14], EQ-5D-5L Health Questionnaire [15], and European Organization for Research and Treatment of Cancer (EORTC) Chemotherapy Induced Peripheral Neuropathy questionnaire (QLQ-CIPN20) [16] were used to grade current QOL, depression, functional status and neuropathic symptoms, respectively.
Interviews
A semi-structured interview of nine questions examined the functional impact of CPN, attempts to ameliorate CPN symptoms and the degree of support needed to manage symptoms (see Appendix 1). The psychosocial effects of neuropathy were also explored, particularly in relation to discontinuation of work and recreational pursuits. Patients were also asked to recall whether or not they were warned of the risk of CPN prior to the commencement of treatment and more broadly were asked about their degree of satisfaction with treatment outcomes. Patients were asked specifically about acceptance or regret of the decision to receive oxaliplatin (see Appendix 1).
Statistical and qualitative analysis
Data collected was used to correlate treatment with CPN outcome data. Pearson correlation coefficients were calculated using STATA 12.0 statistical software [17]. Patient interviews were recorded and transcribed to enable qualitative analysis, using NVivo [18]. Analysis was conducted using a framework approach [19].
Results
Patient characteristics
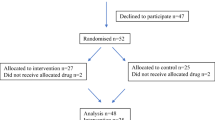
Thirty-nine eligible cases were identified, and 25 patients consented to participate. Many patients who had completed treatment were reluctant to have further intervention with questionnaires and nerve conduction studies, so each element of the study was made optional. Of those consented, 25 patients completed questionnaires, 20 were interviewed and 13 underwent neurological assessment. Eighteen patients were male and 7 female. Of the 25 patients, 20 had colonic and 5 had rectal carcinomas. At the time of oxaliplatin treatment, 17 had stage III disease and 8 had metastatic disease. Seven patients had diabetes. Two patients had previous neuropathic symptoms due to diabetic neuropathy. Two patients had a history of taking medications that could potentially cause peripheral neuropathy (one taking amiodarone but did not have any previous history of peripheral neuropathy, the second patient had a history of taking phenytoin, this latter patient also had diabetes mellitus and had neuropathic symptoms before starting treatment with oxaliplatin). None of the patients had received prior regimens involving neurotoxic chemotherapy prior to receiving oxaliplatin. At the time of the study, none of the patients had spinal metastases, spinal stenosis or carpel tunnel syndrome. Patients’ characteristics have been summarized in Table 1.
Treatment characteristics
The mean starting dose of oxaliplatin was 92 mg/m2. Seven patients underwent dose reduction. The mean oxaliplatin dose given per cycle was 165 mg. The number of cycles of treatment varied between patients, ranging from 3 to 12 cycles, with a mean of 9 cycles. The cumulative dose received ranged from 375 to 2,400 mg, with a mean cumulative dose of 1,515 mg. A complete course of oxaliplatin was given to 13 patients (52 %); oxaliplatin was ceased due to neuropathy in 6 patients (24 %), after a mean of 9 cycles of treatment.
Symptoms of neuropathy
Neuropathic symptoms were explored in 18 patients and varied considerably. At >2 years post-treatment, seven patients reported they never had symptoms, two had infrequent symptoms, six often had symptoms and three always had symptoms. Current neuropathy grade was assessed using Common Terminology Criteria for Adverse Event (CTCAE v 4.03) in 18 patients. Of these, nine did not have CPN symptoms, five had grade I CPN, one had grade II and three had grade III. No patients had grade IV neuropathy (see Fig. 1). Neuropathy grade during treatment was established by case note review. Grade during treatment was unknown in seven patients, grade I in eight patients, grade II in five patients and grade III in four patients. There was no significant correlation between those with higher grades of neuropathy during treatment and CPN of >2 years. Advanced age did not appear to correlate with risk of CPN. The frequency of neuropathic symptoms was not significantly different in diabetic patients (p = 0.43).
Total neuropathy score
Thirteen patients underwent objective neurological assessment according to TNS. Characteristics of patients who did have objective neurological assessments are included in Table 1 as well as patients who did not have the assessment. Scores for the individual components of TNS assessments were consistent with the predominantly sensory features of oxaliplatin-induced CPN; higher scores (indicating worse deficit) were recorded for pinprick and vibration testing. In the cohort of 13 patients who underwent NCS, the sural nerve sensory action potential was absent in 8 patients, whilst the common peroneal muscle action potential was not detected in only 1 patient. In patients with measurable sural nerve potential, reduced sensory nerve conduction velocity was observed in two patients, whilst motor conduction velocity was reduced in only one patient. In our study, modified TNS ranged from 1 to 15 with a mean of 9.5. There was a statistically significant correlation between modified TNS and cumulative oxaliplatin dose (p = 0.023), as shown in Fig. 2.
