Abstract
Trial design
Peripheral neuropathy is a commonly reported adverse effect of oxaliplatin treatment, representing a significant limitation which may require discontinuation of effective therapy. The present study investigated the neuroprotective potential of riluzole in patients undergoing oxaliplatin treatment in a randomised-controlled trial comparing riluzole and placebo-control.
Methods
Fifty-two patients (17 females, 58.1 ± 12.7 years) receiving oxaliplatin treatment were randomised into either a treatment (50 mg riluzole) or lactose placebo group. The primary outcome measure was the total neuropathy score-reduced (TNSr). Secondary outcome measures include nerve excitability measures, 9-hole pegboard and FACT-GOG NTX questionnaire. Patients were assessed at baseline, pre-cycle 10 or 12, 4-week and 12-week post-treatment.
Results
Both the treatment and placebo groups developed objective and patient reported evidence of neurotoxicity over the course of oxaliplatin treatment, although there were no significant differences across any parameters between the two groups. However, across follow-up assessments, the treatment group experienced greater neuropathy, represented by a higher TNSr score at 4-week post-chemotherapy of 8.3 ± 2.7 compared with 4.6 ± 3.6 (p = 0.032) which was sustained at 12-week post-treatment (p = 0.089). Similarly, patients in the treatment group reported worse symptoms with a FACT-GOG NTX score of 37.4 ± 10.2 compared with 43.3 ± 7.4 (p = 0.02) in the placebo group at 4-week post-treatment.
Conclusion
This study is the first to provide an objective clinical investigation of riluzole in oxaliplatin-induced peripheral neuropathy employing both functional and neurophysiological measures. Although the recruitment target was not reached, the results do not show any benefit of riluzole in minimising neuropathy and may suggest that riluzole worsens neuropathy associated with oxaliplatin treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxaliplatin is a first-line treatment for advanced colorectal cancer. Unlike other platinum derivatives, oxaliplatin does not cause renal impairment or ototoxicity [1, 2]. However, oxaliplatin produces prominent peripheral neurotoxicity, including a distinctive pattern of cold-associated acute neurotoxicity in 95% of patients immediately following an infusion that typically resolves over a period of a few days [3, 4]. Oxaliplatin treatment is also related to the development of chronic dose-limiting sensory peripheral neuropathy [5], present in up to 50% of patients at higher cumulative doses [6]. Paraesthesia and numbness are the most commonly reported symptoms [7] and remain a significant limitation to maintaining dose intensity. Neuropathic symptoms that impact on function require dose reductions, dose delays and possibly discontinuation of therapy [8]. This is particularly an issue in the setting of adjuvant therapy where the aim of treatment is cure and long-term neurotoxicity is an unacceptable outcome.
A number of studies have demonstrated that Na+ channels have an important role in mediating axonal degeneration in models of toxic and inflammatory neuropathy [9,10,11]. In particular, the sodium channel isoform Nav1.6 has been implicated in acute oxaliplatin-related neurotoxicity in rodent models, prolonging the repolarisation phase of the action potential and producing hyperexcitability [12, 13]. In the clinical setting, nerve excitability techniques have provided some insight into the pathophysiology of acute oxaliplatin-induced neuropathy [7, 14, 15], also revealing prominent alterations in Na+ channel function.
There is some evidence that the development of acute and chronic oxaliplatin-induced neurotoxicity are linked—with the duration and intensity of acute oxaliplatin neurotoxicity predictive of the development of long-term chronic neuropathy [4, 16]. Furthermore, the degree of acute nerve excitability abnormalities appears to be linked to the subsequent development of chronic neuropathy [17]. Accordingly, there is rationale for the investigation of neuroprotective strategies based on modulation of Na+ channel function for the prevention of chronic neurotoxicity.
