Abstract
This study, conducted on Acacia species (Acacia ehrenbergiana Hayne and Acacia tortilis subsp. raddiana (Savi) Brenan) to determine their adaptive capacity to tolerate drought and suitability for reforestation, revealed that leaf water potential (ψ) decreased in both the species with increase in drought intensity. With increase in the intensity of drought, vessel diameter increased in A. ehrenbergiana, causing a significant decline in vessel frequency mm−2 of the transverse wood surface, while it decreased in A. tortilis, leading to a crowded vessel population. Vessel-wall thickness, in conjunction with inter-vessel pit membrane thickness, showed a positive correlation with drought stress in both the species. Ray dimensions generally decreased in A. ehrenbergiana but increased in A. tortilis under increasing degree of drought. The transverse fiber-wall area decreased in A. ehrenbergiana, thus lowering the density (r = 0.996) and enhancing the vulnerability of wood (r = −0.979) under the drought stress, but increased in A. tortilis, causing a high density (r = −0.979) and low vulnerability of wood (r = 0.869), under the same set of conditions. Correlation of wood density with vulnerability index was stronger in A. ehrenbergiana (r = −0.993) than in A. tortilis (r = −0.753). Diameters and thickness of inter-vessel pit membrane were linearly correlated with increasing intensity of drought in both the species, but its area fraction per vessel segment increased due to water stress in A. ehrenbergiana and decreased in A. tortilis. This study indicated that, on the whole, A. tortilis has a greater capacity to tolerate the harshness of drought than A. ehrenbergiana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At least one-third of the Earth’s land area is covered by water-limited ecosystems and this is likely to increase because of various anthropogenic activities, leading to desertification and climate change (MEA 2005). Soil water availability is a major limiting factor for the phenomena concerning tree growth, species distribution, ecosystem functioning, and the long-term water, carbon and nutrient balance in the arid and semi-arid lands (Reynolds et al. 2004; Otieno et al. 2006).
Water-deficit stress, a situation in which plant water potential and turgor are reduced enough to interfere with normal functions, may occur due to lack of available soil moisture, a very slow absorption or a rapid loss of water, or most often by a combination of all the three (Kramer and Kozlowski 1979). It is determined commonly in terms of leaf water potential, relative water content and gas exchange (Chaves et al. 2003), and its intensity depends on the relative rates of water absorption and water deficit.
In vascular plants, transport efficiency of xylem, which depends largely on vessel width and frequency, vessel-wall properties and inter-vessel pit membrane characteristics, may be reduced not only by vessel collapse, but sometimes even by vessel blockage due to tylosis formation and phenolic deposits within the vessel (Kitin et al. 2010). Xylem conduits have hydraulic limits for the maximum diameters, as they have to withstand strong negative pressures due to transpiration, which could cause cell collapse or embolism within weak vessels (Choat et al. 2008; Kitin et al. 2010). Narrow conduits are known to decrease transport efficiency, but provide a greater hydraulic safety (Corcuera et al. 2004; Mauseth and Stevenson 2004). Pits on vessels or tracheids have a vital role in regulating water transport in trees. Pit membranes are considered responsible for at least 50 % of the hydraulic resistance in the xylem (Wheeler et al. 2005; Hacke et al. 2006). While allowing the inter-vessel passage to water, pit membranes also protect xylem against embolism (Choat et al. 2008). Vessel size and inter-vessel pit structure are thus, important in determining the mean cavitation pressure (MCP) as well as the hydraulic conductivity per xylem cross-sectional area (K xa ), and have a role in safety-efficiency conflict in water-conducting system (Lens et al. 2011). Pit structure is more strongly linked to MCP than pit quantity. Changes in the thickness and porosity of pit membranes influence the total hydraulic resistance in the plant system, thus, affecting xylem vulnerability to water-stress-induced embolism. Membranes with smaller pores are more effective in limiting the embolism spread.
Xylem has to withstand the mechanical stress associated with negative pressure, under which the water moves. Failing this can cause cavitation of water column through air seeding, i.e., by pulling the gas through pit membrane pores from gas-filled cells or intercellular spaces into water-filled xylem conduits (Baas et al. 2004), or due to negative pressures over the walls of the xylem conduit to resist implosion (Cochard et al. 2004; Brodribb and Holbrook 2005). Embolism or gas blockage increases hydraulic resistance, thus reducing water transport, and can result in a reduced stomatal conductance and photosynthesis (Brodribb and Field 2000), and in the dieback of branchlets or the whole plant (Davis et al. 2002). Species with more porous membranes or with larger inter-vessel pit membrane area are more prone to vascular dysfunction under water stress. After facing a cavitation event, a tracheary element may exhibit ‘cavitation fatigue’, i.e., greater susceptibility to further cavitation due to pit membrane damage or tearing (Hacke et al. 2001b).
Water-saving species are known to have rigid cell walls, low osmotic potential, narrow vessels and a reduced transpiration rate to resist embolism in severe drought conditions (Kalapos 1994). Vessel diameter and vessel frequency are partly heritable and partly plastic traits and show a wide inter-specific variation. In short, xylem anatomy largely determines the water-transport efficiency of a species and correlates to its drought tolerance capacity (Tyree et al. 1994; Lens et al. 2011).