Quality of life and functional impact
QOL assessment using WHOQOL BREF [14] showed physical domain scores marginally lower than Australian population norms [20]. In our study, the mean physical domain score was 68.7, compared with an Australian population norm for all ages of 73.5 [21]. Majority of patients, 18/25, felt physical pain impaired their function only a little or not at all, whilst 7/25 felt they were limited either moderately or very much. A higher burden of neuropathic symptoms was seen to relate to poorer quality of life; higher TNS was associated with lower WHOQOL scores, though this did not reach statistical significance (p = 0.091), Fig. 3.
QLQ-CIPN20 [16] was used to examine the experience of symptoms and functional limitations relating to CPN. The tool contains three subscales assessing sensory, motor and autonomic symptoms. Patients were more likely to experience tingling in the feet than hands, and similarly, burning pain was experienced more often in the toes and feet than fingers and hands. Higher scores, implying worse symptoms, were attributed to sensory rather than motor and autonomic symptoms, findings that were consistent with TNS results. A study assessing the validity and reliability of the tool QLQ-CIPN20 compared patients with moderate to severe neuropathy secondary to neurotoxic chemotherapy, with patients without neuropathy [22]. The mean sensory neuropathy score in patients with moderate to severe neuropathy was 20.17, compared with 9.77 in non-neuropathic patients. In our study, the mean sensory neuropathic score was 15.4.
EQ-5D-5L [15] was used as a descriptive profile of global health status. Of 25 patients, 24 reported slight to no impairment in conducting their usual activities, and all reported being able to achieve personal care with only slight to no impairment. The greatest burden of symptoms reported was in relation to pain and discomfort, with 8 patients reporting no pain, 11 slight pain, 5 moderate and 1 severe pain. There was no significant correlation between self-reported CPN symptoms and functional impairment scores.
Patient interviews
Twenty patients participated in interviews, which were analysed qualitatively to examine the bio-psycho-social impact of CPN, depicted in Fig. 4. Many patients described ongoing neuropathic symptoms, though the majority did not feel that neuropathy directly affected their daily function. Of 19 patients, 12 did not recall being warned of CPN (Fig. 5). A number of those who were able to recall prior warning felt the issue was inadequately emphasised.
Due to symptoms of CPN, a minority of interviewees were unable to continue working, though most found they could continue their hobbies. The majority of patients felt that neuropathy had not adversely affected their QOL. Patients had tried varied strategies to manage their symptoms and were most likely to seek help from their GP. Few patients had sought patient support groups, though none had attended.
Despite some patients having significant neuropathic symptoms, majority felt satisfied with their decision to receive oxaliplatin (Fig. 6). The only patient who reported regret had experienced disease relapse.
Discussion
This study combines clinical assessment by oncologists, self-assessment via questionnaires, objective neurological assessment and patient-reported outcomes through the application of a semi-structured interview. It provides a comprehensive view of the long-term neuropathy experience after treatment with oxaliplatin. Assessment of patients using these methods has enabled the collection of data on oxaliplatin-induced CPN from biological, psychological and social perspectives. Our analysis demonstrates the variability of neuropathic symptoms and impact 2 years after treatment. Many patients reported lowered QOL scores, and symptom scales showed varying degrees of functional impairment. Objective measures of neuropathy identified through TNS testing strongly correlated with cumulative oxaliplatin dose (p = 0.023). Sensory nerve action potentials were more frequently absent than muscle action potentials. These findings are consistent with the known axonal toxicity of platinum-based treatments [23, 24]. In our study, modified TNS ranged from 1 to 15 with a mean of 9.5. In a study examining the validity and reliability of TNS, healthy controls were compared with patients with diabetic polyneuropathy. In healthy controls, the TNS mean ± SD was 0.4 ± 0.5; in patients with mild diabetic neuropathy 12 ± 5.2; and those with severe neuropathy 25 ± 7.4 [13].
The present study did not show a significant association between age and risk of CPN, nor with pre-existing diabetes and CPN; it must be noted that the small sample size could limit the power of this observation in identifying the significance of some variables. This is consistent with other reports confirming that advanced age and diabetic status should not preclude oxaliplatin treatment [25–27]. Vincenzi et al [25] identified several factors that predicted the incidence of oxaliplatin CPN, namely pre-treatment anaemia, hypoalbuminaemia and hypomagnesaemia, as well as alcohol consumption. These clinical features are easily accessible and may inform risk counselling.
