Abstract
Key Message
Elevated CO 2 concentration affected biomass partitioning in above-ground biomass, but size and number of water-conducting cells were unchanged in Larix kaempferi, Kalopanax septemlobus and Betula platyphylla.
Abstract
Using a Free-Air CO2 Enrichment (FACE) system, we studied the effect of elevated CO2 on the growth, leaf gas exchange and xylem anatomy of a conifer, Larix kaempferi, and two angiospermous tree species, Kalopanax septemlobus and Betula platyphylla. Two-year-old seedlings were grown at control sites (ambient; 370 ppm) and FACE sites (elevated; 500 ppm) for 5 years. We measured the lumen area and number of water-conducting cells, as well as biomass and leaf gas exchange, and visualized the functional region of water transport using a dye injection experiment. Elevated CO2 did not induce any significant changes in growth or in leaf gas exchange or lumen area of earlywood tracheids in L. kaempferi relative to ambient CO2. In two other tree species, elevated CO2 was found to enhance tree height and total leaf area (LA), with no change in stomatal conductance. In K. septemlobus, there were no changes in lumen area or number of earlywood vessels, or in the functional region of water transport. B. platyphylla also underwent no changes in lumen area or number of vessels, although there was a yearly variation in the size of the vessels. Our results show that 5 years of CO2 exposure did not notably affect the anatomical features of water-conducting cells. This finding suggests that, under elevated CO2, trees respond to changes in water balance due to changes in LA by extending the hydraulically active area of xylem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With increasing atmospheric CO2 concentration, forest trees comprise an important CO2 sink (Körner 2003). The stem is a particularly useful carbon (C) sink because trees accumulate new cells with solid cell walls in their stems over long periods (Hyvönen et al. 2007). Changes in tree growth and in the anatomical features of the secondary xylem in the stem under elevated CO2 concentration might therefore affect the C sink capacity of these trees.
A meta-analysis of Free-air CO2 enrichment (FACE) experiments found increases in the height, stem diameter, leaf area index and above-ground dry mass of trees grown under elevated CO2 concentrations (Ainsworth and Long 2005). Ainsworth and Rogers (2007) suggested that there is an increase in light saturated photosynthesis (A sat) and a decrease in stomatal conductance (g s) in C3 plants grown under elevated CO2.
Many studies have found, however, that long-term CO2 exposure does not induce any change in the lumen size of water-conducting cells of softwoods or hardwoods (e.g., Kilpeläinen et al. 2007; Watanabe et al. 2010). Kilpeläinen et al. (2007) found that the tracheid lumen diameter of latewood of 20-year-old Scots pine grown under elevated CO2 for 6 years in a closed chamber was not significantly changed by elevated CO2, at least during those 6 years, but that in earlywood an increase in lumen diameter took place in only a single year. Kostiainen et al. (2006) reported that the vessel lumen diameter in 7-year-old Betula pendula clones grown in an open-top chamber for 3 years does not respond to elevated CO2. The vessel lumen diameter in Populus tremuloides clones was not significantly altered from ambient values by 3-year CO2 exposure in FACE experiments, although this parameter tends to increase under elevated CO2 (Kaakinen et al. 2004). On the other hand, P. tremuloides showed no effect of elevated CO2 on vessel lumen diameter during 5-year exposure (Kostiainen et al. 2008).
Elevated CO2 reportedly increases the size of tracheary elements; however (Kostiainen et al. 2009, 2014; Watanabe et al. 2010). Kostiainen et al. (2009) found an increase in radial diameter of latewood tracheid in Picea abies grown under elevated CO2 in whole-tree chambers. Watanabe et al. (2010) reported that the earlywood vessel lumen area in Kalopanax septemlobus saplings grown during 3 years of CO2 exposure tends to be greater than in control saplings, but no effect of CO2 on the size of vessels was found in Quercus mongolica, Betula maximowicziana or Acer mono grown in the FACE system. Kostiainen et al. (2014) determined that elevated CO2 enhanced the vessel diameter in Populus tremuloides clones, but not in Betula papyrifera, in their FACE experiments. It is clear that the effect of elevated CO2 on anatomical features of tracheary elements depends on species and on the experimental conditions and that more information is needed to determine the reasons.
