Summary
Objective
Connection between abdominal obesity, type 2 diabetes, and the hypothalamic-pituitary-adrenal (HPA) axis activity remains unclear. The aim of this study was to measure HPA axis activity in 121 type 2 diabetics, in 29 obese subjects, and 19 control subjects.
Research design and methods
Physical examination, anthropometric measures, psychological questionnaire, psychiatric interview, neurological and ophthalmologic examination were performed. Biochemical parameters, urinary free cortisol levels (UFC), cortisol and ACTH levels at 8 and 16 h, cortisol levels after overnight suppression with 1 mg dexamethasone followed by ACTH test in 30 and 60 min were measured. Groups were stratified in relation to obesity, body fat distribution, and chronic complications.
Results
UFC and postdexamethasone cortisol were significantly increased in diabetic patients compared with both obese subjects (p < 0.01) and control group (p < 0.05), regardless to diabetic complications and obesity. Postdexamethasone cortisol was correlated with waist circumference. ACTH-induced cortisol levels were significantly higher in all type 2 diabetic patients. An independent association was found between AUC cortisol in ACTH test and insulin resistance. Multiple regression analysis showed that waist circumference was independently associated with sex, fasting plasma insulin, morning cortisol, and AUC of cortisol in ACTH test (R 2 = 0.334,p < 0.0000).
Conclusions
In type 2 diabetic patients, the HPA axis is clearly hyperactive as evident in increased urinary free cortisol, diminished cortisol suppression after dexamethasone and increased ACTH-induced cortisol levels. Abdominal obesity and the presence of chronic complications increased the HPA axis hyperactivity in type 2 diabetes. Augmentation of positive feedback is associated with insulin resistance and negative feedback with abdominal obesity.
Zusammenfassung
Ziel
Es besteht Unklarheit über die Beziehung zwischen abdominaler Adipositas, Diabetes mellitus Typ 2 und der Hypothalamus-Hypophysen-Nebennieren (HHNN) Achse. Ziel dieser Studie war es, die Aktivität der HHNN-Achse bei 121 Typ 2 Diabetikern, 29 adipösen und 19 Kontrollpersonen zu messen.
Studiendesign und Methodik
Es wurden eine physikalische Untersuchung, anthropometrische Messungen, psychologische Befragungen, psychiatrische Interviews sowie neurologische und ophthalmologische Untersuchungen durchgeführt. Außerdem wurden biochemische Parameter, freie Cortisol Spiegel im Urin (FCU), Cortisol und ACTH Konzentrationen um 8 und um 16 Uhr, sowie Cortisol Konzentrationen nach spätabendlicher Gabe von 1 mg Dexamethason zur Suppression, gefolgt von einem ACTH Test mit Cortisol Messung 30 und 60 min nach der Gabe erhoben. Die Gruppeneinteilung erfolgte in Bezug auf Adipositas, Körper -Fettverteilung und dem Vorhandensein chronischer Komplikationen.
Ergebnisse
Unabhängig von diabetischen Komplikationen und Adipositas waren die FCU und das Post-Dexamethason Cortisol bei den Diabetikern im Vergleich zu den adipösen Patienten (p < 0,01) und der Kontrollgruppe (p < 0,05) erhöht. Die Post-Dexamethason Cortisol Werte waren mit dem Hüftumfang korreliert. Die ACTH stimulierten Cortisol Werte waren bei allen Typ 2 Diabetikern significant höher. Es wurde eine unabhängige Beziehung zwischen der AUC der Cortisolreaktion im ACTH Test und der Insulinresistenz gefunden. Die multiple Regressionsanalyse zeigte, dass der Hüftumfang unabhängig mit dem Geschlecht, dem Nüchtern-Insulin, dem morgendlichem Cortisol und der AUC des Cortisols im ACTH Test assoziiert war (R 2 = 0.334,p < 0.0000).