Species of Acacia, widely distributed in the arid and semi-arid regions of the world, exhibit unique drought resistance and soil-fertilizing abilities (Oba et al. 2001; Aref et al. 2003). Their typically low soil–water uptake in the arid ecosystem is fundamental to their characteristic adaptations that enable them to grow successfully under limited soil–water availability. The present study, based on the seedling anatomy of Acacia ehernbergiana Hayne and Acacia tortilis subsp. raddiana (Savi) Brenan, was undertaken: (1) to identify the anatomical changes that appear in these species under drought condition and affect their tolerance to water stress, and (2) to compare their adaptive capacity to drought conditions, thus assessing their suitability for reforestation purposes. This may help in planning proper utilization of promising species for afforestation and reforestation purposes at regional and national scales.
Materials and methods
Seedlings production
In order to avoid any inter-tree variation, seeds of A. ehrenbergiana and A. tortilis subsp. raddiana were collected from a single tree of each species maintained at the Agricultural Research Station, College of Food and Agricultural Sciences, King Saud University in Riyadh region (N 42°24 E 46°44, Alt. 600 m a.s.l.). These were sown in plastic pots (32 cm × 40 cm) containing a mixture of clay and sand soil (1:2 v/v). Seedlings were grown in a glass house under controlled condition (with 32º C/17 °C +1 °C average day/night temperature, 12-h daylight, and 50 % relative humidity). To maintain the optimum soil-moisture condition, plants were watered once a day from April to June, and later as per the requirement of the experimental design.
Water-deficit treatments
A 2 × 3 factorial experiment was designed to treat plants with three watering regimes, i.e., 100 % of field capacity (control), 50 % FC (moderate deficit irrigation MDI) and 25 % FC (severe deficit irrigation SDI). A bunch of 11 seedlings per species was exposed to each of these irrigation patterns, i.e., 33 seedlings of each species were used in total. To determine the impact of water stress on seedling wood anatomy, sampling was done 3 months after seedling transplantation. Three replicates from each treatment were selected for the anatomical study of each species, and the remaining seedlings were kept for other observations.
Plant water status
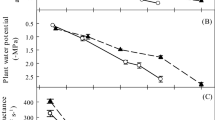
Relative water content (RWC) was tested every 10 days after the onset of water stress, using three leaves (3rd, 4th and 5th leaf) each, from three replicates per treatment. The leaves were weighed for their fresh weight, then made fully turgid by dipping their petioles in water in a beaker kept in the dark overnight, and re-weighed to obtain their turgid weight. After drying at 80 ± 1 °C for 24 h the leaves were re-weighed for their dry weight. RWC was calculated using the following formula of Morgan (1984): RWC = [(Mf−M d)(Mt−M d)−1] × 100, where Mf is the leaf fresh weight; Mt, turgid weight and Md, dry weight. Besides, relative leaf water potential of both the species was measured from ten leaves per replicate per treatment, using a Potential Meter (WP4-T, Decagon Devices, Inc., USA) at pre-dawn every tenth day.
Anatomical studies
For anatomical studies, stem pieces were collected from the third internode for each replicate of each treatment in early hours of the day and fixed on the spot in formalin–acetic acid–alcohol (FAA). After a week, the fixed stem pieces were preserved in an alcohol–glycerol solution (50 % Ethanol + 50 % Glycerol, V:V) for softening.
Sectioning, staining and maceration
Permanent mounts of fine (10-μm thick) transverse, tangential and radial sections of stem pieces obtained on a sliding microtome (AO 860, USA) were prepared, after staining with combinations of haematoxylin and safranin/Bismarck brown (Johansen 1940) or ferric chloride and lacmoid (Cheadle et al. 1953) and dehydrating in ethanol series. These sections were studied for vessel segment dimensions, vessel-wall thickness, vessel density, and ray dimensions. Recommendations of IAWA Committee (1989) were followed for determining and describing the quantitative features of the wood. Two hundred vessel elements and wood rays were measured for each replicate from each treatment, using a micrometer scale on Olympus CX41 (Japan) microscope.
For fiber measurements, wood samples were macerated following the method of Franklin (1945). Matchstick-sized specimens were heated in a test tube containing equal amounts of glacial acetic acid and hydrogen peroxide (35 %), at 70 °C in a water-filled beaker until the specimens became white. The treated specimens were washed thoroughly with tap water and the fibers separated by gentle shaking. Two hundred fibers were measured for each replicate of each treatment.
To analyze the relative proportions of wood components, the transverse and tangential longitudinal wood sections were exposed to digital camera (Olympus DP 72) attached to an Olympus BX 51 microscope and PC. Ten prints for each replicate and treatment were obtained for analyzing the area occupied by vessel lumen and fiber wall in the transverse view and by wood rays in the tangential view. For obtaining area measurements, Scanner Epson Expression 1680 (Scan 300 dpi, Black & White, 130 threshold) and the software Area Scan Ver. 1.0 (by M B Abdulfatah, © 2000) with Image resolution of 300 dpi loaded in PC, were used.
Diameter and area of pit membrane
Inter-vessel pit membrane diameter was measured from 20 randomly selected vessel elements in the tangential and radial views of each replicate of the control, MDI and SDI samples. Twenty-five pit membranes per vessel element were measured at 10 × 100 (in oil immersion) of an Olympus BX 51 microscope attached with 29 inch LCD TV (with pre calibrated micrometer scale as 1 cm = 1.212 μm) to get a markedly enlarged view of horizontal and vertical diameters and cross-sectional thickness of pit membranes. To analyze the pit membrane area on the vessel element walls, tangential and radial sections of each replicate of the control and the stressed plants were exposed to digital camera (Olympus DP 72) attached to the Olympus BX 51 microscope and PC. Ten prints for each replicate were obtained for area analysis. Scanner Epson Expression 1680 (Scan 300 dpi, Black & White, 130 threshold) and software Area Scan Ver. 1.0 (by MB Abdulfatah © 2000), with Image resolution of 300 dpi loaded in PC, were used to analyze the area occupied by the pit membrane on the tangential and radial walls of the vessel elements.