The effect of elevated CO2 on anatomical features of water-conducting cells is believed to be indirect. Kostiainen et al. (2014) stated that the enhanced growth rate of Populus tremuloides clones under elevated CO2 is related to the decrease in cell wall thickness and wood density. Changes due to elevated CO2 in leaf gas exchange, as measured by parameters such as A sat and g s, and in biomass allocation, measured by (for instance) the total leaf area (LA), are believed to affect the formation of water-conducting cells (e.g., Tyree and Alexander 1993; Koike et al. 2015). If g s decreases without any increase in LA under elevated CO2 concentrations, we expect a decrease in the size of water-conducting cells so as to maintain the hydraulic balance within the tree. If, on the other hand, the LA increases due to elevated CO2 concentration, a tree would need more water and the size of water-conducting cells would change in size regardless of changes in g s. Watanabe et al. (2010) suggested that the increase in vessel lumen area in earlywood in K. septemlobus saplings is due to changes in water status and/or the concentration of growth regulators in the stem, via enhancement of the tree height and leaf area. It is therefore necessary to consider the effect of CO2 on anatomical features of water-conducting cells, together with tree growth and leaf gas exchange traits.
Furthermore, changes in leaf gas exchange and biomass allocation under elevated CO2 concentrations might alter the functional region for water transport in the stem, because the water transport pathway in the stem is an important component of the soil–plant–atmosphere continuum (SPAC). Domec et al. (2010) have reported an increase in maximum hydraulic specific conductivity, and a decrease in xylem resistance to embolism, in branches of two diffuse-porous hardwoods, Liquidambar styraciflua and Cornus florida, but found no correlations between physiological changes and anatomical changes under elevated CO2 concentrations. A few detailed studies nevertheless connect tree growth and leaf gas exchange with anatomical features of water-conducting cells and water transport pathways in the xylem of forest trees growing under elevated CO2 concentration (e.g., Norby and Zak 2011; Vaz et al. 2012; Koike et al. 2015).
The present study sought to determine whether elevated CO2 affects the wood structure of three tree species through changes in tree growth and leaf gas exchange during 5 years of CO2 exposure in a FACE system. We looked at the effect of CO2 on (1) tree growth, biomass allocation and leaf gas exchange, (2) anatomical features of water-conducting cells, and (3) the functional region of water transport in the stem. Based on the results, we discuss the structural changes in growth and development of tree saplings exposed to elevated CO2 in relation to water use within the tree.
Materials and methods
FACE experimental design
Our FACE facilities (three enriched CO2 sites with CO2 gas supply system) and control sites (three ambient sites) were set up in the Sapporo Experimental Nursery of Hokkaido University Forests (Sapporo, 43°06′N, 141°20′E), in autumn 2002. Each site was a circular plot, 6.5 m in diameter and 5 m in height. Fertile brown forest soil was provided in half of each site, and infertile volcanic ash soil was provided in the other half (Eguchi et al. 2005a, 2008). The CO2 concentration inside the FACE sites was maintained at around 500 µmol mol−1, corresponding to ambient plus 130 µmol mol−1 (Takagi et al. 2004).
In May 2003, 2-year-old seedlings of one species of conifer and ten species of deciduous broad-leaved trees that grow in cool temperate forests in northern Japan were planted randomly in each soil type. CO2 fumigation took place from July 2003 to August 2007, during the annual growing season (from mid-April to mid-November). These seedlings were grown under natural rainfall. Total precipitation during the growing season from April to September in Sapporo was approximately 460 mm. The experimental design has been described in fuller detail in earlier papers (e.g., Eguchi et al. 2005b; Watanabe et al. 2010).
Materials
We studied three forest tree species. These were one conifer species, Larix kaempferi, and two angiospermous tree species: K. septemlobus, a ring-porous species, and Betula platyphylla var. japonica (abbreviated to B. platyphylla), a diffuse-porous species. L. kaempferi, which is native to central Japan, is a major conifer species used in planting in Hokkaido (Ryu et al. 2009). Kalopanax septemlobus and B. platyphylla are common tree species in cool temperate forests in Northeast Asia (Lee and Kang 2002; Ryu et al. 2009; Zyryanova et al. 2005). Heights and stem basal diameters (mean ± standard error) of seedlings at both sites were 16.5 ± 0.5 cm and 3.0 ± 0.1 mm for L. kaempferi (n = 20), 16.9 ± 0.5 cm and 5.0 ± 0.2 mm for K. septemlobus (n = 22), 15.7 ± 0.3 cm and 2.0 ± 0.1 mm for B. platyphylla (n = 20) (Watanabe et al. 2010).
For each species, we selected from each FACE site and control site a 6-year-old sapling grown in brown forest soil (FACE samples n = 3; control samples n = 3). Before harvesting these saplings, we measured their leaf gas exchange in early July 2007. After measuring the tree height and stem basal diameter, we harvested these saplings in late July 2007, and wood samples were taken from the stem base of the saplings. We measured the fresh weight of these samples and then fixed the samples in FAA (formaldehyde, acetic acid, 50 % ethanol). For measurement of dry mass, we divided each whole sapling into specific organs, as stem, branch and leaves.