Schlussfolgerungen
Die erhöhten FCU Werte, die verminderte Cortisol Suppression durch Dexamethason sowie die gesteigerte Reaktion der Cortisolspiegel auf ACTH Gabe belegen klar eine Hyperaktivität der HHNN-Achse bei Typ 2 Diabetikern. Eine abdominale Adipositas und chronische Komplikationen steigerten die Hyperaktivität der HHNN-Achse bei Typ 2 Diabetikern. Eine Steigerung des positiven Feedbacks ist mit Insulinresistenz und des negativen Feedbacks mit abdominaler Adipositas assoziiert.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a major public health problem. Its prevalence is increasing as the population ages and becomes more obese. Hypertension, hyperlipidemia, obesity, particular visceral obesity, and coronary heart disease are common in type 2 diabetic patients [1]. These are associated with insulin resistance in the metabolic syndrome, but the reason for the association between features remains obscure. Clinical—overt [2] and experimental [3]—cortisol excess is associated with increase in blood pressure and profound metabolic disturbances of intermediate metabolism resulting in characteristic obesity, insulin resistance, and hyperlipidemia, which can lead to diabetes. Therefore, it was suggested that subtle abnormalities in cortisol secretion and action is a missing link between insulin resistance and other features of the metabolic syndrome [4]. In addition, endocrine perturbations, such as cortisol excess, decreased growth hormone secretion, and decrease in male or increase in female free-androgen tissue availability, may have a key role in the development of enlarged abdominal, particularly visceral fat depot [4]. However, the relationship between cortisol secretion, altered cortisol metabolism, and/or increased cortisol tissue sensitivity in patients with type 2 diabetes still remains unclear.
Increased HPA axis activity in diabetic patients has been reported and is especially prevalent in those individuals with poor glycemic control or ketoacidosis [5]. Previously, many type 1 and type 2 diabetics exhibited nonsuppression of HPA axis activity following glucocorticoid administration which suggests that glucocorticoid negative feedback is also impaired in these patients [6,7]. Elevated ACTH levels [8] and salivary cortisol [9] with increased urinary free cortisol levels [10,11] have been described. The presence of chronic diabetic complications has also been associated with activation of the HPA axis [8,10,11]. The previous studies have been conducted in heterogeneous groups with type 1 and type 2 diabetes [6–8], while in other studies obesity was not considered [6–10]. In one population study, the association between degree of severity of several clinical measures of type 2 diabetes and cortisol secretion has been reported [12]. In young Chinese type 2 diabetic patients, the higher cortisol, reduced growth hormone, and hyperinsulinemia were closely associated to central obesity [13]. More recently, the association of the presence of developed diabetic complications as well as diabetes duration and cortisol secretion has been reported [14]. Abdominal obesity, type 2 diabetes and HPA axis activity are closely linked but the exact interactions still need to be clarified.
The aim of our study was to measure the HPA axis activity in the group of type 2 diabetic patients with different patterns of obesity, and in the two control groups of obese and nonobese healthy individuals to examine influence of the diabetic complications and abdominal obesity.