Wood density
Wood density was analyzed by the method of Kocacinar and Sage (2004). Stem segments (4–5 cm long) were taken separately from the 3rd to 6th basal internodes of the seedlings from each replicate and preserved in FAA. These were washed with tap water and cut longitudinally in the middle. The pith as well as the bark and cambium were removed from each sample. Fresh volume of wood was then estimated by immersing the sample in a narrow, graduated cylinder filled with water. The replaced water was carefully removed from the cylinder with a pipette and weighed. Displacement weight (in g) was converted to sample volume by dividing it by the density of water at 20° C (0.998 g cm−3). Dry weight of the tissue was then determined after drying the material at 70° C for 48 h. Wood density (g cm−3) was calculated by dividing the dry weight by the fresh volume of the wood block.
Vulnerability factor
Data were collected on vessel diameter and vessel frequency mm−2 of the wood to determine vulnerability factor (VF) of xylem by dividing the mean vessel diameter by the number of vessels mm−2, as described by Carlquist (1977).
Histochemistry
Histochemical analysis of thin TS, TLS and RLS of freshly collected stem pieces of both the species was done following the vanillin-hydrochloric acid method of Gardner (1975), to identify the compound accumulated in the axial parenchyma, ray parenchyma and vessel lumen, and on the pits of the vessel walls.
Statistical analysis
The experimental layout was completely randomized with two factors (species and watering regimes). The data were analyzed by variance analysis (ANOVA) and the means separated by LSD (generally at p < 0.05), using the SAS statistical package (SAS 2004).
Results
Relative water content and water potential
RWC and water potential showed a significant decrease with increase in drought intensity in both the species studied. In A. ehrenbergiana, RWC ranged from a maximum of 89.5 % in the control (100 % FC) to the minimum (77.4 %) under severe deficit irrigation (25 % FC). Water potential was −1.7 MPa and −2.4 MPa in samples from plants grown under MDI (50 % FC) and SDI (25 % FC), respectively, as compared with −1.0 MPa in the control (100 % FC), thus exhibiting a decline of 70 and 140 % under drought conditions. RWC analyzed for A. tortilis varied from 91.2 % in the control to 72.2 % under SDI. Leaf water potential was measured as −1.1 MPa in the control, −1.8 MPa under MDI and −2.6 MPa under SDI. It, thus, decreased by 63.6 and 136.3 % under the deficit-irrigation regimes.
Drought effects on wood anatomy
Acacia ehrenbergiana
Microscopic analysis of the wood of A. ehrenbergiana seedlings revealed that vessel element length was significantly less, up to 11.72 %, under severe deficit irrigation in comparison with the control. However, the mean vessel diameter was markedly increased under both MDI and SDI, showing a significant negative correlation with leaf water potential (Tables 1, 2). The vessels developed thicker walls under drought stress: the total wall thickness between two neighboring vessels was 7.68 μm under MDI and 7.74 μm under SDI, while it was only 5.04 μm in the controls (Table 1). A negative significant correlation (r = −8755) was observed for the inter-treatment variation in inter-vessel-wall thickness and water potential (Table 2). The vessel-wall thickness to vessel-lumen diameter ratio (VWT/VLD) was higher under drought stress (0.34 and 0.35 for MDI and SDI, respectively) in comparison with the control (0.23). Drought also had a profound effect on vessel density (vessel number per mm2 of wood), which was significantly less under MDI (198.59 mm−2) and SDI (178.20 mm−2) than in the control (247.71 mm−2). The reduction in vessel density was nearly 20 and 28 % under MDI and SDI, respectively (Table 1; Fig. 1a, b).
Transverse view of the stem wood of Acacia ehrenbergiana and Acacia tortilis subsp. raddiana seedlings: sections a, b exhibit density as well as distribution pattern of vessels in A ehrenbergiana, while sections c, d depict the same features in A. tortilis subsp raddiana, under control (100 % FC) and drought (25 % FC) conditions. Vessels are more crowded and form bigger clusters in the control (a) than in the drought-affected (b) plants of A. ehrenbergiana, but these are scattered and less frequent in the control (c) and more abundant, mostly in radially elongate clusters, often surrounded by tannin depositions in the drought-affected (d) plants of A. tortilis subsp. raddiana. Scale bar on A = 125 μm and applies to all the four views
The height and width of rays, as seen in TLS, decreased with the increasing intensity of drought. The rays were tall and wide in the control population, whereas short and narrow in plants grown under SDI. Fibers were significantly shorter under both MDI and SDI in comparison with the control (Table 1). Area fraction of wood rays (in tangential view), and of the vessel lumen and fiber wall (in transectional view), decreased with the increase in drought intensity. The ray area was dropped by 13 and 31 % under MDI and SDI, respectively. Similarly, area fraction of vessel lumen was reduced by about 13 and 22 %, while that of the transverse fibers wall by 5 and 12 % under MDI and SDI, respectively (Fig. 2), showing a positive correlation with water potential (Table 2). Also, it had a positive correlation with vessel-lumen area and a negative one with xylem vulnerability (Table 3).