For the dye injection experiments (Sano et al. 2005), we selected three saplings of K. septemlobus from each control and FACE site. The tree height, stem basal diameter and leaf gas exchange were measured prior to dye injection, in early July 2007.
Methods
Leaf gas exchange measurements
Leaf gas exchange (the light saturated net photosynthetic rate per leaf area, P sat) and stomatal conductance (g s) were measured using an open gas exchange system (LiCor-6400; Li-Cor Inc., Lincoln, USA). Measurements were made on mature sun leaves of all saplings of each tree species at a given site in early July 2007, under both ambient and elevated CO2 concentration (ambient: 370 µmol mol−1, elevated: 500 µmol mol−1). The saturating photosynthetic photon flux (PPF) at the upper leaf surface was 1500 µmol m−2s−1. The leaf temperature was maintained at 25 °C (Watanabe et al. 2008).
Measurement of dry mass, leaf mass per area and LA
The organs of the samples were dried in an oven, at a temperature of 75 °C for leaves and 105 °C for stem and branch. The dry mass of each organ and the total above-ground dry mass were then measured. The leaf mass per (unit) area (LMA) was measured based on 20 leaves per sapling, and the total leaf area per sapling (LA) was calculated by dividing the leaf dry mass by the LMA value. Biomass partitioning (the percentage of each organ contributing to the total biomass) was estimated from the dry mass of each organ.
For K. septemlobus saplings undergoing dye injection, all leaves were stripped from the plant after dye injection, and the LA was measured with a leaf area meter (LI-3100C; Li-Cor, Lincoln, USA) in early August 2007.
Measurement of ring width
The FAA-fixed samples were washed with fresh water and the wood samples were cut into small pieces. Transverse sections, approximately 16 µm thick, were cut from these samples by a sliding microtome. These sections were stained with 1 % safranin in 30 % ethanol, and were mounted permanently.
Photographs of transverse sections were taken from pith to bark using a light microscope (AxioSkop; Carl Zeiss Inc, Germany) in conjunction with a digital camera (Digital Sight DS-5M; Nikon, Japan). Digital images were synthesized using Adobe Photoshop software (Adobe Systems Inc, USA). We measured the ring width that formed during 2004–2006 from synthesized micrographs, using Image J (Rasband 1997–2012).
Measurement of lumen size in tracheids and vessels
Photographs of transverse sections were obtained from secondary xylem formed in 2006 and 2007 using a light microscope (AxioSkop; Carl Zeiss Inc, Germany) and a digital camera (Digital Sight DS-5M; Nikon, Japan). Digital images were synthesized using Adobe Photoshop software (Adobe Systems Inc, USA). The micrograph areas of each synthesized photograph were approximately 0.19 mm2 for L. kaempferi and 2.62 mm2 for K. septemlobus and B. platyphylla. In L. kaempferi, earlywood tracheids were selected. In K. septemlobus, wide vessel elements were selected from the earlywood. In B. platyphylla, vessel elements were selected from the central part of the annual ring. We measured the lumen area of each tracheid and vessel from synthesized micrographs using Image J (Rasband 1997–2012).
For L. kaempferi, we calculated the total tracheid lumen area per micrograph (mm2; TLA), the mean tracheid lumen area of a single tracheid (µm2; MLA), the percentage of tracheid lumen area per unit micrograph area (%; PLA) and the number of tracheids per square mm (NT). For K. septemlobus and B. platyphylla, we calculated the total vessel lumen area per micrograph (mm2; TLA), the mean lumen area of a single vessel (µm2; MLA), the percentage of vessel lumen area per unit micrograph area (%; PLA), and the number of vessels per square mm (NV); see Watanabe et al. (2008, 2010). In all species we also calculated the relative frequency of lumen area comprising water-conducting cells in different size classes. Measured vessels in ring-porous species and diffuse-porous species were distinguished from latewood vessels and from other types of cells (axial parenchyma cells or wood fibers) by means of a threshold value for the vessel lumen area, respectively (5024 µm2 for ring-porous woods; 314 µm2 for diffuse-porous woods). These threshold values were established from a histogram of porous area in all annual rings of one sample (data not shown).
We calculated the square of the lumen area (squared lumen area) of water-conducting cells as the index of the fourth power of the diameter, using the Hagen-Poiseuille formula to estimate the water transport capability of tracheids and vessels in samples.
Dye injection method
We undertook dye injection according to the method of Sano et al. (2005) in late July and early August 2007. An aqueous solution of 0.5 % (w/v) Acid fuchsin (Wako Chemicals, Tokyo, Japan) was prepared. This solution was filtered through a 0.22 µm filter (GV, Millipore, Billerica, USA). A receptacle was set at the base of each sapling and the aqueous solution was poured into this receptacle. A notch was cut with a chisel below the solution surface in each funnel, and the solution was allowed to flow into the xylem through the notch for about 50 min (see Fig. 2 in Sano et al. 2005). After the dye had been introduced, we poured liquid nitrogen into another receptacle located 1 m above the first. After 5 min, two discs were cut from each frozen part of each sapling and maintained frozen. Following shaving of the surface of the freeze-dried discs, we observed the dye distribution in the stem and photographed it with a digital camera.