Subjects and methods
Subjects
We recruited 121 type 2 diabetic patients and 30 obese subjects from outpatient clinic of University Hospital Osijek and 19 healthy subjects with no family history of diabetes and obesity. Informed and written consent was obtained from all participants and the study was approved by Clinical Research Ethic Committee of our University. Exclusion criteria were: use of drugs known to affect steroid hormone secretion or metabolism, including oral contraceptive pills, for minimum 3 months; no glucocorticoid therapy in any route, clinical history of endocrine disease or any other intercurrent illness prior to investigation; clinical history of hospitalization due to acute diabetic complication or any hypoglycemia in patients diary in the last 3 months; body weight loss in last 3 months; any psychiatric disorder (questionnaire and interview by psychiatrist); abnormal renal function (creatinine clearance < 1.6 ml/s and 24-h proteinuria > 150 mg); abnormal hepatic and thyroid testing and excessive alcohol consumption (usage of more than 400 g ethanol per week). Women were examined in early folicular phase of menstrual cycle. Because exogenous insulin treatment might lead to a false high plasma levels and HOMA index could not be calculated, insulin-treated patients were not included in the study. The Croatian version of the questionnaire “Center for Epidemiological Studies Depression Scale” was employed to quantify depressive symptoms (all had score above 23) together with interview by psychiatrist to exclude any psychiatric disorder. All patients were in good physical health. Diabetic patients maintained their diabetic diet and treatment: 33 (27.3 %) patients were on diet alone, 88 (72.7 %) patients received oral hypoglycemic agents (60 glibenclamid (gliburide), 19 repaglinid, 19 metformin and/or 20 acarbosis). All patients had a complete physical exam including neurological and ocular assessments. Patients with proliferative or laser treated retinopathy and painful diabetic neuropathy were not included because of known association with HPA axis hyperreactivity [8]. Hypertension was defined as blood pressure > 140/90 mmHg, or receiving antihypertensive treatment. 48 diabetic patients (39.6 %) had hypertension and 37 received regular antihypertensive treatment (ACE inhibitor, and/or calcium channel blocker).
Obese subjects were physically fit, and had normal physical examination and electrocardiogram. Seven had hypertension (24.1 %) and only two (6.9 %) received antihypertensive treatment (calcium channel antagonist). Nineteen healthy controls were also studied. They were recruited among hospital personnel. None had family history of diabetes or obesity, had not been treated for psychiatric disorders or had a first or second degree relative who had undergone psychiatric treatment. Physical examination, electrocardiogram, and routine laboratory tests were normal. Alcohol consumption, physical activity, and smoking were estimated by standard questionnaire completed by personal interview. Smoking habits were recorded as the average number of cigarettes smoked per day. Smoking status was randomly distributed between groups.
Study protocol
Study was performed on three consecutive days. On day 1, at 0800 hours after an overnight fast, blood samples were drawn to determine routine laboratory tests including cholesterol, triglycerides, HDL-C, LDL-C, urea, creatinine, HbA1c, basal hormone profiles, including cortisol and ACTH. In addition, at 1600 hours samples were drawn to determine cortisol and ACTH. On that same day 24-h urine was collected to determine free cortisol excretion, creatinine clearance and 24-h proteinuria. On day 2, blood samples were drawn to determine glucose and insulin levels. A standard oral glucose tolerance test with 75 g of glucose was performed on nondiabetic subjects. On day 2 at 2300 hours, each subject received 1 mg dexamethasone orally. On day 3 at 0800 hours, an antecubital vein was cannulated and 30 min later ACTH was injected as an intravenous bolus at the dose of 250 µg. Blood samples for cortisol level analysis were collected at 0, 30, and 60 min after the administration of ACTH. After dexamethasone administration, a plasma cortisol level of 50 nmol/l or greater was considered a nonsuppressed response [15]. Seven patients in diabetic group and one subject in obese group had nonsuppressed response, and therefore a standard low dose dexamethasone suppression test (0.5 mg 6 hourly for 2 days) was performed. Postdexamethasone plasma cortisol was measured at 0800 hours, 6 h after the last dose of dexamethasone and all subjects had suppressed cortisol response. One obese subject with nonsuppressed response was excluded from study and remaining 29 were analyzed.
Methods
Measurements of plasma glucose, HbA1c, lipids, renal function, creatinine clearance, and 24-h urinary protein excretion were performed by routine assays using automatic analyzer Olympus AV 640. Commercial available RIA kits were used for the hormone analysis: plasma insulin (Sorin Biomedica, Perugia, Italy, the intra-assay variability 10.6 %, the interassay variability 7.0 %), plasma and urinary cortisol (CIS Bio International, Gif-Sur-Yvette, Cedex, France, the intraassay variability 2.6 %, the interassay variability 6.5 %), and ACTH (CIS Bio International, Gif-Sur-Yvette, Cedex, France, the intraassay variability 6.1 %, the interassay variability 5.3 %).