Effect of the moderate and severe deficit irrigations (50 and 25 % FC, respectively) on the relative proportion of wood rays, vessels and fibers, as measured in % per mm−2 of the transverse surface area of wood of the a Acacia ehrenbergiana and b A. tortilis subsp. raddiana seedlings, in comparison with the control (100 % FC)
Wood density was inversely proportional to the drought intensity, being significantly low under deficit irrigation. It fell by nearly 6 and 11 % under MDI and SDI, respectively, as compared with the control (r = 0.9962). It had a positive correlation with fiber area and a negative one with vulnerability factor (Table 3). Wood as a means of hydraulic conductivity showed increased vulnerability to embolism under severe drought. A significantly higher vulnerability factor (r = −0.9797) was obtained for drought treatments (Tables 1, 2).
Acacia tortilis subsp. raddiana
In the A. tortilis seedlings, vessel element length as well as vessel diameter declined with increase in the intensity of water deficit. The radial, tangential and mean diameters reduced significantly under both the deficit-irrigation regimes (Table 1; Fig. 1c, d). A positive correlation was obtained for inter-treatment variation in vessel diameter and water potential (Table 2). The total wall thickness between two neighboring vessels was quite high under MDI (8.54 μm) and SDI (8.60 μm), as compared with the control (5.76 μm), and correlated negatively to water potential. The vessel-wall thickness to vessel-lumen diameter ratio was enhanced under water stress, being 0.45 and 0.46 for MDI and SDI, respectively, in comparison with 0.26 for the control. Changes in vessel density were prominent; the density being 142 and 165 % higher under MDI and SDI, respectively, than in the control. It showed a negative and highly significant correlation with water potential (Table 2).
Ray height increased with the intensity of drought. The rays were significantly tall and broad under MDI, whereas tall and narrow under SDI. Fiber length was adversely affected, being significantly shorter under MDI (579 μm) and SDI (503 μm) than in the control (599 μm) (Table 1).
The area fraction of wood rays showed a significant decline under SDI. On the contrary, vessel-lumen-area fraction increased over the control by 79 and 90 % under MDI and SDI, respectively. Transverse fiber-wall area also increased up to 10 and 16 % under MDI and SDI, respectively (Fig. 2) and showed a highly significant negative correlation with water potential (Table 2). Fiber area had a positive correlation with wood density (r = 0.9418) and a negative one with vulnerability factor (Table 3).
Wood density was positively correlated to water stress (r = −0.9798), showing a significant increase of nearly 3 and 12 % under MDI and SDI, respectively. The higher wood density curtailed vulnerability to embolism (r = −0.7530); vulnerability factor was significantly reduced by deficit irrigation (Table 1), showing a positive correlation with leaf water potential (Table 2).
Inter-vessel pit membrane
The effect of deficit irrigation on inter-vessel pit membrane diameter was significant in both the species (Table 4). The diameter increased in line with the intensity of drought, the horizontal diameter being more sensitive than the vertical one. Inter-treatment variation in the mean diameter was more closely related to leaf water potential (r = −0.9398) in A. ehrenbergiana than in A. tortilis (Table 2). Inter-vessel pit membrane thickness also showed an increase with growing intensity of water deficit. It ranged between 0.606 and 1.060 μm in A. ehrenbergiana and 0.424–0.848 μm in A. tortilis, thus the former having a thicker pit membrane than the latter (Table 4). It showed a negative and significant correlation with water potential in both the species (Table 2). Inter-vessel pit membrane thickness was strongly correlated to the total inter-vessel-wall thickness in both the species (Table 3). Area fraction of pit membrane per vessel segment was higher under deficit irrigation than in the control of A. ehrenbergiana (r = −0.8852), while it was significantly lower in A. tortilis (r = 0.6427) possibly owing to the small size of vessel elements. Pit membrane area fraction increased from 39 % (in the control) to nearly 44 % (under SDI) in A. ehrenbergiana, while it decreased from about 45 % (control) to 38 % (MDI) in A. tortilis (Fig. 3).
Deposition on vessel pits
In both the species studied, wood cells in the control plants (at 100 % FC) were free from any specific depositions. However, under MDI and SDI conditions, a chemical substance accumulated in ray parenchyma, in axial parenchyma surrounding vessels or vessel groups, in the vessel lumen and on the pits of the vessel walls. The substance deposited was identified as tannin. The pattern of accumulation was similar in both the species, as evident from transitional stages of deposition. Synthesis/accumulation of tannin started in living parenchyma cells (ray parenchyma and the axial parenchyma surrounding vessels or vessel groups) in the xylem (Fig. 4a, d). It then deposited on the lateral walls, especially on the pits of the vessels (Figs. 4a, c, 5a, b), blocking completely the pit aperture, pit cavity and pit membrane (Fig. 4b, d). Groups of vessels, as seen in cross sections, showed these depositions on pits of the peripheral walls of vessels (Fig. 4c, d). Tannin deposition progressed through inter-vessel pits to the inner surface of the wall of the adjacent vessel, and from outer vessels to inner ones through the inter-vessel pit membranes, or from parenchyma to vessels through the intervening pit membranes. Finally, tannins were found deposited on walls of the inner vessels (Figs. 4a, b, 5a–f). In some cases, vessel lumens were partially or completely blocked by these depositions (Figs. 4d, 5f), irrespective of vessel width. Such depositions on vessels were seen mostly in the middle to outer part of the wood in transverse section. Few vessels in A. tortilis were found collapsed under SDI treatment (Fig. 5e). Tannin deposition resulting in blockage of vessel pits, hardly seen in the control samples, increased with the intensity of drought stress in both the species.
