Abstract
Purpose
Oxaliplatin-induced neuropathy is a dose-related side effect which occurs in almost 40 % of patients treated with oxaliplatin. Aim of the present study was to identify reliable clinical factors predicting its development and duration.
Methods
One hundred sixty-nine completely resected colorectal cancer patients treated with adjuvant Folfox IV regimen were retrospectively included. The following pre-treatment clinical parameters were collected: hypocalcaemia, hypomagnesaemia, hypoalbuminaemia, anaemia, diabetes, chronic renal failure (CRF), folate deficiency, vitamin B12 deficiency, number of cycles received and habit to alcohol consumption. Incidence, grade (NCI-CTCAE v.3) and duration of neuropathy were recorded.
Results
Incidence of neuropathy was found to be higher in patients with pre-treatment anaemia (p = 0.001), hypoalbuminaemia (p = 0.01) and hypomagnesaemia (p = 0.001) as well in those with habit to alcohol consumption (p = 0.003). Neuropathy durations were conversely associated with age, being longer in younger patients (p = 0.03), and again with hypoalbuminaemia (p = 0.04) and hypomagnesaemia (p = 0.002). No correlation was found with gender, hypocalcaemia, diabetes and CRF. The correlation between vitamin B12 and folate levels and the development of neurotoxicity were not analysed because of the high number of missing data in the population.
Conclusions
Age, anaemia, hypoalbuminaemia, hypomagnesaemia and alcohol consumption are reliable and easily assessable clinical factors predicting incidence and length of oxaliplatin-induced neuropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxaliplatin, together with 5-fluorouracil and leucovorin (Folfox regimen) or with capecitabine (Xelox regimen), is currently a mainstay of adjuvant chemotherapy in resected colorectal cancer (CRC) patients. The Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) trial pointed out that adding oxaliplatin to a regimen of fluorouracil and leucovorin improves the disease-free survival (DFS) of stage III (HR for recurrence 0.76) or high-risk stage II (HR for recurrence 0.80) resected CRC patients [1]. A more recent analysis has also proven an impact of oxaliplatin-based therapy on overall survival [2]. Haller et al. have shown similar results comparing fluorouracil and leucovorin (3-year DFS rate of 66.5 %) versus oxaliplatin plus capecitabine (3-year DFS rate of 70.9 %) regimen in stage III CRC patients [2].
Oxaliplatin-induced peripheral neuropathy is the main concern about the use of this drug. In fact, oxaliplatin can cause both acute neuropathy (transient, distal paresthesias during or shortly after the first minutes of infusion) and chronic sensory neuropathy (distal paresthesia and numbness which can lead to functional disability, in the case of grade 3 toxicity). These neurosensory symptoms increase in intensity with cumulative doses, persist between cycles and affect the quality of life in the majority of patients.
In the MOSAIC trial, the incidence of grade (G) 2–3 peripheral neuropathy during the treatment was 40 % and it persisted 18 months after treatment termination in 3.9 % of all patients [1]. Despite some promising efforts in the last years, an effective treatment for oxaliplatin-induced peripheral neuropathy is not available yet.
Moreover, there are not available data regarding clinical parameters potentially able to predict the onset and persistence of peripheral neuropathy during and after oxaliplatin treatment, respectively. Interestingly, Attal et al. have described how the presence of hyperalgesia to cold and heat stimuli is a reliable early clinical marker of oxaliplatin neurotoxicity [3]. Moreover, a recently published prospective study by Won et al. identified several polymorphisms associated with severe oxaliplatin-induced chronic peripheral neuropathy in two independent data sets, and it also evaluated these polymorphisms as potential predictive markers [4]. The results from this study underlined how a genome-wide pharmacogenomic approach could be useful for identifying novel predictive polymorphism that may be used in personalised chemotherapy. Aim of the present retrospective study was to evaluate some pre-treatment clinical parameters that may influence peripheral nerves function (age, sex, hypocalcaemia, hypomagnesaemia, hypoalbuminaemia, anaemia, diabetes, chronic renal failure, folate and vitamin B12 deficiency and the habit to alcohol consumption) as predictive factors of development and duration of oxaliplatin-induced chronic peripheral neuropathy.
Patients and methods
Patient population
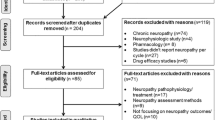
In order to perform our study, we decided to retrospectively include all available completely resected CRC patients, treated between 2000 and 2011 at the University Campus Bio-Medico of Rome (Roma), Ospedale S. Giovanni di Dio (Frattaminore), Ospedale S. Salvatore (Pesaro) and Ospedale Giovanni II (Bari). As additional inclusion criteria, all the patients had to be treated with adjuvant Folfox IV regimen, which comprised a 2-h infusion of 200 mg of leucovorin/m2 of body surface area followed by a bolus of 400 mg/m2 of fluorouracil and then a 22-h infusion of 600 mg/m2 of fluorouracil given on two consecutive days. Oxaliplatin (85 mg/m2) was administered with a 2-h infusion on day 1. This schedule was repeated every 14 days, for 12 cycles.