Definitions
Obesity was defined as a BMI > 30 kg/m2. Abdominal obesity was defined as a waist circumference > 102 cm for men and > 88 cm for women. Hypertension was defined as blood pressure > 140/90 mmHg, or receiving antihypertensive treatment. Beta-cell function and insulin sensitivity were assessed by the relationship between fasting glucose and insulin concentrations analyzed by homeostasis model assessment (HOMA). HOMA-IR index was calculated as a fasting insulin (mIU/l) and glucose (mmol/l) product divided by 22.5. HOMA-B index was calculated according the formula [16]:
In all diabetic patients, the presence of autonomic neuropathy was evaluated by performing deep-breathing, lying to standing and postural tests [17], and the presence of peripheral somatic neuropathy was evaluated by measuring the diabetic neuropathy score [18]. All diabetic patients were examined by ophthalmologist and background retinopathy was diagnosed by fundus ocular examination.
Statistical analysis
All data are expressed as mean ± SD. Mann-Whitney test or parametric t-test was used for between group comparisons. Analysis of variance (ANOVA) was used to determine the significance of difference between three or more groups. For data which was not normally distributed analysis of variance was performed with Kruskal Wallis ANOVA. Relationships between variables were analyzed using Spearman’s coefficients of correlations. Stepwise multiple regression analysis with forward selection was used to examine the independent relationships among variables. Results of regression analysis were given as beta coefficients and R2. All statistical analysis was performed using the program packages SAS 8.2 and StatSoft, Statistica 6.0.
Results
Clinical, biochemical, and hormonal characteristics from the diabetic, obese, and control group are summarized in the Table 1. Duration of diabetes was 7.1 ± 5.6 years, ranging from newly diagnosed (0 years) in five patients to 20 years in two patients. Groups were matched for age, smoking, and physical activity. Significantly, more diabetic patients had hypertension (48 or 39.6 %) compared with obese subjects (7 or 24.1 %) and none in control group, although systolic and diastolic blood pressure were similar in all three groups. As expected, diabetic patients were more obese with higher insulin resistance, fasting glucose, and triglyceride values than the control group. Obese subjects had normal response in OGTT, but significantly higher fasting plasma glucose, HbA1c, fasting insulin and higher HOMA-IR than controls. Diabetic patients had significantly lower afternoon ACTH values and significantly higher urinary free cortisol, postdexamethasone cortisol and cortisol following ACTH, both at 30 and 60 min, and increased AUC of cortisol response to ACTH.
Stratification in relation to obesity and body fat distribution
Table 2 summarizes clinical, biochemical, and hormonal characteristics of the subjects after stratification in subgroups according to obesity and obesity type. Subgroups were matched for age, smoking, and physical activity. Diabetic patients with abdominal obesity (AO) had higher triglyceride and higher fasting insulin values, increased HOMA-IR, and decreased HOMA-B index compared to any other subgroup. HOMA-IR was the highest in the diabetic group with abdominal obesity but even normal weight diabetic patients (NW) had significantly higher HOMA-IR index than subjects with abdominal obesity (p < 0.000) and control group (p < 0.000). Urinary free cortisol was elevated in all three diabetic subgroups, significantly in subgroups with abdominal obesity and normal weight compared to nondiabetics with abdominal obesity (p < 0.05) and control subjects (p < 0.05). Dexamethasone suppression was blunted in subgroups with abdominal obesity and normal weight diabetic patients compared to peripheral obese subgroup (p < 0.05,p < 0.005) and control group (p < 0.001). Dexamethasone suppression was also blunted in nondiabetic abdominal obese subjects but not significantly. Cortisol response to ACTH (in 30 min) as well as AUC of cortisol response to ACTH was significantly elevated in all three diabetic subgroups as compared to both obese groups and control group.