Stem wood in transverse sections of Acacia ehrenbergiana seedlings: tannin accumulation is visible in axial parenchyma (arrow) adjacent to vessels and through pit pairs on the wall of vessels at the lower side (a), heavy accumulations covering the vessel walls as indicated by arrows (b), depositions appearing mainly on walls forming the outer boundary (arrow) of a cluster of vessels (c), the lumen of some vasicentric axial parenchyma cells and a vessel (arrows) is almost filled with tannin depositions (d). Scale bar on A = 50 μm and applies to all views
Stem wood of Acacia tortilis subsp. raddiana seedlings, showing the progressive deposition of tannin in vessels as seen in transverse sections (a–f): arrows indicate deposition on pit membranes (a), later covering the whole pit pairs and the inner part of the wall (b). Tannin accumulation may be uneven (c) or uniform (d) on the wall of vessels. Heavy accumulations inside the lumen of the vessel segment may damage the water conduit by lumen blockage or wall rupture (e–f). Scale bar on A = 50 μm and applies to all views
Discussion
Water deficit affects every aspect of plant growth and modifies plant resistance to drought (Auge et al. 2003). Plants adopt different strategies for survival and growth under the conditions of limited water supply or high evaporative demands (Jones 2004; Tambussi et al. 2007). Variations in the annual wood production in arid-zone plants are attributable by about 90 % to differences in soil–water availability (Zahner 1968). Water stress reduces RWC and leaf water potential (Merchant et al. 2007) as well as the extension and radial growths in woody plants (Corcuera et al. 2004). A study of Acacia species revealed that A. tortilis resisted water stress and maintained its pace of branch development, leaf production and total leaf area, possibly by allocating more carbon to roots. Its ability to reallocate more carbon to roots, thus enhancing the root growth, was a dehydration-avoiding strategy that might matter in its survival during drought (Otieno et al. 2001, 2005). Preconditioning enhanced the ability of seedlings to survive water stress. Moreover, seedlings of A. tortilis and A. xanthophloea developed drought-resisting characteristics better under prolonged moderate drought than under severe short-spanned stress (Otieno et al. 2005).
Early wood vessels having large diameters contribute maximally to water flow up the stem, but are susceptible to embolization due to frost or drought (Tyree and Cochard 1996; Al-Khalifah et al. 2006). Increased vessel widths covering larger transectional area of wood, as observed in A. ehrenbergiana, was reported in Eucalyptus globulus under low water-stress condition (Leal et al. 2003). Water transport may be impeded not only by xylem collapse, but also by decreased lignin deposition on vessel walls and increased phenolic accumulation and tylosis development in vessel lumen, as observed in the low-lignin transgenic poplar genotypes (Kitin et al. 2010). Cavitation resistance is also influenced by calcium; removal of calcium from cell wall increased xylem vulnerability to cavitation in both conifers and angiosperms, though it showed no effect on hydraulic conductance (Herbette and Cochard 2010).
The reduced vessel area proportion under stressful condition is due partly to a shift towards small diameter vessels (Thomas et al. 2004) or sparsely distributed vessels (Mahmooduzzafar et al. 2010). Narrow vessels are supposed to be positively correlated to xeromorphism (Carlquist 1977), and woods with lesser vessel-lumen area are stronger and have a higher density (Wagner et al. 1998). Accordingly, the water-deficient A. tortilis with short, narrow, crowded and thick-walled vessel elements, high proportion of transverse fiber-wall area and high wood density, is less vulnerable to drought stress than A. ehrenbergiana.
Plant resistance to drought is often correlated with xylem vulnerability to cavitation; species maintaining functional xylem conduits even under extreme drought conditions have a greater chance of survival (Maherali et al. 2004). Further, resistance to xylem cavitation is strongly correlated to inter-vessel-wall thickness (Cochard et al. 2008); a greater wall thickness under MDI and SDI conditions than in the controls of Acacia species in our study suggests that both species can survive under extreme water stress by avoiding wall implosion. Vessel-wall reinforcement is required to prevent wall implosion and cavitation, when xylem pressure is highly negative (Hacke et al. 2001a). Vessel-wall thickenings (sculpturing) may have a link with drought adaptation, as they strengthen the vessel wall in more arid-adapted taxa, which experience greater negative xylem pressures. These thickenings may also reduce the contact angle between water and vessel wall to nearly zero, causing increased wall wettability, which could decrease embolism chances and enhance refilling (Kohonen and Helland 2009). Variations in the transverse-area fraction of fiber wall and vessel lumen (e.g. a decrease in A. ehrenbergiana and an increase in A. tortilis) under increasing intensity of drought are likely to be phenotypic traits, whereas the relationship between transverse fiber-wall area and vessel-lumen area in A. ehrenbergiana (r = 0.9849) and A. tortilis (r = 0.9532) seems to be a genotypic trait. A highly significant negative correlation was observed between the inter-treatment variations for wood density and vulnerability factor in both A. ehrenbergiana (r = −0.9934) and A. tortilis (r = −0.7530). The A. ehrenbergiana wood weakened with increased water stress (0.6618 g cm−3 at MDI and 0.6290 g cm−3 at SDI), but was still strong enough to resist drought. On the other hand, the A. tortilis wood became stronger with the increasing wood density, under both MDI (0.6232 g cm−3) and SDI (0.6762 g cm−3) conditions.