After observation of the dye distribution, the cross-sectional area of all discs was scanned with a flatbed scanner (EPSON GT-X970, SEIKO EPSON Corp., Nagano, Japan). The area of red-colored vessels in all images was quantified using Image J (Rasband 1997–2012) and the proportion of colored vessel area to cross-sectional area of discs was calculated.
Statistical analysis
To determine the effect of CO2 on tree growth (tree height, stem basal diameter and biomass), on parameters of leaf gas exchange (P sat and g s), and on wood properties (ring width, total lumen area, mean lumen area, proportion, number of vessels, squared lumen area and the proportion of colored vessel area to cross-sectional area), we performed t tests at a marginal probability level p < 0.10 (KaleidaGraph 4.1.3, Synergy Software, 2011).
For the vessel frequency, the Mann–Whitney U test was performed to determine the effect of CO2 (SPSS 10.0.5. J, SPSS, Tokyo, Japan).
Results
Tree growth and above-ground dry mass
Table 1 shows the tree growth and dry mass of all species. For L. kaempferi the tree height and stem basal diameter did not differ significantly between the FACE and control sites. For K. septemlobus and B. platyphylla, in contrast, the tree height of saplings growing at the FACE site was significantly greater than at the control site, although there were no differences between the sites in stem basal diameter.
The dry mass of each organ and total above-ground dry mass of L. kaempferi and K. septemlobus did not differ significantly between the FACE and control sites. For B. platyphylla, the leaf dry mass and stem dry mass were significantly greater at the FACE site than at the control site, but the branch dry mass did not differ. There was no effect of elevated CO2 on LMA in any of the three species examined. LA significantly increased at the FACE site in B. platyphylla. For K. septemblobus, LA was 87 % greater at the FACE site than at the control site, although there was no significant difference between control and the FACE site (P = 0.111).
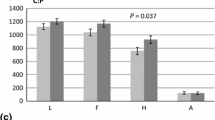
Figure 1 shows biomass partitioning in the three species. The percentage of stem dry mass to total above-ground biomass increased in L. kaempferi at the FACE site relative to control. In K. septemlobus and B. platyphylla, the percentage of leaf dry mass increased at the FACE site relative to controls. However, there were no significant differences between FACE and control sites in all organs in the three species.
Percentage of stem dry mass, branch dry mass and leaf dry mass to total above-ground biomass in saplings of all species grown at control sites and FACE sites. a Larix kaempferi, b Kalopanax septemlobus, c Betula platyphylla. Statistical results in all organs were not significant between control and FACE sites in the three species
Leaf gas exchange traits
There were no significant effects of elevated CO2 on P sat and g s in the three species examined (Table 1).
Ring width and anatomical features of water-conducting cells
Table 2 shows the width of rings formed from 2004 to 2006. We harvested all saplings before growth ring formation was complete in 2007, so we did not measure the ring width in that year. The growth rings of L. kaempferi that formed in 2004 were significantly wider at the FACE site than at controls. There was no significant effect of elevated CO2 on the width of rings formed in 2005 and 2006 in this species, however. The ring width for K. septemlobus and B. platyphylla did not differ between FACE and control sites during 2004–2006. The total ring width for the 3 years did not differ significantly between the FACE and control sites in any of the three species studied, although the mean values were all higher at the FACE site.
Table 2 sets out the anatomical features of water-conducting cells produced in 2006 and 2007. For L. kaempferi, all anatomical features of tracheids (namely TLA, MLA, PLA and NT in earlywood) exhibited no significant difference between FACE and control sites in 2006 or 2007. For K. septemlobus, the PLA and NV values increased in earlywood at the FACE site in 2006 relative to controls, but the TLA and MLA were not different in that year; in 2007, no parameters of vessels differed significantly between the FACE and control sites. For B. platyphylla in 2006, the TLA and PLA values at the FACE site were smaller than at the control site, but MLA and NV did not differ between the sites; in 2007, no vessel parameters differed significantly between FACE and control sites. The squared lumen area of vessels did not differ between the FACE and control sites in K. septemlobus or B. platyphylla.