Stratification in relation to chronic diabetic complications
Table 3 shows clinical, biochemical, and hormonal characteristics of the subjects after stratification in subgroups according to presence or absence of chronic complications. In the group with chronic complications 10 patients had diabetic retinopathy, 24 had diabetic neuropathy and 31 had both retinopathy and neuropathy. Diabetic patients with chronic complications were significantly older (p < 0.05) and duration of diabetes was significantly longer (p < 0.001) than in diabetics without complications. Triglycerides were significantly higher in both diabetic subgroups (p < 0.05), while HDL cholesterol was significantly lower only in diabetics with complications (p < 0.05, data not shown) than in control group. Fasting plasma glucose, HBA1c concentrations and HOMA-IR were significantly higher in both diabetic subgroups than in non diabetic groups. Only in subgroup with both diabetic neuropathy and retinopathy fasting glucose, HbA1c levels and HOMA-IR were significantly higher than in diabetics without complications and both non diabetic groups (p < 0.001 for each, data not shown). Morning ACTH values were increased in diabetics with complications compared to diabetics without complications (p < 0.001). Diabetic patients with neuropathy had higher morning ACTH levels (p < 0.05) and those with retinopathy had higher afternoon ACTH levels (p < 0.05) than diabetics without complications (data not shown). Urinary free cortisol was elevated in both diabetic groups, while postdexamethasone cortisol was higher in diabetic patients without complications than in both nondiabetic groups. Cortisol values in response to ACTH (at 30 and 60 min) and AUC of cortisol response to ACTH in both diabetic subgroups were significantly higher than in both nondiabetic groups.
Relationships between variables
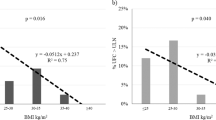
Postdexamethasone cortisol was positively correlated only with waist circumference (r = 0.244,p < 0.01) and WHR (r = 0.232,p < 0.01). AUC of cortisol response to ACTH was positively correlated with age (r = 0.272,p < 0.01), HbA1c (r = 0.339,p < 0.001), fasting glucose (r = 0.405,p < 0.01), duration of diabetes (r = 0.365,p < 0.001), neuropathy (r = 0.216,p < 0.01), retinopathy (r = 0.178,p < 0.05) and with HOMA-IR (r = 0.406,p < 0.001). No correlations were found between AUC of cortisol response to ACTH and BMI, waist circumference, and sex. In the first model of stepwise multiple regression analysis with forward selection, dependent variable was the AUC of cortisol response to ACTH and independent variables were HOMA-IR and BMI. AUC of cortisol response to ACTH was significantly and independently associated with HOMA-IR (R 2 = 0.1127,p < 0.0000). In the second model of multiple regression analysis in all study subjects, the dependent variables were BMI and waist circumference. The most important independent determinants of variability of waist circumference were sex, fasting insulin, morning cortisol, and AUC of cortisol response to ACTH (R 2 = 0.334,p < 0.0000). The only independent determinant variability of BMI was fasting insulin (R 2 = 0.189,p < 0.0000). Table 4 shows results of the second model of multivariate regression analysis in three examined groups separately. In obese subjects and diabetic patients, the sex, fasting insulin, and morning cortisol were independently associated with waist circumference.
Discussion
The present study strongly suggests hyperactivity of the HPA axis in the type 2 diabetic patients as evident in increased urinary free cortisol levels, blunted negative and increased positive feedback, even in type 2 diabetic patients without chronic complications and in normal weight diabetic patients. Moreover, augmentation of positive feedback of the HPA axis is associated with insulin resistance. In this study, type 2 diabetic patients had similar degree of obesity, waist circumference, and fasting insulin levels as obese subjects, but significantly increased insulin resistance and decreased beta-cell function compared with obese subjects. These features are the very same as those of the metabolic syndrome associated with type 2 diabetes [2]. Our type 2 diabetic patients and nondiabetic subjects had plasma cortisol and ACTH concentrations within the acceptable normal range. Morning ACTH levels were slightly but significantly higher in the patients with diabetic neuropathy, and afternoon levels were slightly higher in the subgroup with retinopathy, while diabetic patients without complications had the lowest ACTH levels. Increased cortisol production demonstrated by higher urinary free cortisol levels together with slightly higher ACTH levels may suggest central dysregulation of the HPA axis.