Riluzole is a neuroprotective agent currently used for the treatment of neurodegenerative diseases, particularly amyotrophic lateral sclerosis, where it prolongs survival [18, 19]. Riluzole acts to reduce axonal excitability and repetitive firing via suppression of persistent Na+ currents, enhancement of calcium-dependent K+ currents and reduction of neurotransmitter release [20]. The effects on Na+ channels promote neuroprotection by blocking inward movement of Na+ ions through persistent Na+ channels to prevent the reverse activation of axonal Na+/Ca2+ exchanger and subsequent Ca2+-mediated excitotoxicity [19]. Accordingly, riluzole has demonstrated neuroprotective properties in multiple neuronal cell populations, including enhancing neurite growth in dorsal root ganglion neurons [21]. Furthermore, in animal models, riluzole has shown efficacy in preventing the development of oxaliplatin-induced neurotoxicity symptoms including cold [22] and mechanical allodynia [23] with no evidence of tumour promotion [23, 24]. To examine the neuroprotective potential of riluzole in oxaliplatin neurotoxicity, we carried out a randomized controlled trial to assess whether treatment with riluzole results in a reduction in the development of chronic neuropathy. We hypothesised that riluzole treatment would be neuroprotective during oxaliplatin treatment and result in better neurophysiological and patient reported outcomes when compared with placebo-control.
Methods
Trial design
A randomised, double-blinded placebo-controlled trial was undertaken to determine the effect of riluzole on the development of neuropathy in patients receiving oxaliplatin chemotherapy. The trial was prospectively registered with the Australian and New Zealand Clinical Trials Registry (12611000514909, ‘neu-horizons’; 18/05/2011) and was conducted with ethical approval from the South Eastern Sydney and Illawarra Area Health Service—Human Research Ethics Committee, in accordance with the 1964 Helsinki Declaration. Patient recruitment and assessments were undertaken at the Prince of Wales Hospital, Sydney, Australia. Potential patients were given information to read regarding the benefits and risks of the study and provided written informed consent. Patients were recruited from July 2011 to December 2015, with the last follow-up assessment completed in September 2016. The study is reported in accordance with the Consolidated Standards for Reporting Trials Statement (CONSORT).
Participants
The inclusion criteria were (i) 18–80 years of age; (ii) planned oxaliplatin treatment; (iii) able to provide written informed consent and (iv) histological or cytological confirmation of cancer. Exclusion criteria included (i) baseline clinical and nerve conduction evidence of pre-existing neuropathy; (ii) past history of neurotoxic chemotherapy, (iii) concurrent use of anticonvulsant medications that modulate nerve function, (iv) elevated hepatic transaminase levels, (v) administration of another investigational drug within 30 days prior to randomisation, (vi) a history of severe hypersensitivity reaction to riluzole or any of the tablet components, (vii) significant pre-existing neurological or psychiatric disorder and (viii) pregnancy or lactation.
Intervention
Patients were randomly allocated to one of two groups: an intervention group that was prescribed riluzole 50 mg twice daily prior to the second oxaliplatin dose, continuing to the end of treatment, and for 2-week post-completion of treatment or matched daily lactose placebo. Randomisation was performed in a 1:1 ratio stratified by the frequency of oxaliplatin treatment (2 weekly vs 3 weekly). Each oxaliplatin cycle comprises of one oxaliplatin treatment dose. All patients had liver function tests performed at baseline and at monthly levels. Riluzole was discontinued if liver function testing demonstrated elevation in alanine aminotransferase levels to greater than five times the upper limit of normal.
Outcomes
Neurophysiological and functional assessments were conducted at baseline, during treatment prior to cycle 10 and 12, and at 4- and 12-week post-treatment. The primary outcome measure was the severity of neuropathy assessed at 4-week post-treatment using a validated scoring system, the total neuropathy score-reduced (© Johns Hopkins University) [25, 26]. This scale is used to evaluate neuropathy across eight different clinical domains, graded from zero to four for each domain covering sensory neuropathic symptoms, examination findings such as pin-prick sensitivity, vibration sense, strength, tendon jerk reflexes and nerve conduction results of the sural and tibial nerves. The scores obtained in the different categories were added to give a total neuropathy score (TNSr; range 0–32). All assessments were conducted by a single technician qualified in neurophysiological assessment.