Figure 2 shows a histogram of the lumen area of water-conducting cells. In L. kaempferi, mid-size tracheids (450–600 µm2) are significantly more common at FACE sites than at control sites, but only in 2007 (Fig. 2a, b). For K. septemlobus, in 2006 medium size vessels (10,000–14,000 µm2) were less common at the FACE site than in controls, but no such differences between the FACE site and controls was observed in xylem formed in 2007 (Fig. 2c, d). For B. platyphylla, no such differences between the FACE site and controls were observed in xylem formed in 2006 and 2007 (Fig. 2e, f).
Distribution of lumen area of water-conducting cells into different size classes in saplings of all species in both 2006 and 2007. a Larix kaempferi in 2006, b L. kaempferi in 2007, c Kalopanax septemlobus in 2006, d K. septemlobus in 2007, e Betula platyphylla in 2006, f B. platyphylla in 2007. Actual P values are shown when P < 0.10 (n = 3)
Dye distribution
Saplings selected for dye injection were taller at the FACE sites than at control sites. The basal diameter of the stem and LA did not differ significantly between FACE and control sites. Elevated CO2 did not affect P sat and g s (Table 3).
Figure 3 shows the dye distribution in saplings grown at the FACE and control sites. In all K. septemlobus saplings, at both FACE (Fig. 3b) and control sites (Fig. 3a), vessels in earlywood and whole latewood in the current year and some of the latewood vessels of the previous year were colored red with Acid fuchsin. The average proportion of colored vessel area to cross-sectional area was approximately 1.25 times higher in FACE saplings (10.0 ± 1.2 %) than in control saplings (8.1 ± 0.8 %), although there were no significant differences between control and FACE sites (P = 0.29).
Micrographs of dye distribution in transverse sections of secondary xylem in saplings of Kalopanax septemlobus grown at control site (a) and at FACE site (b) after injection of Acid fuchsin solution. Red-colored vessels are active in water conduction. Earlywood vessels formed in the current year and latewood vessels formed in both current year and the previous year were stained by Acid fuchsin, but earlywood vessels formed in the previous year were not stained by Acid fuchsin at both control site and FACE site. White arrowheads; earlywood vessels formed in 2007, black arrowheads; earlywood vessels formed in 2006. Scale bars 1 mm
Discussion
Tree growth and leaf gas exchange traits under elevated CO2
In our FACE experiments, an elevated CO2 concentration significantly enhanced the height of two angiospermous tree species, but did not significantly enhance the basal diameter of the stem (Table 1). This is consistent with a meta-analysis of FACE experiments (Ainsworth and Long 2005; Pinkard et al. 2010).
The meta-analysis of Ainsworth and Long (2005) found a 28 % increase in total above-ground dry matter production due to elevated CO2 concentration. In the present study, the total above-ground biomass of saplings of L. kaempferi, K. septemlobus and B. platyphylla was, respectively, 24, 18 and 86 % greater at the FACE site than at the control site. The significant increase in total above-ground dry mass of B. platyphylla under elevated CO2 could be due to an increase in leaf dry mass and stem dry mass (Table 1). In particular, the leaf dry mass in B. platyphylla under elevated CO2 was approximately three times greater than in control saplings. The increase in leaf dry mass and LA without change in LMA suggests an increase in leaf size at elevated CO2, and/or an increase in leaf number per sapling (Table 1). This increase in leaf dry mass might be caused by an improvement in growth conditions, such as light, due to the increase in tree height under elevated CO2.
There were no significant differences in the above-ground biomass partitioning in the three species due to small number of saplings tested in this study (Fig. 1). However, our results showed the possibility that the raised CO2 concentration altered the above-ground biomass partitioning in the three tree species (Fig. 1).
Meta-analyses of tree FACE experiments indicate that elevated CO2 enhances A max and reduces g s, improving the water use efficiency (WUE) (Ainsworth and Rogers 2007; Pinkard et al. 2010). In the present study, however, P sat and g s did not change in the three species examined during 5 years of CO2 exposure (Table 1). It is possible that long-term CO2 exposure leads to photosynthetic acclimation to elevated CO2 (Pinkard et al. 2010). The absence of any significant response in g s in the present study might be due to the relatively low elevated CO2 concentration (about 500 ppm) compared to other FACE experiment sites, such as Duke Forest (CO2 concentration; ambient plus 200 ppm) (Ainsworth and Long 2005).
Ring width after 5 years of CO2 exposure
In our study, elevated CO2 significantly enhanced the annual ring width only in 1 year, 2004 (the second growth season), in saplings of L. kaempferi during the period 2003–2007 (Table 2). The review by Yazaki et al. (2005) found that elevated CO2 did enhance growth ring width in softwoods and hardwoods in the short term. Some studies nevertheless found that the response of radial growth to long-term CO2 fumigation differs from the response to short-term CO2 fumigation (e.g., Norby et al. 2001; Körner et al. 2005; Kilpeläinen et al. 2007; Koike et al. 2015). Kilpeläinen et al. (2007) found that elevated CO2 increases growth ring width in 4 years in 20-year-old Scots pine trees (Pinus sylvestris) during the period 1997–2002. Our results also indicate that long-term CO2 exposure does not stimulate radial growth in trees. Enhancement of the annual ring width which we observed in 2004 might be due to increases in the assimilation rate under elevated CO2 concentration with light saturation (A growth) in 2003 in saplings of L. kaempferi (Eguchi 2008).