All our type 2 diabetic patients had increased activity of the HPA axis regardless of chronic complications and obesity, but the presence of chronic complications exaggerated the HPA axis hyperactivity. In some previous studies, the HPA axis hyperactivity was reported even in uncomplicated diabetic patients and alterations were more pronounced in the presence of neuropathy [19]. In some other studies, the HPA axis hyperactivity was reported in neuropathy [8], retinopathy and macrovascular disease [10,11]. Chiodini et al. [14] have found that cortisol secretion correlated with the presence and number of chronic diabetic complications; however, contrary to our results, in uncomplicated type 2 diabetic patients the activity of the HPA axis was normal. There are several possible explanations for observed differences. Our subjects were 5–10 years younger and had much better metabolic control compared with diabetic subjects in this study (HbA1c in subgroup without diabetic complications was 7.3 vs. 9.3 %). Furthermore, in our study, insulin therapy was exclusion criteria, because HOMA index could not be calculated, and in Chiodini study 11 % of patients without complications and 22 % of patients with chronic complications received insulin therapy, which might interfere with the results. It is well established that insulin crosses blood-brain barrier [20]. The hippocampus is one of the brain regions with the highest density of insulin receptors [21] and a mediator of negative feedback effects of corticosteroids on HPA activity [22]. During high-insulin clamp study, cortisol and ACTH levels were elevated suggesting that insulin acutely stimulates the HPA secretory capacity [23]. In animal studies, hyperactivation of the HPA axis in streptozotocin-diabetes was associated with decreased pituitary and adrenal sensitivity, and insulin treatment partially restored the pituitary-adrenal response [24]. In addition, in rats, the diabetes impaired HPA response to hypoglycemia and insulin treatment normalized HPA but not deficient epinephrine response [25]. It is possible that hyperinsulinemia which is commonly observed in insulin resistance may be partly responsible for increased HPA axis activity and insulin therapy might interfere with the results.
The rate of cortisol secretion is controlled by central drive to the HPA axis and by negative feedback suppression by glucocorticoids. In our diabetic patients, the postdexamethasone cortisol levels were higher in all subgroups, regardless of chronic complications, obesity, or obesity type. Previously, some diabetic patients reported to have nonsuppressed response to overnight dexamethasone suppression [6,7]. In both types of diabetes, Hudson et al. [7] did not show any difference in postdexamethasone plasma cortisol values in the morning but significant elevation in the afternoon which was associated with HbA1c concentrations. In our study, 7 diabetic patients had nonsuppressed response to dexamethasone or 5.7 %, similar to Kaye et al. [26] who did not find association between postdexamethasone cortisol levels and Hba1c. In our subjects, the postdexamethasone plasma cortisol concentrations were significantly correlated only with waist circumference.