Secondary outcome measures included functional, neurophysiological and quality of life measures. The nine-hole pegboard test (Smith and Nephew Rehabilitation, Inc., USA) was included as a functional measure of upper limb dexterity. Neurophysiological studies included nerve conduction studies and nerve excitability techniques. Nerve conduction studies were administered using previously described methods [27, 28] with a Medelec Synergy system (Viasys Healthcare, USA) to obtain peak motor and sensory amplitude of the tibial and sural nerves, respectively. Nerve excitability techniques provide information regarding ion channel function in peripheral nerves [29]. Nerve excitability studies were conducted on the median nerve at the wrist recording sensory compound action potentials from digit 2 using QtracS threshold-tracking protocol (copyright Institute of Neurology, UCL) [30], with skin temperature maintained ≥ 32°. Excitability analysis was comprised of parameters of hyperpolarizing threshold electrotonus 90–100 ms, refractoriness at 2.5 ms and superexcitability at 7 ms [31]. The FACT-GOG-NTX [32] is a validated questionnaire to assess the severity of neurotoxicity and impact on quality of life. It contains 13-items with each question is graded on a 0–4 scale, a score of ‘0’ representing ‘not at all’ and a score of 4 representing ‘very much’ inquiring neuropathic symptoms over a recent 7-day period. The total is tallied and reverse-scored so that the maximum score of 52 indicates no neuropathic symptoms.
Randomisation and masking
Participants were randomised at the initial study visit, after they had provided informed consent and met the study inclusion criteria. Patients were randomised in a 1:1 ratio stratified according to the frequency of oxaliplatin treatment (2 weekly vs 3 weekly) and centrally randomised through the National Health and Medical Research Centre (NHMRC) Clinical Trials Centre. All participants and investigators were blinded to the treatment allocation.
Statistical methods
Appropriate sample size was determined from the incidence of neuropathy in a previous open-label Na+ channel blocker trial with a neuropathy incidence of 31% in the treatment arm compared with 75% in the control arm for patients treated with oxaliplatin [33]. In order to detect this extent of difference, it was determined that 90 patients were required to detect differences between placebo and riluzole treatment arms to achieve at least 80% power and a two-sided type I error rate of 10%. Due to a short-fall in accrual and funding restrictions, the data presented is for a total of 48 patients who were recruited.
All efficacy analyses were performed on the Full Analysis Set (FAS). All randomised subjects who received at least one treatment were eligible for inclusion in the FAS in accordance with the intention-to-treat analysis principle. Subjects with missing neuropathy assessment data were considered treatment failures and included in the primary analysis. Normally distributed data were presented as mean ± standard deviation; otherwise, data were presented as median and interquartile range. Change across time-points was analysed using t test, Wilcoxon-signed rank test or chi-squared tests. Due to an appreciable number of missing data due to withdrawal or death, a mixed model for repeated measures modelling has been used where applicable. Analyses were completed using SAS 9.3 (SAS Institute Inc., Cary, NC) and a conservative significance threshold of p < 0.1 was selected to determine whether the actions of riluzole would necessitate further investigation.
Results
Fifty-two patients (32 males and 20 females) were recruited and consecutively randomised into the treatment group (n = 27) and control group (n = 25) (Fig. 1). Four patients (two in each group) withdrew participation before any study drug was administered and were therefore excluded from the final analysis. The final analysis included 48 patients. The mean age ± SD for the cohort was 58 ± 12.6 years. The intervention and control groups did not differ in terms of age, sex or primary location of cancer tumour. Chemotherapy treatment duration was similar across both groups with 19.2 ± 6.6 weeks for the intervention group and 17.9 ± 6.1 weeks for the control group. Dose intensity did not differ between groups (p = 0.42, Table 1). There were no adverse events reported for the duration of the study. A majority of patients received riluzole/placebo before the first treatment of oxaliplatin. Ten patients received riluzole/placebo prior to the 2nd cycle of oxaliplatin, of whom 6 patients were from the riluzole group and 4 from the control group. Additional clinical characteristics of patients included in the final analyses are represented in Table 1.
Primary outcome measure
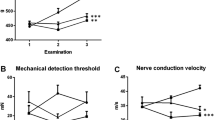
The baseline TNSr was 0.7 ± 1.6 and 0.5 ± 1.2 for the intervention and control groups, respectively, indicating no neuropathy at baseline. The mixed model repeated measures analysis revealed a significant difference between the two groups following chemotherapy treatment at 4-week post-treatment and 12-week post-treatment. The TNSr at 4-week post-chemotherapy was 8.3 ± 2.7 for the intervention group and 4.6 ± 3.6 for the control group (p = 0.032), consistent with greater neuropathy severity in the intervention arm (Fig. 2a). The TNSr remained worse for the intervention group at 12-week post-chemotherapy with a score of 8.5 ± 2.5 in comparison with 6.5 ± 3.9 for the control group (p = 0.09).