Size and number of water-conducting cells after 5 years of CO2 exposure
Our FACE study determined that the three species did not undergo any significant changes in the size of their water-conducting cells, although saplings of all species showed yearly variation at both control and FACE sites (Table 2; Fig. 2). Kilpeläinen et al. (2007) and Kostiainen et al. (2014) have also found yearly variations in the mean lumen diameter of tracheary elements in Scots pine and in two diffuse-porous woods under elevated CO2. The formation of tracheary elements is influenced by external and internal conditions (Denne and Dodds 1981), so it is necessary to consider internal factors such as plant growth regulators and also external factors such as elevated CO2 concentrations and precipitation.
Functional region of water transport in stem under elevated CO2
Watanabe et al. (2010) have proposed that the enhanced LA of K. septemlobus as a result of elevated CO2 induced a change in vessel size, although g s did not change after 3 years of CO2 exposure. It is also possible that an increase in LA under elevated CO2 would lead to changes in the functional region for water transport. The dye injection method is capable of visualizing the water-conducting pathway in the stem (Sano et al. 2005; Umebayashi et al. 2008). We, therefore, investigated whether there are changes in the functional region for water transport through vessels in K. septemlobus, using the dye injection method together with measurement of the size of vessels.
The dye distribution revealed that water conducts through earlywood vessels and latewood vessels of K. septemlobus in the current year, and through part of the latewood vessels in the previous year, in both controls and under elevated CO2. This result is consistent with previous studies that visualized the water transport pathway in various tree species using dye injection (Umebayashi et al. 2008; Sano et al. 2011). Our results suggest that the functional region for water transport in the stem of K. septemlobus does not significantly change under elevated CO2.
Do changes in biomass allocation and leaf gas exchange traits due to elevated CO2 induce changes in anatomical features of water-conducting cells?
Our results indicate that elevated CO2 does not affect tree growth, leaf gas exchange, anatomical features or water transport ability of L. kaempferi saplings. Yazaki et al. (2001, 2004) also found no obvious changes in anatomical features of tracheids in L. sibirica and L. kaempferi seedlings at elevated CO2. Domec et al. (2010) suggested that physiological traits in some conifers would respond to elevated CO2 after the replacement of a large proportion of pre-treatment hydraulic active sapwood by wood produced under elevated CO2. In our study, L. kaempferi saplings produced a large proportion of sapwood under elevated CO2, but we found no changes in leaf gas exchange traits. It appears that xylem formation of L. kaempferi is less sensitive to elevated CO2.
In K. septemlobus and B. platyphylla, the elevated CO2 concentration enhanced the LA value after 5 years of CO2 exposure, although g s did not change (Table 1). Enhanced LA would involve more water to leaves, and the anatomical features of water-conducting cells would be influenced by it. No obvious changes in the size or water transport ability of earlywood vessels or in the functional region of water transport were found under elevated CO2 in K. septemlobus, however (Table 2; Fig. 3).
Kalopanax septemlobus saplings grown under elevated CO2 might respond to changes in the water demand of leaves as a result of enhanced LA by increasing the proportion of hydraulically active area. There were also no significant changes in size or water transport ability in B. platyphylla after 5 years exposure to CO2 (Table 2). B. platyphylla uses almost all of the vessels in several tree rings for water transport (Utsumi et al. 1998). This species might, therefore, respond to changes in demand of water to leaves due to enhanced LA by increasing the functional region of water transport.
Our study has shown that enhanced LA did not induce changes in the anatomical features of water-conducting cells in the three tree species examined under elevated CO2. The wood structure of these trees, including the size of tracheary elements, would be less sensitive to elevated CO2. Trees might respond to changes in water balance due to changes in LA by extending the hydraulically active area of xylem, rather than by making changes in the vessel size and number of vessels, under elevated CO2.