Data collection
The peripheral neurotoxicity developed during the treatment was evaluated according to NCI-CTCAE version 3 [5] (Table 1) and was recorded. The length of the neurotoxicity and the total dose of oxaliplatin received were calculated, and every eventual treatment administered for the treatment of neurotoxicity was recorded. We retrospectively collected the pre-treatment using the following parameters: hypocalcaemia, hypomagnesaemia, hypoalbuminaemia, anaemia, diabetes, chronic renal failure (CRF), folate and vitamin B12 deficiency and the habit to alcohol consumption (Table 2).
Statistical analysis
The incidence of neuropathy was described as the rate of neurotoxicity occurring during the entire treatment duration. The severity was described as the highest common criteria toxicity recorded during the treatment. Stratified permutation tests were carried out to explore the association between clinical categorical variables and incidence/severity of oxaliplatin-induced neuropathy. The duration of oxaliplatin-induced neurotoxicity was described as the median value (and 95 % confidence interval) and was determined using Kaplan–Meier product limit method. The differences in terms of duration of neurotoxicity according to the different clinical variables were evaluated using the log-rank test. A multivariate analysis was performed to assess the specific weight of each clinical variable on the onset of neurotoxicity. SPSS software (version 17.00, SPSS, Chicago) was used for statistical analysis. A p value of <0.05 was considered to indicate statistical significance.
Results
Patient population
One hundred sixty-nine completely resected CRC patients treated with adjuvant Folfox IV regimen were retrospectively included in our study. Patient characteristics are shown in Table 3.
Among the enrolled patients, 50 % were under 60 years (84/169), while 50 % were over (85/169). Males represented 45 % of the sample (76/169), while female, 55 % (93/169).
As for comorbidities, 18 % of patients were diabetic (29/169), and 9 % were affected by chronic kidney failure (15/169). Of all patients, 8 % were found to be used to alcohol consumption (13/169).
Hypoalbuminaemia was found to be the most common findings among laboratory parameters, being present in 80 % of patients (136/169); anaemia was detected in 55 % of patients (93/169); hypocalcaemia, in 20 % (34/169); and hypomagnesaemia, in 26 % (44/169). Finally, 10 % of patients received ≤6 cycles of Folfox IV (18/169), while 90 % (151/169), 7 to 12 cycles.
Incidence of peripheral neuropathy
Table 4 shows the incidence of peripheral neurotoxicity developed during the treatment and its correlation with clinical potential predictive factors. No grade 4 peripheral neuropathy was recorded.
In the present study, age (p = 0.78) and gender (p = 0.97) were not found to be associated with the development of peripheral neuropathy. In fact, G2–G3 neurotoxicity was recorded in 52 % of patients both in the older (44/85) and in the younger group (4/84). Similarly, it was observed in 50 % (38/76) of male patients and in 54 % (50/93) of female patients.
On the contrary, G2–G3 neurotoxicity was observed in 56 % (52/93) of anaemic patients and in 47 % (36/76) of non-anaemic patients, and the difference was found to be statistically significant (p = 0.001). Furthermore, the incidence of G2–G3 neurotoxicity was also significantly higher in patients with hypoalbuminaemia (50 %, 68/136) than in those with normal level of albumin (61 %, 20/33) (p = 0.01).
Regarding electrolytes levels, calcium levels were not significantly associated with the onset of neuropathy. Forty-nine percent (66/135) of patients with normal calcium levels and 65 % (22/34) of patients with hypocalcaemia (p = 0.1) presented G2–G3 neurotoxicity. Interestingly, a reduction in magnesium plasmatic levels resulted to be highly associated with the severity of neurotoxicity: 42 % (53/125) of patients with normal magnesium levels developed G2–G3 peripheral neuropathy versus 80 % (35/44) of patients with hypomagnesaemia (p = 0.001).
Diabetic patients did not show a higher incidence of peripheral neuropathy when compared with non-diabetic patients (p = 0.33). In particular, G2–G3 neurotoxicity was observed in 51 % (72/140) of non-diabetic population and in 55 % (16/29) of the diabetic population. As for chronic renal failure, the incidence of G2–G3 neurotoxicity was 49 % (76/154) among patients with normal renal function versus 80 % (12/15) in the population with renal impairment (p = 0.05).