Cavitation resistance shows positive correlation with xylem density, vessel-wall thickness to lumen diameter ratio and the transverse fiber-wall area, whereas negative correlation with fiber-lumen area (Hacke et al. 2001a; Crous et al. 2012). Since drought acclimation of woody plants involves thickening of vessels and other xylem cells (Pratt et al. 2007), which contributes to wood density also, the latter becomes linked to drought acclimation (Al-Khalifah et al. 2006; Christensen-Dalsgaard and Ennos 2012). In other words, plants subjected to drought may be expected to develop stems having stiffer and stronger xylem tissue with a higher density (Christensen-Dalsgaard and Ennos 2012). In a study of Acacia species, Searle and Owen (2005) found some association between basic density and percentage heartwood at the species level, but none at the provenance and within-species level. Although wood density decreased in A. ehrenbergiana and increased in A. tortilis, vessel-wall thickness to vessel-lumen ratio increased in both the species, with growing drought stress.
The wood- and vessel-strength parameters strongly scale with mean cavitation pressure (MCP). Greater cavitation resistance is associated with features that may minimize mechanical stresses on aspirated pit membrane. The more cavitation-resistant Acer species possessed shorter vessels as well as more frequent and longer radial vessel multiples (Lens et al. 2011). In addition, fibers have a role in buttressing vessel walls against implosion under extreme negative pressure. The positive correlation between cavitation resistance and fiber-wall area seems to suggest a mechanical role of fibers against cavitation (Jacobsen et al. 2005). Thus, the significant negative correlations between transverse fiber-wall area and xylem-vulnerability factor in A. ehrenbergiana (r = −0.9644) and A. tortilis (r = −0.9303) are indicative of a positive role of fibers in drought resistance. In A. tortilis, transverse fiber-wall area increased under increasing water stress. In A. ehrenbergiana, despite a decline under deficit-irrigation regimes, the fiber-wall area maintained a sizeable fraction (over 50 %) under stressful condition.
Lens et al. (2011) brought out significance of the vessel length and inter-vessel pit structure in relation to MCP and hydraulic conductivity per xylem cross-sectional area (K xa ). The role of vessel length and inter-vessel pit structure in determining the MCP and K xa was, thus, decisive in the safety-efficiency struggle in Acer. The characteristics of inter-vessel pit membrane in hardwood species may vary with environment (Jansen et al. 2009). We recorded a significant effect of drought on diameter and thickness of inter-vessel pit membranes. Under the same irrigation deficit, A. ehrenbergiana had a thicker pit membrane than A. tortilis. Thicker pit membranes are less porous and more drought resistant (Jansen et al. 2009). Pit membrane area per vessel normally shows a strong negative correlation with cavitation. It also has a link with small surface of narrow and short vessels (Wheeler et al. 2005; Hacke et al. 2006). In our study, A. ehrenbergiana showed increased area fraction of pit membrane under MDI and SDI, but A. tortilis avoided this adaptation. Moreover, despite having a somewhat increased average of vessel-lumen width, A. ehrenbergiana has a relatively low vessel-lumen-area fraction in comparison to A. tortilis, a positive adaptation for survival under stress.
In addition to the presence of vestured pits, which is a common feature of vessels in acacias (Nair and Mohan Ram 2008; Jansen et al. 2009; Damunupola et al. 2011), the blockage of peripheral pits (pit aperture, pit chamber and pit membrane including pit pores) of a group of vessels, as observed in the present investigation, could be an additional protective device against drought. These depositions possibly protect the inner vessels, irrespective of the vessel element dimensions and pit characteristics, from air seeding, cavitation and embolism. Participation of the axial and ray parenchyma in tannin deposition on pits confirms the possible role of living xylem cells in the embolism-check/repair process (Sperry et al. 2006).
Vulnerability index of wood, calculated to determine the impact of ecological domain on water-carrying ability of wood, demonstrated a greater tendency of both the species studied towards xeromorphism under drought condition. Vulnerability of water-transport system in angiosperms is linearly related to pit aperture and/or pit membrane pore diameter (Choat et al. 2003; Sperry and Hacke 2004). It may also have a positive correlation with stress level and/or plant age (Mahmooduzzafar et al. 2010). Narrow and numerous vessels, having intervascular-pit resistance, ensure a successful plant performance in xeric conditions or under severe drought (Carlquist 1984; Tyree and Zimmermann 2002). The successful growth and survival of Acacia seedlings under severe drought conditions endorse all these views.
Conclusions
Since, an increase in vessel frequency, inter-vessel-wall thickness, vessel-wall thickness to vessel-lumen diameter ratio (VWT/VLD), pit membrane diameter and thickness, ray dimensions and wood density, and a reduction in vessel diameter, pit membrane area fraction per vessel element, and vulnerability factor of wood are indicative of a smooth water transport and successful plant growth and survival. It may be surmised that A. ehrenbergiana can grow and survive better than A. tortilis subsp. raddiana in normal tropical atmosphere, but A. tortilis has a greater ability to adapt to increased water stress and can, therefore, perform better than A. ehrenbergiana under harsh drought conditions. Tannin deposition on vessel pits is an additional adaptive feature against drought in both the species; the significance of this feature in relation to air seeding, cavitation and embolism needs to be confirmed.