In our diabetic patients, the plasma cortisol levels after stimulation with ACTH were significantly higher regardless of the presence of chronic complications. This intense response to ACTH drive was mostly pronounced in patients with abdominal obesity. Furthermore, the exaggerated response to ACTH was independently associated with insulin resistance. In young Chinese type 2 diabetic patients, the higher plasma cortisol and reduced growth hormone level was associated with abdominal obesity [13]. In one population study in type 2 diabetic patients, the cortisol was related to fasting glucose, postprandial glucose, HbA1c, blood pressure as well as to relative abdominal mass [12]. Fraser et al. [27] demonstrated that in general population cortisol excretion rate did not correlate with blood pressure but correlated strongly with BMI, WHR, and negatively with HDL cholesterol [27]. In The Edinburgh Type 2 Diabetes Study in type 2 diabetic patients, elevated fasting cortisol was associated with ischemic heart disease and its risk factors [28]. We can speculate that hyperinsulinemia and insulin resistance commonly observed in abdominal obesity can increase the HPA axis activity, but when overt type 2 diabetes develops, even without complications, the HPA axis is clearly hyperactive. Enhanced cortisol secretion has deleterious effect on glucose metabolism and might worsen metabolic control of diabetes leading to chronic complications. Another hypothesis postulated by Convit [29], Bruehl et al. [30] proposed a model suggesting that early on in the course of type 2 diabetes, the hippocampus is damaged and this may be in part a result of the endothelial dysfunction that accompanies insulin resistance. Primary damage of the hippocampus leads to a disruption of the HPA axis, which in turn results in elevated cortisol levels, creating a vicious cycle. Bruehl et al. [30] recently showed that the HPA axis hyperreactivity in type 2 diabetes is associated with HbA1c and with deficits in declarative memory. Furthermore, in elderly type 2 diabetic patients, the diminished cortisol suppression after dexamethasone and dyslipidemia were associated with decreased cognitive function whereas obesity was negatively related to hippocampal volume [31]. Obesity, altered HPA axis activity, and metabolic control of diabetes have important impact on chronic diabetic complications and on hippocampal structure and function.
This article provides additional evidence about hyperactive HPA axis in type 2 diabetes mellitus. We confirmed that even uncomplicated type 2 diabetes mellitus activated the HPA axis, and the presence of chronic complications and abdominal obesity further increased the HPA hyperactivity. Blunted negative feedback of the HPA axis correlated with waist circumference and augmentation of positive feedback is associated with insulin resistance. Further investigations, both clinical and experimental, are required to clarify the possible role of cortisol secretion in pathogenesis of type 2 diabetes mellitus and development of chronic complications of diabetes.
References
DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94.
Boscaro M, Barzon I, Fallo F, Sonino N. Cushing’s syndrome. Lancet. 2001;357:783–91.
Connell JMC, Whitworth JA, Davies DL, Lever AF, Richards AM, Fraser R. Effects of ACTH and cortisol administrations on blood pressure, electrolyte metabolism, atrial natriuretic peptide and renal function in normal man. J Hypertens. 1986;5:425–33.
Bjorntorp P, Holm G, Rosmond R. Hypothalamic arousal, insulin resistance and type 2 diabetes mellitus. Diabetic Med. 1999;16:373–81.
Hüther KJ, Scholz HR. The plasma concentrations and rate of plasma clearance and plasma production of cortisol and corticosterone in healthy persons and in subjects with asymptomatic and clinical diabetes mellitus. Horm Metab Res. 1969;1:253.
Cameron OG, Kronfol Z, Greden JF, Carroll BJ. Hypothalamic-pituitary-adrenocortical activity in patients with diabetes mellitus. Arch Gen Psychiatry. 1984;41:1090–5.
Hudson JI, Hudson MS, Rotschils AJ, Vignati L, Schatzberg AF, Melby JC. Abnormal results of dexamethasone suppression tests in nondepressed patients with diabetes mellitus. Arch Gen Psychiatry. 1984;41:1086–9.
Tsigos C, Young RJ, White A. Diabetic neuropathy is associated with increased activity of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 1993;76:554–8.
Liu H, Bravata DM, Cabaccan J, Raffa H, Ryzen E. Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin Endocrinol (Oxf). 2005;63:642–9.
Roy MS, Roy A, Brown S. Increased urinary free cortisol outputs in diabetic patients. J Diabetes Complicat. 1998;12:24–7.
Dacou-Voutetakis C, Peppa-Patrikiou M, Dracopoulou M. Urinary free cortisol and its nyctohemeral variations in adolescent and young adults with IDDM: relation to endothelin 1 and indices of diabetic angiopathy. J Pediatr Endocrinol. 1998;11:437–45.
Oltmanns KM, Dodt B, Raspe HH, Schweiger U, Born J, Fehm H, et al. Cortisol correlates with metabolic disturbances in a population study of type 2 diabetic patients. Eur J Endocrinol. 2006;154:325–31.