The alcohol consumption, defined as reported in Table 2, found to be associated with an increased incidence of G2–G3 neurotoxicity: in fact, it occurred in 69 % (9/13) of patients with a higher alcohol intake compare with versus 51 % (79/156) in the other group (p = 0.003).
Finally, the incidence of G2–G3 peripheral neuropathy appeared to be positively correlated with the number of chemotherapy cycles (p = 0.0001). In fact, no G2–G3 neurotoxicity was developed by patients receiving ≤6 cycles of Folfox IV, while it was developed by 42 % (64/151) of patients treated with 7–12 cycles. All these results have been confirmed in the multivariate analysis, including the correlation between CRF and the development of neurotoxicity (p = 0.04). The correlation between vitamin B12 and folate levels and the development of neurotoxicity were not analysed because of the higher number of missing data in the population (due to the retrospective design of the study).
Duration of peripheral neuropathy
In the present study, the duration of neuropathy was significantly longer in younger patients (p = 0.03), regardless of gender (p = 0.30) and presence of anaemia (p = 0.79), diabetes (p = 0.4) and renal impairment (p = 0.22). Among the other parameters evaluated, the duration of peripheral neurotoxicity was significantly associated with low albumin levels (p = 0.04) and with the presence of hypomagnesaemia (p = 0.002), while no difference was observed between patients with low or normal calcium levels (p = 0.21).
Finally, the habit of alcohol consumption did not show any impact on the duration of the neuropathy (p = 0.9). Figure 1 shows the correlation between age, hypoalbuminaemia, hypomagnesaemia and length of peripheral neuropathy (in weeks).
Discussion
Despite the efforts done in the last decade, chronic peripheral neuropathy is still a recognised complication of oxaliplatin-based regimen, which can lead to dose reduction or treatment discontinuation, with possible significant impact on patients’ quality of life. Currently available data show that oxaliplatin-induced neuropathy is a dose-related side effect which occurring in almost 50 % of patients treated with oxaliplatin. Unfortunately, no reliable predictive factors have been identified yet, and a standardised approach in terms of prevention and treatment has still not being defined.
With the present study, for the first time, we aimed to identify potential clinical parameters, easily obtainable by a pre-treatment assessment of the patient and complete blood test, to predict the development and the length of peripheral neuropathy. In the present series, the incidence of G2–G3 neurotoxicity in patients treated with adjuvant Folfox IV is 42 %, which is consistent with previous reports. Among the 169 colorectal cancer patients enrolled, the incidence of oxaliplatin-induced neuropathy was higher in anaemic patients (p = 0.001) and in those with pre-treatment low levels of albumin (p = 0.01) and magnesium (p = 0.001).
While no data are currently available on the possible relation between anaemia or serum albumin level and peripheral neuropathy development, the role of magnesium supplementation in the prevention of peripheral neuropathy with different aetiologies has been extensively investigated. For example, low magnesium levels in type 1 diabetes are associated with electromyographical signs of polyneuropathy [6], and a long-term oral supplementation seems to influence favourably the natural evolution of neuropathy in these patients [7]. Similarly, the results from the present study prove a strong correlation between hypomagnesaemia and the development of oxaliplatin-induced neurotoxicity. These data appear in concordance with recent reports from two clinical trials, the North Central Cancer Treatment Group (NCCTG) N04C7 [8] and the French Neuroxa study [9], supporting intravenous magnesium supplementation as an effective neuroprotectant against oxaliplatin-induced neuropathy. Unfortunately, the NCCTG N04C7 trial was closed after 104 of 300 planned patients were enrolled—this was due to a report from a previous phase III trial, the CONcePT [10], which initially showed that the Ca/Mg infusion arm was associated with a decreased response rate to oxaliplatin. However, the final results of this trial, probably due to early closure and lack of statistical power, were unable to demonstrate a substantial decrease in neuropathy in patients receiving Ca/Mg versus those receiving placebo. Furthermore, data regarding the lower response rate in the experimental arm were subsequently found to be incorrect.
Regarding the incidence of oxaliplatin-induced neuropathy, we found that alcohol consumption promote the development of oxaliplatin-induced neuropathy (p = 0.003). Chronic alcohol consumption is known to affect the peripheral nervous system. The pathogenesis of this damage is complex and still poorly understood; direct toxic effect, nutritional deficiency and oxidative stress are probably involved. Interestingly, alcohol consumption was proved to enhance anti-retroviral painful peripheral neuropathy [11]. These evidences suggest that the avoidance, or at least a moderate intake, of alcohol consumption should be probably recommended to those patients who are going to start an oxaliplatin-based treatment.