Abbreviations
- FAA:
-
Formalin–acetic acid–alcohol
- FC:
-
Field capacity
- K xa :
-
Hydraulic conductivity per xylem cross-sectional area
- MCP:
-
Mean cavitation pressure
- MDI:
-
Moderate deficit irrigation
- RLS:
-
Radial longitudinal section
- RWC:
-
Relative water content
- SDI:
-
Severe deficit irrigation
- TLS:
-
Tangential longitudinal section
- VF:
-
Vulnerability factor
- VLD:
-
Vessel-lumen diameter
- VWT:
-
Vessel-wall thickness
References
Al-Khalifah NS, Khan PR, Al-Abdulkader AM, Nasroun T (2006) Impact of water stress on the sapwood anatomy and functional morphology of Calligonum comosum. IAWA J 27:299–312
Aref IM, El-Juhany LI, Hegazy SS (2003) Comparison of the growth and biomass production of six Acacia species in Riyadh, Saudi Arabia after 4 years of irrigated cultivation. J Arid Environ 54:783–790
Baas P, Ewers FW, Davi SD, Wheeler EA (2004) Evolution of xylem physiology. In: Poole I, Hemsley A (eds) Evolution of plant physiology, Linnaean Society Symposium Series. Elsevier Academic Press, London, pp 273–295
Brodribb TJ, Field TS (2000) Stem hydraulic supply is linked to leaf photosynthetic capacity: evidence from New Caledonian and Tasmanian rainforests. Plant, Cell Environ 23:1381–1388
Brodribb TJ, Holbrook NM (2005) Water stress deforms tracheids peripheral to the leaf vein of a tropical conifer. Plant Physiol 137:1139–1146
Carlquist S (1977) Ecological factors in wood evolution: a floristic approach. Am J Bot 64:887–896
Carlquist S (1984) Wood anatomy and relationships of Pentaphylaceae: significance of vessel features. Phytomorphology 34:84–90
Chaves MM, Maroco JP, Periera S (2003) Understanding plant responses to drought from genes to the whole plant. Funct Plant Biol 30:239–264
Cheadle VI, Gifford EM Jr, Esau K (1953) A staining combination for phloem and contiguous tissues. Stain Technol 28:49–53
Choat B, Ball M, Luly J, Holtum J (2003) Pit membrane porosity and water stress-induced cavitation in four co-existing dry rainforest tree species. Plant Physiol 131:41–48
Choat B, Cobb A, Jansen S (2008) Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytol 177:608–626
Christensen-Dalsgaard KK, Ennos AR (2012) Effects of drought acclimation on the mechanical properties of Ochroma pyramidale, Betula pendula and Acacia karroo tree seedling stems. Forestry 85(2):215–223
Cochard H, Froux F, Mayr S, Coutand C (2004) Xylem collapse in water stressed pine needles. Plant Physiol 134:401–408
Cochard H, Barigah ST, Kleinhents M, Eshel A (2008) Is xylem cavitation resistance a relevant criterion for screening drought resistance among Prunus species? J Plant Physiol 165:976–982
Committee IAWA (1989) IAWA list of microscopic features for hardwood identification. IAWA Bull ns 10:219–332
Corcuera L, Camarero JJ, Gil-Pelegrin E (2004) Effects of a severe drought on Quercus ilex radial growth and xylem anatomy. Trees 18:83–92
Crous CJ, Jacobs SM, Esler KJ (2012) Wood anatomical traits as a measure of plant responses to water availability: invasive Acacia mearnsii De Wild. Compared with native tree species in fynbos riparian ecotones. South Africa. Trees 26:1527–1536
Damunupola JW, Ratnayake K, Joyce DC, Irving DE (2011) Characterisation of xylem conduits and their possible role in limiting the vase life of cut Acacia holosericea (Mimosaceae) foliage stem. Funct Plant Biol 38:614–623
Davis SD, Ewers FW, Portwood KA, Sperry JS, Crocker MC, Adams GC (2002) Shoot dieback during prolonged drought in Ceanothus (Rhamnaceae) chaparral in California: a possible case of hydraulic failure. Am J Bot 89:820–828
Franklin GL (1945) Preparation of thin sections of synthetic resins and wood-resin composites and a new macerating method for wood. Nature 155:51
Gardner RO (1975) Vanillin-hydrochloric acid as a histochemical test for tannin. Stain Technol 50:315–317
Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA (2001a) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126:457–461
Hacke UG, Stiller V, Sperry JS, Pittermann J, McCulloh KA (2001b) Cavitation fatigue, embolism and refilling cycles can weaken the cavitation resistance of xylem. Plant Physiol 125:770–786
Hacke UG, Sperry JS, Wheeler JK, Castro L (2006) Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol 26:689–701
Herbette S, Cochard H (2010) Calcium is a major determinant of xylem vulnerability to cavitation. Plant Physiol 153:1932–1939
Jacobsen AL, Ewers FW, Pratt RB, Paddock WA III, Davis SD (2005) Do xylem fibers affect vessel cavitation resistance? Plant Physiol 139:546–556
Jansen S, Choat B, Pletsers A (2009) Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am J Bot 96:409–419
Johansen DA (1940) Plant microtechnique. McGraw Hill, New York
Jones H (2004) What is water use efficiency? In: Bacon MA (ed) Water use efficiency in plant biology. Blackwell Publishing, Oxford
Kalapos T (1994) Leaf water potential-leaf water deficit relationship for ten species of a semi-arid grassland community. Plant Soil 160:105–112
Kitin P, Voelker SL, Meinzer FC, Beeckman H, Strauss SH, Lachenbruch B (2010) Tyloses and phenolic deposits in xylem vessels impede water transport in low-lignin transgenic poplars: a study by cryo-fluorescence microscopy. Plant Physiol 154:887–898
Kocacinar F, Sage RF (2004) Photosynthetic pathway alters hydraulic structure and function in woody plants. Oecologia 139:214–223