Lee ZSK, Chan JCN, Yeung VTF, Chow CC, Lau MSW, Ko GTC, et al. Plasma insulin, growth hormone, cortisol, and central obesity among young Chinese type 2 diabetic patients. Diabetes Care. 1999;22:1450–7.
Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, et al. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care. 2007;30:83–8.
Putigano P, Duini A, Toja P, Inviti C, Bonfanti S, Raedelli G, et al. Salivatory cortisol measurement in normal-weight, obese and anorectic women: comparison with plasma cortisol. Eur J Endocrinol. 2001;145:165–71.
Haffner SM, Gonzales C, Mietinen H, Kennedy E, Stern MP. A prospective analysis of the HOMA model. The Mexico city diabetes study. Diabetes Care. 1996;19:1138–41.
Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J. 1985;285:916–8.
Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Green DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–9.
Chiodera P. Low-dose ovine corticotropin-releasing hormone stimulation test in diabetes mellitus with or without neuropathy. Metabolism. 1995;44:538–42.
Akama SF, Cascio CS, Du JZ, Levin N, Dallman MF. Reset of feedback in the adrenocortical system: an apparent shift in sensitivity of adrenocorticotropin to inhibition by corticosterone between morning and evening. Endocrinology. 1986;119:2325–32.
Pavlovcik RA, Phillips MI, Kappy MS, Raiyada MK. Insulin inhibits pyramidal neurons in hypocampal slices. Brain Res. 1984;309:187–91.
Jacobsen L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–34.
Fruehwald-Schuletes B, Kern W, Bong W, Wellhoener P, Kerner W, Born J, et al. Supraphysiological hyperinsulinemia acutely increases hypothalamic-pituitary-adrenal secretory activity in humans. J Clin Endocrinol Metab. 1999;84:3041–6.
Chan O, Inouye K, Vranic M, Matthew SG. Hyperactivation of the hypothalamo-pituitary-adrenocortical axis in streptozotocin-diabetes is associated with reduced stress responsiveness and decreased pituitary and adrenal sensitivity. Endocrinology. 2002;143:1761–8.
Chan O, Chan S, Inouye K, Shum K, Matthew SG, Vranic M. Diabetes impairs hypothalamo-pituitary-adrenal (HPA) response to hypoglycemia, and insulin treatment normalizes HPA but not epinephrine response. Diabetes. 2002;51:1681–9.
Kaye TB, Rubin RA, Goldfine AB, Rajamani K, Kinsley BT, Vischer UM, et al. Effect of glycemic control on the overnight dexamethasone suppression test in patients with diabetes mellitus. J Clin Endocrinol Metab. 1992;74:640–4.
Fraser R, Ingram MC, Anderson NH, Morrison C, Davies E, Connell JMC. Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension. 1999;33:1364–8.
Reynolds RM, Labad J, Strachan MW, Braun A, Fowkes FG, Lee AJ, et al. Elevated fasting plasma cortisol is associated with ischemic heart disease and its risk factors in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. J Clin Endocrinol Metab. 2010;95:1602–8.
Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol Aging. 2005;26(Suppl 1):31–5.
Bruehl H, Rueger M, Dziobek I, Sweat V, Tirsi A, Javier E, et al. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J Clin Endocrinol Metab. 2007;92:2439–45.
Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009;1280:186–94.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prpić-Križevac, I., Canecki-Varžić, S. & Bilić-Ćurčić, I. Hyperactivity of the hypothalamic-pituitary-adrenal axis in patients with type 2 diabetes and relations with insulin resistance and chronic complications. Wien Klin Wochenschr 124, 403–411 (2012). https://doi.org/10.1007/s00508-012-0191-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-012-0191-4
Schlüsselwörter
- Cortisol Sekretion
- Diabetes mellitus Typ 2
- Insulinresistenz
- Abdominale Adipostas
- chronische Komplikationen