In accordance with previous reports [12, 13], our retrospective analysis confirms that, although the rationale is strong, both diabetes mellitus and age are not associated with an increased risk of chemotherapy-induced peripheral neuropathy, and they should not be considered as deterrents to oxaliplatin-based treatments. However, it must be noted that diabetic patients enrolled in this series are patients with newly diagnosed diabetes or under anti-diabetic treatment, and therefore, their serum glucose levels remain usually into the range. We believe that, despite the absence of correlation, a long-term control of glycaemia should be achieved in diabetic patients treated with oxaliplatin, in order to avoid the worsening of peripheral symptoms.
Regarding the duration of peripheral neuropathy, it was found to be longer in those patients with low albumin levels (p = 0.04) and hypomagnesaemia (p = 0.002). Moreover, a correlation with age was found, being younger patients at higher risk for a prolonged duration of peripheral symptoms.
Conclusion
In conclusion, the results from our analysis allow to potentially identify, prior to treatment start, a subgroup of patients with an increased risk of developing a severe and long-lasting oxaliplatin-induced peripheral neuropathy. Particular attention should be paid to young patients, with a pre-treatment detection of anaemia, hypoalbuminaemia, hypomagnesaemia and habit to alcohol consumption. In these patients, a preventive restore of normal magnesium levels should be considered and a limited consumption of alcohol should be recommended. Furthermore, in order to prevent the late sequelae of peripheral nervous system injury, an early treatment of oxaliplatin-induced neuropathy with one of the compound today available (e.g., duloxetine [14], venlafaxine [15]) should be undertaken. The main limitation of the present study is represented by its retrospective design. Further prospective studies are needed to confirm these preliminary data and to validate the use of our identified clinical parameters as predictors of oxaliplatin-induced chronic peripheral neuropathy.
References
André T, Boni C, Mounedji-Boudiaf L et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. N Engl J Med 350:2343–2351
Haller DG, Tabernero J, Maroun J et al (2011) Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 29:1465–1471
Attal N, Bouhassira D, Gautron M et al (2009) Thermal hyperalgesia as a marker of oxaliplatin neurotoxicity: a prospective quantified sensory assessment study. Pain 144:245–252
Won HH, Lee J, Park JO et al (2012) Polymorphic markers associated with severe oxaliplatin-induced, chronic peripheral neuropathy in colon cancer patients. Cancer 118(11):2828–2836
Cancer Therapy Evaluation Program, Common terminology criteria for adverse events, version 3.0, DCTD, NCI, NIH, DHHS March 31, 2003 (http://ctep.cancer.gov), Publish Date: August 9, 2006
Engelen W, Bouten A, De Leeuw I, De Block C (2000) Are low magnesium levels in type 1 diabetes associated with electromyographical signs of polyneuropathy? Magnes Res 13:197–203
De Leeuw I, Engelen W, De Block C, Van Gaal L (2004) Long-term magnesium supplementation influences favourably the natural evolution of neuropathy in Mg-depleted type 1 diabetic patients (T1dm). Magnes Res 17:109–114
Grothey A, Nikcevich DA, Sloan JA et al (2011) Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol 29:421–427
Gamelin L, Boisdron-Celle M, Delva R et al (2004) Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusion: a retrospective study of 161 patients receiving oxaliplatin combined with 5-fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res 10:4055–4061
Grothey A, Hart LL, Rowland KM et al (2008) Intermittent oxaliplatin administration and time-to-treatment-failure (TTF) in metastatic colorectal cancer (mCRC): final results of the phase III CONcePT trial. J Clin Oncol 26:2008
Ferrari LF, Levine JD (2010) Alcohol consumption enhances antiretroviral painful peripheral neuropathy by mitochondrial mechanisms. Eur J Neurosci 32:811–818
Ramanathan RK, Rothenberg ML, de Gramont A (2010) Incidence and evolution of oxaliplatin-induced peripheral sensory neuropathy in diabetic patients with colorectal cancer: a pooled analysis of three phase III studies. Ann Oncol 21:754–758
Goldberg RM, Tabah-Fisch I, Bleiberg H et al (2006) Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 24:4085–4091
Yang YH, Lin JK, Chen WS et al (2012) Duloxetine improves oxaliplatin-induced neuropathy in patients with colorectal cancer: an open-label pilot study. Support Care Cancer 20(7):1491–1497
Durand JP, Deplanque G, Montheil V et al (2012) Efficacy of venlafaxine for the prevention and relief of oxaliplatin-induced acute neurotoxicity: results of EFFOX, a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol 23:200–205
Conflict of interest
Authors have not any conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vincenzi, B., Frezza, A.M., Schiavon, G. et al. Identification of clinical predictive factors of oxaliplatin-induced chronic peripheral neuropathy in colorectal cancer patients treated with adjuvant Folfox IV. Support Care Cancer 21, 1313–1319 (2013). https://doi.org/10.1007/s00520-012-1667-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-012-1667-5