Total Neuropathy Score and patient reported neuropathy symptoms. a Total neuropathy score continued to increase beyond the chemotherapy treatment period for both groups and was significantly higher for the riluzole group in the follow-up period. b Sural amplitude decreased following chemotherapy with no differences between the riluzole and control groups. c Tibial motor activity remained relatively stable across the assessment period. d Functional Assessment of Cancer Therapy-Gynaecologic Oncology Group-Neurotoxicity (FACT-GOG) questionnaire was reported as having worsening symptoms for the riluzole group in comparison to the control group at 4-week post-chemotherapy which resolved at 12-week post-chemotherapy
Secondary outcome measures
There was a progressive reduction in the peak sural nerve sensory amplitude between both groups from a baseline mean ± SD of 11.9 ± 4.5 μV for the intervention group and 15.6 ± 6.6 μV for the control group to 3.5 ± 2.7 μV and 4.1 ± 4.0 μV at 12-week post-chemotherapy but no statistically significant was noted in the extent of peak decline between groups (Fig. 2b). Tibial nerve conduction responses were stable across the treatment and follow-up period. However, mixed model analyses revealed a statistically significant difference in tibial amplitude at 4-week post-chemotherapy with 11.7 ± 3.7 mV for the intervention group compared with 9.5 ± 4.0 mV (p = 0.057, Fig. 2c) for the control group, although mean values for both groups were within the normative range. Sensory nerve excitability studies were conducted on the median nerve to examine nerve membrane potential. Following repeated measures analysis, refractoriness at 2.5 ms was reduced for both groups indicating altered nerve excitability during treatment but at 4 week post-treatment, refractoriness remained lower for the intervention group at 7.1 ± 15.1% threshold while the control group recovered to 18.7 ± 19.7% (p = 0.049; Supplementary Fig. 1b). There were no other statistical differences at any time points for any measures of nerve excitability (Supplementary Table 1).
Functional testing using the 9-hole peg test at baseline was 23.3 ± 5.3 s duration for the intervention group and 23.1 ± 5.9 s for the control group (Supplementary Fig. 1d). There were no significant differences across any time points and following repeated measures. Patient reported outcomes for neuropathy were assessed using the FACT-GOG-NTX. The baseline mean score for two groups was similar with patients in the intervention group scoring 49.4 ± 2.6 points and control group scoring 50.6 ± 1.8 points. There was a consistent worsening of FACT-GOG scores for both groups during treatment, with a statistically significant difference between the two groups only at 4-week post-chemotherapy. The intervention group reported worse perceived neuropathic symptoms with a reduced score of 37.4 ± 10.2 points in comparison with the control group with 43.3 ± 7.4 points (p = 0.02, Fig. 2d). This difference did not persist at 12-week post-chemotherapy.
Discussion
The present study was the first to investigate the neuroprotective potential of riluzole in patients receiving oxaliplatin treatment. Although there was convincing evidence to suggest the actions of riluzole may be beneficial in oxaliplatin chemotherapy treatment, we were unable to demonstrate neuroprotection that was superior to placebo. The primary end point of 4-week post-chemotherapy suggested that there were worsening objective and subjective outcomes in the riluzole group as represented by a greater TNSr and lower FACT-GOG score, respectively.
Analysis of secondary outcomes measures demonstrated no significant change in the nine-hole peg test, a measure of upper limb functional dexterity. Nerve excitability measures demonstrated a difference only in refractoriness, a marker of recovery from inactivation of nodal transient Na+ channels. However, previous work has failed to demonstrate an effect of riluzole alone on refractoriness or the relative refractory period [34]. Accordingly, changes in refractoriness independent of any change in threshold electrotonus or superexcitability may be due to inter-individual patient factors such as ion channel properties or skin temperature fluctuations [31], reflected by a single time-point difference at 4-week post-chemotherapy which did not persist at 12-week post-chemotherapy. Similarly, tibial nerve amplitude did not vary greatly across the testing period and this was expected as oxaliplatin produces a sensory neuropathy [7]. The significant difference observed at 4-week post-chemotherapy may be a reflection of the sample size as there were no sustained differences at 12-week post-chemotherapy and mean values for both groups remained within the normal range. Although we did not observe any significant changes in patients treated with riluzole during the test period, neuropathic symptoms did not appear to improve by the 12-week period and future studies may benefit from observing the rate of recovery across the two test groups in a longer follow-up period.