Author contribution statement
Yoko Watanabe: writing of the article, collection and assembly of data, analysis and interpretation of data. Keita Wakabayashi, Satoshi Nakaba, Satoshi Kitaoka, Takami Satomura and Norikazu Eguchi: collection and assembly of data. Makoto Watanabe: Analysis of data. Kentaro Takagi: conception and design of the study. Yuzou Sano: collection and assembly of data, analysis and interpretation of data. Ryo Funada: conception and design of the study. Takayoshi Koike: conception and design of the study, final approval of the article.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Denne MP, Dodd RS (1981) The environmental control of xylem differentiation. In: Barnett JR (ed) Xylem cell development. Castle House, Kent, pp 236–255
Domec JC, Schäfer K, Oren R, Kim HS, McCarthy HR (2010) Variable conductivity and embolism in roots and branches of four contrasting tree species and their impacts on whole-plant hydraulic performance under future atmospheric CO2 concentration. Tree Physiol 30:1001–1015
Eguchi N (2008) Study on the changes in CO2 fixation and storage capacity of deciduous tree species native to cool temperate zone with increasing ambient CO2 concentration. Doctoral dissertation of The Graduate School of Agriculture of Hokkaido University, Sapporo, Japan (in Japanese)
Eguchi N, Funada R, Ueda T, Takagi K, Hiura T, Sasa K, Koike T (2005a) Soil moisture condition and growth of deciduous tree seedlings native to northern Japan grown under elevated CO2 with FACE system. Phyton 45:133–138
Eguchi N, Koike T, Ueda T (2005b) Free Air CO2 enrichment experiment in Northern Japan. Vaisala News 169:15–16
Eguchi N, Karatsu K, Ueda T, Funada R, Takagi K, Hiura T, Sasa K, Koike T (2008) Photosynthetic responses of birch and alder saplings grown in a free air CO2 enrichment system in northern Japan. Trees 22:437–447
Hyvönen R, Ågren GI, Linder S, Persson T, Cotrufo MF, Ekblad A, Freeman M, Grelle A, Janssens IA, Jarvis PG, Kellomäki S, Lindroth A, Loustau D, Lundmark T, Norby RJ, Oren R, Pilegaard K, Ryan MG, Sigurdsson BD, Strömgren M, van Oijen M, Wallin G (2007) The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytol 173:463–480
Kaakinen S, Kostiainen K, Ek F, Saranpää P, Kubiske ME, Sober J, Karnosky DF, Vapaavuori E (2004) Stem wood properties of Populus tremuloides, Betula papyrifera and Acer saccharum saplings after 3 years of treatments to elevated carbon dioxide and ozone. Global Change Biol 10:1513–1525
Kilpeläinen A, Gerendiain AZ, Luostarinen K, Peltola H, Kellomäki S (2007) Elevated temperature and CO2 concentration effects on xylem anatomy of Scots pine. Tree Physiol 27:1329–1338
Koike T, Watanabe M, Watanabe Y, Agathokleous E, Mao QZ, Eguchi N, Takagi K, Satoh F, Kitaoka S, Funada R (2015) Ecophysiology of deciduous trees native to Northeast Asia grown under FACE (Free Air CO2 Enrichment). J Agr Met 71:174–184
Körner Ch (2003) Carbon limitation in trees. J Ecol 91:4–17
Körner Ch, Asshoff R, Bignucolo O, Hättenschwiler S, Keel SG, Peláez-Riedl S, Pepin S, Siegwolf RTW, Zotz G (2005) Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science 309:1360–1362
Kostiainen K, Jalkanen H, Kaakinen S, Saranpää P, Vapaavuori E (2006) Wood properties of two silver birch clones exposed to elevated CO2 and O3. Global Change Biol 12:1230–1240
Kostiainen K, Kaakinen S, Warsta E, Kubiske ME, Nelson ND, Sober J, Karnosky DF, Saranpää P, Vapaavuori E (2008) Wood properties of trembling aspen and paper birch after 5 years of exposure to elevated concentrations of CO2 and O3. Tree Physiol 28:805–813
Kostiainen K, Kaakinen S, Saranpää P, Sigurdsson BD, Lundqvist SO, Linder S, Vapaavuori E (2009) Stem wood properties of mature Norway spruce after 3 years of continuous exposure to elevated [CO2] and temperature. Global Change Biol 15:368–379
Kostiainen K, Saranpää P, Lundqvist SO, Kubiske ME, Vapaavuori E (2014) Wood properties of Populus and Betula in long-term exposure to elevated CO2 and O3. Plant Cell Environ 37:1452–1463
Lee DK, Kang HS (2002) Distribution of Kalopanax septemlobus and its growth in Northeast Asia. Eurasian J For Res 5:85–94
Norby RJ, Zak DR (2011) Ecological lessons from Free-Air CO2 Enrichment (FACE) Experiments. Ann Rev Ecol Evol Syst 42:181–203
Norby RJ, Todd DE, Fults J, Johnson DW (2001) Allometric determination of tree growth in a CO2-enriched sweetgum stand. New Phytol 150:477–487