Through the interview process, patients described a broad range of neuropathic symptoms, though importantly, the majority was able to continue to function normally. Patients did not require significant input from family members to support them with daily activities and, consequently, were able to maintain independence. A small number of patients were unable to resume work and expressed significant regret about losing their skills, and to a degree, identity and livelihood. Given the potential impact on social, psychological and financial wellbeing, awareness of this potential risk and its implications is critical.
Surprisingly, a substantial proportion of patients described a lack of adequate information about the risk of CPN. The majority of patients denied or were unable to recall any discussion of the expected, dose-dependent side effect prior to treatment. This is in stark contrast with expected practice; each patient treated with oxaliplatin should receive pre-treatment education about its risks and benefits. Whilst it is possible that patients were not warned of the risk of neuropathy, it may be more likely that methods used to inform patients of the risk were ineffective, and thus, information was poorly retained.
Despite the broad ranging impact of neuropathy, patients generally felt satisfied with their decision to be treated with oxaliplatin. Patients were grateful for receiving oxaliplatin, as they attributed their survival to the drug. One patient who reported regret experienced disease relapse, and felt oxaliplatin had caused CPN, without preventing recurrence. Despite the fact that many patients with resectable colon cancer are cured by their surgery, interviewees frequently believed they would have succumbed to cancer had they not received oxaliplatin.
There are a number of factors that may confound our results. The present study did not show a significant association between age and risk of CPN, nor with pre-existing diabetes and CPN, though this study had low power to determine this. Due to a small sample size, statistical significance was difficult to report on. Many patients who had completed treatment were reluctant to have further intervention, and given patients did not complete all parts of the study, our data set is incomplete and there is the potential for selection bias.
There are a limited number of studies examining patient perspectives on the impact of chemotherapy-induced CPN [28–31], though ours is the first to combine subjective and objective quantitative measures, with exploratory qualitative research. Despite an awareness of the consequences of CPN, ongoing systematic assessment of these symptoms has not been widely adopted. Furthermore, attention to pre-treatment counselling and a focus on effective strategies of providing informed consent are of paramount importance. The various tools used in this study were straightforward to apply and provide a comprehensive assessment of the impact of neuropathy. Whilst the WHOQOL BREF [14] and EQ-5D-5L [15] give an insight into the longer term effects on global health measures, the QLQ-CIPN20 [16] can provide clinicians with a standardized assessment of acute and chronic neuropathic symptoms and may be useful in the clinic setting to evaluate the current impact and portend future symptoms of neuropathy. Despite patients’ acceptance of this long-term toxicity, more effective strategies to prevent and manage this predictable and potentially disabling toxicity are needed.
References
Bécouarns Y et al (2001) Oxaliplatin: available data in non-colorectal gastrointestinal malignancies. Crit Rev Oncol Hematol 40(3):265–272, ISSN 1040–8428. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/11738949 >
Pasetto LM et al (2006) Oxaliplatin-related neurotoxicity: how and why? Crit Rev Oncol Hematol 59(2):159–168, ISSN 1040–8428. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/16806962 >
Quasthoff S, Hartung HP (2002) Chemotherapy-induced peripheral neuropathy. J Neurol 249(1):9–17, ISSN 0340–5354. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/11954874 >
Grothey A, Goldberg RM (2004) A review of oxaliplatin and its clinical use in colorectal cancer. Expert Opin Pharmacother 5(10):2159–2170, ISSN 1744–7666. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/15461551 >
Grothey A (2005) Clinical management of oxaliplatin-associated neurotoxicity. Clin Colorectal Cancer 5(Suppl 1):S38–S46, ISSN 1533–0028. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/15871765 >
de Gramont A et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18(16):2938–2947, ISSN 0732-183X. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/10944126 >
Grothey A (2003) Oxaliplatin-safety profile: neurotoxicity. Semin Oncol 30(4 Suppl 15):5–13, ISSN 0093–7754. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/14523789 >
André T et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350(23):2343–2351, ISSN 1533–4406. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/15175436 >
Krishnan AV et al (2006) Oxaliplatin and axonal Na+ channel function in vivo. Clin Cancer Res 12(15):4481–4484, ISSN 1078–0432. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/16899592 >
Land SR et al (2007) Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol 25(16):2205–2211, ISSN 1527–7755. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/17470850 >