It is difficult to pinpoint the exact mechanisms underlying the greater neuropathy severity in the riluzole arm. Riluzole is known to target a number of pathways, including Ca2+ channels, glutamatergic pathways and both persistent and transient Na+ channels [19, 34,35,36]. In addition to Na+ channels, riluzole causes reversible Ca2+ channel blockade and specifically targets the alpha-2 subunit [37] as well as having effects on K+ channels including TREK channels [24]. Several previous trials have examined the effects of other Na+ channel blockers on oxaliplatin neurotoxicity. A previous study of the Na+ channel blocker, oxcarbazepine, demonstrated maintenance of nerve conduction peak amplitude in sural and peroneal nerves [38], although long-term follow-up assessments were not available to assess the long-term effects of neuropathy outcomes. Oxcarbazepine has a different effect to riluzole on Na+ channels and acts on processes relating to high frequency firing [39, 40]. However, its analogue carbamazepine did not exhibit beneficial effects on oxaliplatin treated patients, with no evidence of preservation of nerve conduction amplitudes [41]. In previous animal studies, riluzole has shown efficacy in preventing the development of oxaliplatin-induced neurotoxicity [22,23,24]. However, this is the first study to investigate riluzole in combination with oxaliplatin treatment in a clinical cohort, and previous investigations have been on rodent models which are often not predictive of outcomes in neuroprotective trials. While it remains possible that there may be a synergistic effect of riluzole and oxaliplatin on sodium channel kinetics which may exacerbate axonal degeneration, previous trials of sodium channel blockers in oxaliplatin-treated patients have not demonstrated worsening of neuropathy outcomes. However, there are many individual factors which govern the development and severity of CIPN, including multiple genetic contributors which are not fully characterized. Given the limited sample size, it is possible that intrinsic variation in neuropathy outcomes developed between the cohorts led to more severe neuropathy in the riluzole arm.
The present study design incorporated multiple methods of neuropathy assessment, including patient reported outcomes and objective assessment tools, in line with recommended trial protocols [42]. However, our study did not identify any benefits of riluzole treatment—with treated patients developing worse patient reported and clinical outcomes at 4-week post-oxaliplatin treatment. While it is acknowledged that sample size considerations may impact the study power, it is important to note that several prior neuroprotection trials have identified unexpected worsening of neuropathy compared with placebo in chemotherapy-treated patients [43, 44]. This is an important consideration for future trials, as the possibility of detrimental effects may necessitate the addition of interim analyses. Of note, there is currently an ongoing study of riluzole treatment in oxaliplatin-treated patients [45] which may benefit from such analyses.
In conclusion, although there was a good rationale to suggest that riluzole could prevent oxaliplatin-induced peripheral neuropathy, this was not supported by the findings of this randomised trial. Using both functional and neurophysiological testing methods, we identified no benefit of riluzole on the severity of neuropathy during chemotherapy treatment and up to 12 week post-treatment.