Pinkard EA, Beadle CL, Mendham DS, Carter J, Glen M (2010) Determining photosynthetic responses of forest species to elevated [CO2]: alternatives to FACE. For Ecol Manag 260:1251–1261
Rasband WS (1997–2012) ImageJ, US National Institutes of Health, Bethesda, MD. http://imagej.nih.gov/ij/
Ryu K, Watanabe M, Shibata H, Takagi K, Nomura M, Koike T (2009) Ecophysiological responses of the larch species in northern Japan to environmental changes as a basis for afforestation. Landscape Ecol Eng 5:99–106
Sano Y, Okamura Y, Utsumi Y (2005) Visualizing water-conduction pathways of living trees: selection of dyes and tissue preparation methods. Tree Physiol 25:269–275
Sano Y, Morris H, Shimada H, De Craene LPR, Jansen S (2011) Anatomical features associated with water transport in imperforate tracheary elements of vessel-bearing angiosperms. Ann Bot 107:953–964
Takagi K, Eguchi N, Ueda T, Sasa K, Koike T (2004) CO2 control in a FACE system for tree saplings. J Agr Met Hokkaido 56:9–16 (in Japanese)
Tyree MT, Alexander JD (1993) Plant water relations and the effects of elevated CO2: a review and suggestions for future research. Vegetatio 104(105):47–62
Umebayashi T, Utsumi Y, Koga S, Inoue S, Fujikawa S, Arakawa K, Matsumura J, Oda K (2008) Conducting pathways in north temperate deciduous broadleaved trees. IAWA J 29:247–263
Utsumi Y, Sano Y, Fujikawa S, Funada R, Ohtani J (1998) Visualization of cavitated vessels in winter and refilled vessels in spring in diffuse-porous trees by cryo-scanning electron microscopy. Plant Physiol 117:1463–1471
Vaz M, Cochard H, Gazarini L, Graça J, Chaves MM, Pereira JS (2012) Cork oak (Quercus suber L.) seedlings acclimate to elevated CO2 and water stress: photosynthesis, growth, wood anatomy and hydraulic conductivity. Trees 26:1145–1157
Watanabe Y, Tobita H, Kitao M, Maruyama Y, Choi DS, Sasa K, Funada R, Koike T (2008) Effects of elevated CO2 and nitrogen on wood structure related to water transport in seedlings of two deciduous broad-leaved tree species. Trees 22:403–411
Watanabe Y, Satomura T, Sasa K, Funada R, Koike T (2010) Differential anatomical responses to elevated CO2 in saplings of four hardwood species. Plant Cell Environ 33:1101–1111
Yazaki K, Funada R, Mori S, Maruyama Y, Abaimov AP, Koike T (2001) Growth and annual ring structure of Larix sibirica grown at different carbon dioxide concentrations and nutrient supply rates. Tree Physiol 21:1223–1229
Yazaki K, Ishida S, Kawagishi T, Fukatsu E, Maruyama Y, Kitao M, Tobita H, Koike T, Funada R (2004) Effects of elevated CO2 concentration on growth, annual ring structure and photosynthesis in Larix kaempferi seedlings. Tree Physiol 24:941–949
Yazaki K, Maruyama Y, Mori S, Koike T, Funada R (2005) Effects of elevated carbon dioxide concentration on wood structure and formation in trees. In: Omasa K, Nouchi I, De Kok LJ (eds) Plant responses to air pollution and global change. Springer, Tokyo, pp 89–97
Zyryanova OA, Yabarov VT, Abaimov AP, Koike T, Sasa K, Terazawa M (2005) Problems in the maintenance and sustainable use of forest resources in Priamurye in the Russian Far East. Eurasian J For Res 8:53–64
Acknowledgments
We thank Dr. A. Sumida for help with the measurement of leaf area using a leaf area meter (LI-3100C; Li-Cor, Lincoln, USA) and Dr. T. Umebayashi for his technical advice about image analysis. Thanks are also due to Mr. T. Ueda of DALTON Co ltd. for maintenance of the FACE system. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (Nos. 21580170, 21114008, 26292075), a Grant-in-Aid (Research Project for Parameterization of Terrestrial Ecosystem for Integrated Global Modeling, Research Revolution 2002) from the Ministry of Education, Culture, Sports, Science and Technology, Japan and a Grant-in-Aid for Young Scientists (H19-Y-079) from the Northern Advanced Centre for Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Communicated by J. Major.
Rights and permissions
About this article
Cite this article
Watanabe, Y., Wakabayashi, K., Kitaoka, S. et al. Response of tree growth and wood structure of Larix kaempferi, Kalopanax septemlobus and Betula platyphylla saplings to elevated CO2 concentration for 5 years exposure in a FACE system. Trees 30, 1569–1579 (2016). https://doi.org/10.1007/s00468-016-1390-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1390-9