Pietrangeli A et al (2006) Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol 56(1):13–16, ISSN 0014–3022. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/16825773 >
Trotti A et al (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13(3):176–181, ISSN 1053–4296. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/12903007 >
Cornblath DR et al (1999) Total neuropathy score: validation and reliability study. Neurology 53(8):1660–1664, ISSN 0028–3878. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/10563609 >
(1995) The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med 41(10):1403–1409. ISSN 0277–9536. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/8560308 >
Herdman M et al (2011) Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 20(10):1727–1736, ISSN 1573–2649. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/21479777 >
Postma TJ et al (2005) The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer 41(8):1135–1139, ISSN 0959–8049. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/15911236 >
STATACORP. Stata: Release 12. College Station, TX.: Statacorp LP.: Statistical Software. p. 2011
NVivo (2006) Qualitative data analysis software: QSR International Pty, Ltd
Smith J, Firth J (2011) Qualitative data analysis: the framework approach. Nurse Res 18(2):52–62, ISSN 1351–5578. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/21319484 >
Hawthorne G, Richardson J, Osborne R (1999) The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Qual Life Res 8(3):209–224, ISSN 0962–9343. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/10472152 >
Hawthorne G, Herrman H, Murphy B (2006) Interpreting the WHOQOL-BREF: preliminary population norms and effect sizes. Soc Indic Res 77:37–59
Lavoie Smith EM et al (2013) Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual Life Res 22(10):2787–2799, ISSN 1573–2649. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/23543373 >
Höke A, Ray M (2014) Rodent models of chemotherapy-induced peripheral neuropathy. ILAR J 54(3):273–281, ISSN 1930–6180. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/24615440 >
Park SB et al (2008) Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem 15(29):3081–3094, ISSN 0929–8673. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/19075655 >
Vincenzi B et al (2013) Identification of clinical predictive factors of oxaliplatin-induced chronic peripheral neuropathy in colorectal cancer patients treated with adjuvant Folfox IV. Support Care Cancer 21(5):1313–9, ISSN 1433–7339. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/23196819 >
Ramanathan RK et al (2010) Incidence and evolution of oxaliplatin-induced peripheral sensory neuropathy in diabetic patients with colorectal cancer: a pooled analysis of three phase III studies. Ann Oncol 21(4):754–758, ISSN 1569–8041. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/19887466 >
Goldberg RM et al (2006) Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 24(25):4085–4091, ISSN 1527–7755. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/16943526 >
Bennett BK et al (2012) Impact of oxaliplatin-induced neuropathy: a patient perspective. Support Care Cancer 20(11):2959–2967, ISSN 1433–7339. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/22426503 >
Tofthagen C (2010) Patient perceptions associated with chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs 14(3):E22–E28, ISSN 1538-067X. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/20529785 >
Tofthagen C (2010) Surviving chemotherapy for colon cancer and living with the consequences. J Palliat Med 13(11):1389–1391, ISSN 1557–7740. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/21091028 >
Leonard GD et al (2005) Survey of oxaliplatin-associated neurotoxicity using an interview-based questionnaire in patients with metastatic colorectal cancer. BMC Cancer 5:116, ISSN 1471–2407. Disponível em: < http://www.ncbi.nlm.nih.gov/pubmed/16168057 >
Author information
Authors and Affiliations
Corresponding author
Additional information
Key points
• Mild to moderate oxaliplatin-induced neuropathy >2 years post-treatment is common.
• The psychosocial impact of chronic peripheral neuropathy is significant and varies considerably.
• Majority of patients did not recall being warned of the risks of chronic peripheral neuropathy. Many of those who recall being warned did not feel sufficient emphasis was placed on the issue.
Appendix 1
Appendix 1
Interview questions
-
1.
How is this affecting your life?
-
2.
How is this affecting your work?
-
3.
How does your family support you with symptoms of peripheral neuropathy?
-
4.
Were you well informed about the risk of chronic peripheral neuropathy before receiving the treatment?
-
5.
Do you find that there are enough resources to support groups for chronic peripheral neuropathy?
-
6.
Have you tried anything to make the symptoms go better? Has any of your attempts worked
-
7.
How do you feel about living with chronic peripheral neuropathy?
-
8.
Do you feel that having symptoms of peripheral neuropathy outweighs clinical benefit of oxaliplatin? Do you regret receiving this treatment?
Rights and permissions
About this article
Cite this article
Padman, S., Lee, J., Kumar, R. et al. Late effects of oxaliplatin-induced peripheral neuropathy (LEON)—cross-sectional cohort study of patients with colorectal cancer surviving at least 2 years. Support Care Cancer 23, 861–869 (2015). https://doi.org/10.1007/s00520-014-2423-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2423-9