References
McKeage MJ, Hsu T, Screnci D, Haddad G, Baguley BC (2001) Nucleolar damage correlates with neurotoxicity induced by different platinum drugs. Br J Cancer 85(8):1219–1225. https://doi.org/10.1054/bjoc.2001.2024
Amptoulach S, Tsavaris N (2011) Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract 2011:843019–843015. https://doi.org/10.1155/2011/843019
Cassidy J, Misset JL (2002) Oxaliplatin-related side effects: characteristics and management. Semin Oncol 29(5 Suppl 15):11–20. https://doi.org/10.1053/sonc.2002.35524
Argyriou AA, Cavaletti G, Briani C, Velasco R, Bruna J, Campagnolo M, Alberti P, Bergamo F, Cortinovis D, Cazzaniga M, Santos C, Papadimitriou K, Kalofonos HP (2013) Clinical pattern and associations of oxaliplatin acute neurotoxicity: a prospective study in 170 patients with colorectal cancer. Cancer 119(2):438–444. https://doi.org/10.1002/cncr.27732
Staff NP, Grisold A, Grisold W, Windebank AJ (2017) Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol 81(6):772–781. https://doi.org/10.1002/ana.24951
Velasco R, Bruna J, Briani C, Argyriou AA, Cavaletti G, Alberti P, Frigeni B, Cacciavillani M, Lonardi S, Cortinovis D, Cazzaniga M, Santos C, Kalofonos HP (2014) Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry 85(4):392–398. https://doi.org/10.1136/jnnp-2013-305334
Krishnan AV, Goldstein D, Friedlander M, Kiernan MC (2005) Oxaliplatin-induced neurotoxicity and the development of neuropathy. Muscle Nerve 32(1):51–60. https://doi.org/10.1002/mus.20340
Pietrangeli A, Leandri M, Terzoli E, Jandolo B, Garufi C (2006) Persistence of high-dose oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol 56(1):13–16. https://doi.org/10.1159/000094376
Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG (2004) Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain 127(Pt 2):294–303. https://doi.org/10.1093/brain/awh032
Kapoor R, Davies M, Blaker PA, Hall SM, Smith KJ (2003) Blockers of sodium and calcium entry protect axons from nitric oxide-mediated degeneration. Ann Neurol 53(2):174–180. https://doi.org/10.1002/ana.10443
Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C (2000) The chemotherapeutic oxaliplatin alters voltage-gated Na(+) channel kinetics on rat sensory neurons. Eur J Pharmacol 406(1):25–32. https://doi.org/10.1016/s0014-2999(00)00667-1
Deuis JR, Zimmermann K, Romanovsky AA, Possani LD, Cabot PJ, Lewis RJ, Vetter I (2013) An animal model of oxaliplatin-induced cold allodynia reveals a crucial role for Nav1.6 in peripheral pain pathways. Pain 154(9):1749–1757. https://doi.org/10.1016/j.pain.2013.05.032
Sittl R, Lampert A, Huth T, Schuy ET, Link AS, Fleckenstein J, Alzheimer C, Grafe P, Carr RW (2012) Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proc Natl Acad Sci U S A 109(17):6704–6709. https://doi.org/10.1073/pnas.1118058109
Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC (2008) Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem 15(29):3081–3094. https://doi.org/10.2174/092986708786848569
Heide R, Bostock H, Ventzel L, Grafe P, Bergmans J, Fuglsang-Frederiksen A, Finnerup NB, Tankisi H (2018) Axonal excitability changes and acute symptoms of oxaliplatin treatment: in vivo evidence for slowed sodium channel inactivation. Clin Neurophysiol 129(3):694–706. https://doi.org/10.1016/j.clinph.2017.11.015
Pachman DR, Qin R, Seisler D, Smith EM, Kaggal S, Novotny P, Ruddy KJ, Lafky JM, Ta LE, Beutler AS, Wagner-Johnston ND, Staff NP, Grothey A, Dougherty PM, Cavaletti G, Loprinzi CL (2016) Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Support Care Cancer 24(12):5059–5068. https://doi.org/10.1007/s00520-016-3373-1
Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC (2009) Oxaliplatin-induced neurotoxicity: changes in axonal excitability precede development of neuropathy. Brain 132(Pt 10):2712–2723. https://doi.org/10.1093/brain/awp219
Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V (1996). Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 347 (9013):1425–1431. doi:https://doi.org/10.1016/s0140-6736(96)91680-3