Kohonen MM, Helland A (2009) On the function of wall sculpturing in xylem conduits. J Bionic Engin 6:324–329
Kramer PJ, Kozlowski TT (1979) Physiology of woody plants. Academic Press, New York
Leal S, Pereira H, Grabner M, Wimmer R (2003) Clonal and site variation of vessels in 7-year old Eucalyptus globulus. IAWA J 24:185–195
Lens F, Sperry JS, Christman MM, Choat B, Rabaey D, Jansen S (2011) Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytol 190:709–723
Maherali H, Pockman WT, Jackson RB (2004) Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 85:2184–2199
Mahmooduzzafar Hegazy SS, Aref IM, Iqbal M (2010) Anatomical changes in the wood of Syzygium cumini exposed to coal-smoke pollution. J Food Agric Environ 8:959–964
Mauseth JD, Stevenson JF (2004) Theoretical considerations of vessel diameter and conductive safety in populations of vessels. Internat J Plant Sci 165:359–368
MEA (2005) Ecosystems and human well-being: Desertification synthesis. Millennium Ecosystem Assessment (MEA). World Resources Institute, Washington, DC
Merchant A, Callister A, Arndt S, Tausz M, Adams M (2007) Contrasting physiological responses of six Eucalyptus species to water deficit. Ann Bot 100:1–9
Morgan JM (1984) Osmoregulation and water stress in higher plants. Annu Rev Plant Physiol 35:299–319
Nair MNB, Mohan Ram HY (2008) Vestured pits and vestured vessel member walls in some Indian dicotyledonous woods. Bot J Linn Soc 100:323–336
Oba G, Nordal I, Stenseth NC, Stave J, Bjora CS, Muthondeki JK, Bii WKA (2001) Growth performance of exotic and indigenous tree species in saline soils in Turkana, Kenya. J Arid Environ 47:499–511
Otieno DO, Kinyamario JI, Omenda TO (2001) Growth features of Acacia tortilis and Acacia xanthophloea seedlings and their response to cyclic soil drought stress. Afr J Ecol 39:126–132
Otieno DO, Schmidt MWT, Adiku S, Tenhunen J (2005) Physiological and morphological responses to water stress in two Acacia species from contrasting habitats. Tree Physiol 25:361–371
Otieno DO, Kurz-Besson C, Liu J, Schmidt MWT, Vale-Lobo DR, David TS, Siegwolf R, Pereira JS, Tenhunen JD (2006) Seasonal variations in soil and plant water status in a Quercus suber L. stand: roots as determinants of tree productivity and survival in the Mediterranean-type ecosystem. Plant Soil 283:119–135
Pratt RB, Jacobsen AI, Ewers FW, Davis SD (2007) Relationships among xylem transport, biomechanics and storage in stems and roots of nine Rhamnaceae species of the California chaparral. New Phytol 174:1–12
Reynolds JF, Kemp PR, Ogle K, Ferna′ndez RJ (2004) Modifying the pulse reserve paradigm for deserts of North America: precipitation pulses, soil water and plant responses. Oecologia 141:194–210
SAS (2004) SAS guide to applications development, 2nd edn. SAS Institute, Cary, North Carolina, USA
Searle SD, Owen JV (2005) Variation in basic wood density and percentage heartwood in temperate Australian Acacia species. Aust For 68:126–136
Sperry JS, Hacke UG (2004) Analysis of circular bordered pit function. I. Angiosperm vessels with homogenous pit membranes. Am J Bot 91:369–385
Sperry JS, Hacke UG, Pittermann J (2006) Size and function in conifer tracheids and angiosperm vessels. Am J Bot 93:1490–1500
Tambussi EA, Bort J, Araus JL (2007) Water use efficiency in C3 cereals under Mediterranean conditions: a review of physiological aspects. Ann Appl Biol 150:307–321
Thomas DS, Montagu KD, Conroy JP (2004) Changes in wood density of Eucalyptus camaldulensis due to temperature-the physiological link between water viscosity and wood anatomy. Forest Ecos Manag 193:157–165
Tyree MT, Cochard H (1996) Summer and winter embolism in oak: impact on water relations. Ann Sci For 53:173–180
Tyree MT, Zimmermann MH (2002) Xylem structure and the ascent of sap, 2nd edn. Springer, Berlin
Tyree MT, Davis SD, Cochard H (1994) Biophysical perspectives of xylem evolution: is there a trade off to hydraulic efficiency for vulnerability to dysfunction? IAWA J 15:335–360
Wagner KR, Evers FW, Davis SD (1998) Trade off between hydraulic efficiency and mechanical strength in the stems of four co-occurring species of chaparral shrubs. Oecologia 117:53–62
Wheeler JK, Sperry JS, Hacke UG, Hoang N (2005) Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: a basis for a safety versus efficiency trade-off in xylem transport. Plant, Cell Environ 28:800–812
Zahner R (1968) Water deficits and growth of trees. In: Kozlowski TT (ed) Water deficit and plant growth. Academic Press, New York, pp 191–252
Acknowledgments
The authors acknowledge sponsorship of this work by the Deanship of Scientific Research, College of Food and Agricultural Sciences, King Saud University, Riyadh. MI thanks the King Saud University of Riyadh for facilities provided during his affiliation as visiting professor with its Department of Plant Production in the College of Food & Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Luettge.
Rights and permissions
About this article
Cite this article
Aref, I.M., Ahmed, A.I., Khan, P.R. et al. Drought-induced adaptive changes in the seedling anatomy of Acacia ehrenbergiana and Acacia tortilis subsp. raddiana . Trees 27, 959–971 (2013). https://doi.org/10.1007/s00468-013-0848-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-013-0848-2