Cheah BC, Vucic S, Krishnan AV, Kiernan MC (2010) Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem 17(18):1942–1199. https://doi.org/10.2174/092986710791163939
Bellingham MC (2011) A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther 17(1):4–31. https://doi.org/10.1111/j.1755-5949.2009.00116.x
Leinster VH, Robson LG, Shortland PJ (2010) Differential effects of riluzole on subpopulations of adult rat dorsal root ganglion neurons in vitro. Neuroscience 166(3):942–951. https://doi.org/10.1016/j.neuroscience.2009.12.058
Yamamoto S, Egashira N, Tsuda M, Masuda S (2018) Riluzole prevents oxaliplatin-induced cold allodynia via inhibition of overexpression of transient receptor potential melastatin 8 in rats. J Pharmacol Sci 138(3):214–217. https://doi.org/10.1016/j.jphs.2018.10.006
Yamamoto S, Ushio S, Egashira N, Kawashiri T, Mitsuyasu S, Higuchi H, Ozawa N, Masuguchi K, Ono Y, Masuda S (2017) Excessive spinal glutamate transmission is involved in oxaliplatin-induced mechanical allodynia: a possibility for riluzole as a prophylactic drug. Sci Rep 7(1):9661. https://doi.org/10.1038/s41598-017-08891-1
Poupon L, Lamoine S, Pereira V, Barriere DA, Lolignier S, Giraudet F, Aissouni Y, Meleine M, Prival L, Richard D, Kerckhove N, Authier N, Balayssac D, Eschalier A, Lazdunski M, Busserolles J (2018) Targeting the TREK-1 potassium channel via riluzole to eliminate the neuropathic and depressive-like effects of oxaliplatin. Neuropharmacology 140:43–61. https://doi.org/10.1016/j.neuropharm.2018.07.026
Cavaletti G, Jann S, Pace A, Plasmati R, Siciliano G, Briani C, Cocito D, Padua L, Ghiglione E, Manicone M, Giussani G (2006) Multi-center assessment of the total neuropathy score for chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst 11(2):135–141. https://doi.org/10.1111/j.1085-9489.2006.00078.x
Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, Joh T (1999) Total neuropathy score: validation and reliability study. Neurology 53(8):1660–1664. https://doi.org/10.1212/wnl.53.8.1660
Burke D, Skuse NF, Lethlean AK (1974) Sensory conduction of the sural nerve in polyneuropathy. J Neurol Neurosurg Psychiatry 37(6):647–652. https://doi.org/10.1136/jnnp.37.6.647
Buschbacher RM (1999) Tibial nerve motor conduction to the abductor hallucis. Am J Phys Med Rehabil 78(6 Suppl):S15–S20. https://doi.org/10.1097/00002060-199911001-00004
Krishnan AV, Lin CS, Park SB, Kiernan MC (2009) Axonal ion channels from bench to bedside: a translational neuroscience perspective. 89 (3):288-313. https://doi.org/10.1016/j.pneurobio.2009.08.002
Kiernan MC, Lin CS, Andersen KV, Murray NM, Bostock H (2001) Clinical evaluation of excitability measures in sensory nerve. Muscle Nerve 24(7):883–892. https://doi.org/10.1002/mus.1085
Bostock H, Cikurel K, Burke D (1998) Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve 21(2):137–158. https://doi.org/10.1002/(sici)1097-4598(199802)21:2<137::aid-mus1>3.0.co;2-c
Calhoun EA, Welshman EE, Chang CH, Lurain JR, Fishman DA, Hunt TL, Cella D (2003). Psychometric evaluation of the functional assessment of cancer therapy/gynecologic oncology group-neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 13 (6):741–748. doi:https://doi.org/10.1111/j.1525-1438.2003.13603.x
Argyriou A, Chroni E, Polychronopoulos P, Iconomou G, Koutras A, Makatsoris T, Gerolymos M, Gourzis P, Assimakopoulos K, Kalofonos HJN (2006) Efficacy of oxcarbazepine for prophylaxis against cumulative oxaliplatin-induced neuropathy. 67 (12):2253-2255. https://doi.org/10.1212/01.wnl.0000249344.99671.d4
Vucic S, Lin CS, Cheah BC, Murray J, Menon P, Krishnan AV, Kiernan MC (2013) Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis. Brain 136(Pt 5):1361–1370. https://doi.org/10.1093/brain/awt085
Lazarevic V, Yang Y, Ivanova D, Fejtova A, Svenningsson P (2018) Riluzole attenuates the efficacy of glutamatergic transmission by interfering with the size of the readily releasable neurotransmitter pool. Neuropharmacology 143:38–48. https://doi.org/10.1016/j.neuropharm.2018.09.021
Huang CS, Song JH, Nagata K, Yeh JZ, Narahashi T (1997) Effects of the neuroprotective agent riluzole on the high voltage-activated calcium channels of rat dorsal root ganglion neurons. J Pharmacol Exp Ther 282(3):1280–1290
Hebert T, Drapeau P, Pradier L, Dunn RJ (1994) Block of the rat brain IIA sodium channel alpha subunit by the neuroprotective drug riluzole. Mol Pharmacol 45(5):1055–1060
Argyriou AA, Chroni E, Polychronopoulos P, Iconomou G, Koutras A, Makatsoris T, Gerolymos MK, Gourzis P, Assimakopoulos K, Kalofonos HP (2006) Efficacy of oxcarbazepine for prophylaxis against cumulative oxaliplatin-induced neuropathy. Neurology 67(12):2253–2255. https://doi.org/10.1212/01.wnl.0000249344.99671.d4
Ichikawa K, Koyama N, Kiguchi S, Kojima M, Yokota T (2001) Inhibitory effect of oxcarbazepine on high-frequency firing in peripheral nerve fibers. Eur J Pharmacol 420(2–3):119–122. https://doi.org/10.1016/s0014-2999(01)01007-x
Schmidt D, Elger CE (2004) What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs? Epilepsy Behav 5(5):627–635. https://doi.org/10.1016/j.yebeh.2004.07.004
von Delius S, Eckel F, Wagenpfeil S, Mayr M, Stock K, Kullmann F, Obermeier F, Erdmann J, Schmelz R, Quasthoff S, Adelsberger H, Bredenkamp R, Schmid RM, Lersch C (2007) Carbamazepine for prevention of oxaliplatin-related neurotoxicity in patients with advanced colorectal cancer: final results of a randomised, controlled, multicenter phase II study. Investig New Drugs 25(2):173–180. https://doi.org/10.1007/s10637-006-9010-y
Gewandter JS, Brell J, Cavaletti G, Dougherty PM, Evans S, Howie L, McDermott MP, O'Mara A, Smith AG, Dastros-Pitei D, Gauthier LR, Haroutounian S, Jarpe M, Katz NP, Loprinzi C, Richardson P, Lavoie-Smith EM, Wen PY, Turk DC, Dworkin RH, Freeman R (2018) Trial designs for chemotherapy-induced peripheral neuropathy prevention: ACTTION recommendations. Neurology 91(9):403–413. https://doi.org/10.1212/WNL.0000000000006083
Cassidy J, Paul J, Soukop M, Habeshaw T, Reed NS, Parkin D, Kaye SB (1998) Clinical trials of nimodipine as a potential neuroprotector in ovarian cancer patients treated with cisplatin. Cancer Chemother Pharmacol 41(2):161–166. https://doi.org/10.1007/s002800050723
Hershman DL, Unger JM, Crew KD, Minasian LM, Awad D, Moinpour CM, Hansen L, Lew DL, Greenlee H, Fehrenbacher L, Wade JL 3rd, Wong SF, Hortobagyi GN, Meyskens FL, Albain KS (2013) Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol 31(20):2627–2633. https://doi.org/10.1200/JCO.2012.44.8738
Kerckhove N, Busserolles J, Stanbury T, Pereira B, Plence V, Bonnetain F, Krakowski I, Eschalier A, Pezet D, Balayssac D (2019). Effectiveness assessment of riluzole in the prevention of oxaliplatin-induced peripheral neuropathy: RILUZOX-01: protocol of a randomised, parallel, controlled, double-blind and multicentre study by the UNICANCER-AFSOS Supportive Care intergroup. BMJ Open 9 (6):e027770. doi:https://doi.org/10.1136/bmjopen-2018-027770
Funding
This study was supported by a National Health and Medical Research Council of Australia (NHMRC) Project Grant (#1007628) and a Cancer Institute NSW Program Grant (14/TPG/1–05). SBP is supported by a NHMRC Career Development Fellowship (#1148595). MCK is supported by a NHMRC Practitioner Fellowship (#1156093); and by ForeFront, a large collaborative research group dedicated to the study of neurodegenerative diseases and funded by the National Health and Medical Research Council of Australia Program Grant (#1132524).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 367 kb)
Rights and permissions
About this article
Cite this article
Trinh, T., Park, S.B., Murray, J. et al. Neu-horizons: neuroprotection and therapeutic use of riluzole for the prevention of oxaliplatin-induced neuropathy—a randomised controlled trial. Support Care Cancer 29, 1103–1110 (2021). https://doi.org/10.1007/s00520-020-05591-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05591-x