Abstract
Key message
Striking hydro-climatic differences of 2 years (wet; dry) dramatically control the increment pattern of L. huasango in varying extent, even causing a “growth collapse” during the La Niña drought 2010/2011.
Abstract
We present the first multi-year long time series of local climate data in the seasonally dry tropical forest in Southern Ecuador and related growth dynamics of Loxopterygium huasango, a deciduous tree species. Local climate was investigated by installing an automatically weather station in 2007 and the daily tree growth variability was measured with high-resolution point dendrometers. The climatic impact on growth behaviour was evaluated. Hydro-climatic variables, like precipitation and relative humidity, were the most important factors for controlling tree growth. Changes in rainwater input affected radial increment rates and daily amplitudes of stem diameter variations within the study period from 2009 to 2013. El Niño Southern Oscillation (ENSO) related variations of tropical Pacific Ocean sea surface temperatures influenced the trees’ increment rates. Average radial increments showed high inter-annual (up to 7.89 mm) and inter-individual (up to 3.88 mm) variations. Daily amplitudes of stem diameter variations differed strongly between the two extreme years 2009 (wet) and 2011 (dry). Contrary to 2009, the La Niña drought in 2011 caused a rapid reduction of the daily amplitudes, indicating a total cessation (‘growth collapse’) of stem increment under ENSO-related drought conditions and demonstrating the high impact of climatic extreme events on carbon sequestration of the dry tropical forest ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endangered ecosystem “seasonally dry tropical forest” features an exceptional biodiversity and great endemism richness (Eamus 1999; Estrada-Medina et al. 2013) which is characterised by a small number of evergreen species and a majority of deciduous tree species (Bullock et al. 1995). A distinct seasonality of rainfall patterns, up to five humid months, is the decisive factor controlling phenological phases and seasonal tree growth rhythms (Borchert et al. 2005; Reich and Borchert 1982; Sayer and Newbery 2003).
In general, seasonally dry tropical forests are characterized by a maximum annual precipitation below 1600 mm that is unevenly distributed throughout the year, resulting in a long dry period of at least 5–6 months with monthly precipitation sums below 100 mm (Mayle 2004; Pennington et al. 2006).
The seasonality of important climatic factors (e.g. precipitation, solar radiation, relative humidity, temperature, and vapour pressure) is reflected by the trees’ physiological responses (e.g. phenological and growth cycles, competitive behaviour). However, these responses vary considerably along the pan-tropics, depending on region and tree species (Wagner et al. 2014).
In comparison with the subtropical Andes and the temperate zones of South America (Boninsegna et al. 2009) little is still known about the growth behaviour of trees inhabiting in the dry tropical areas of South America. Dry forests have received rather little attention in scientific literature, although drylands cover 41 % of the earth’s surface and host ca. 20 % of major centres of plant biodiversity (Maestre et al. 2012). Only few studies focus on this ecosystem along the equatorial Pacific region, including Ecuador and Peru (Mayle 2004; Linares-Palomino and Ponce-Alvarez 2005; Linares-Palomino et al. 2010; Volland-Voigt et al. 2011; Särkinen et al. 2012). Hence, detailed knowledge about the interactions between climatic conditions and tree growth patterns in the seasonally dry tropical forests of equatorial South America is largely lacking.
Since the study site “Laipuna Conservation and Development Area” is located in a region strongly influenced by the El Niño Southern Oscillation (ENSO) phenomenon, trees need to develop different growth strategies to cope with these climatic extreme conditions. A widely used method for studying relations between tree growth and climate variability is the well-established measurement of stem diameter variations with high-resolution electronic point dendrometers (e.g. Worbes 2002; Rozendaal and Zuidema 2011; Steppe et al. 2015). This approach has been proven to be suitable to record long-term, seasonal, and diurnal diameter growth variations of tropical trees (Wimmer et al. 2002; Biondi et al. 2005; Deslauriers et al. 2007; Bräuning et al. 2008, 2009a, b; Biondi and Hartsough 2010; Volland-Voigt et al. 2011; Krepkowski et al. 2011, 2013; Drew et al. 2014).
The climatic influence of ENSO along the Ecuadorian and Peruvian coasts includes basin-wide warming/cooling of sea surface temperatures of the Pacific Ocean and therefore wetter El Niño and drier La Niña conditions (Trenberth 1997; Vuille and Bradley 2000; Vuille et al. 2000; Takahashi 2004; Bendix et al. 2011). The strong La Niña event 2010–2011 (Boening et al. 2012; Cotrill 2012; Tobin 2012; Tobin and Skinner 2012; Hoyos et al. 2013) was characterised by a severe drought along the Peruvian and Ecuadorian costs, and extreme rainfall events associated with flooding in the higher Andean region, respectively.
It is precisely for these reasons that the aim of our study is to analyse the response of the seasonally dry tropical forest ecosystem to inter-annual variations of different climatic forcing factors. Specifically, we studied growth rhythm and growth rate of the tree species Loxopterygium huasango that is a valuable timber species and an important constituent of the dry forest ecosystem. In detail, we address the following research questions: (1) Is tree growth of L. huasango affected by the amount of local precipitation? (2) Is positive radial increment restricted to any hydro-climatic season (wet, dry, or both seasons)? (3) How are tree growth and consequently carbon sequestration influenced by La Niña events?
Methods
Study site and local climate
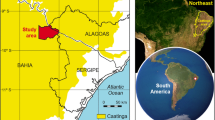
The study site Reserva Laipuna, officially named “Laipuna Conservation and Development Area”, is located at the south-western slope of the Andes (4°12′S, 79°53′W) at altitudes between 590 and 1.480 m asl (Fig. 1). The area belongs to the seasonally dry topical forest belt, distributed along the Pacific coast line in Ecuador and Peru (Bullock et al. 1995; Linares-Palomino et al. 2010; Särkinen et al. 2012). Since the Andes block moist easterly air masses originating from the Atlantic Ocean (Garreaud et al. 2009), the regional precipitation regime shows only one single rainy season with rainfall brought from the Pacific by westerly winds, typically lasting from January to the end of May (Volland-Voigt et al. 2011). A long dry season occurs between June and December. The terrain is hilly with deeply incised gorges, and bedrock mainly consists of acidic Cretaceous intrusive granite. Consequently, very young, acidic, dry, and little developed Torriorthens soils dominate in the study area which are characterized by low fertility (Ministerio de Recursos Naturales y Energeticos 1982; Ministerio de Agricultura y Ganaderia 1984; United States Department of Agriculture, Natural Resources Conservation Service 1999).
In May 2007, we installed a climate station (THIES Clima Germany) near the valley of Rio Catamayo (590 m asl) in a distance of ca. 500 m from the studied trees. The measured climate variables included air temperature, precipitation, relative humidity, and wind direction and wind speed 2.5 m above ground. Each climate variable was recorded at 10-min intervals and hourly arithmetic means (for wind direction vector mean) were stored by a data logger.
Tree species and wood anatomy
Loxopterygium huasango Spruce ex Engl. (Anacardiaceae) is a common deciduous broadleaved tree species of the Ecuadorian and Peruvian seasonally dry tropical forest and occurs at altitudes between 900 to 1100 m asl (Burnham and Carranco 2004; Rodríguez et al. 2005; Linares-Palomino and Ponce-Alvarez 2009). Tree heights may attain up to 25 m (Gonzáles Estrella et al. 2005). The species is shedding all leaves during the latter part of the dry season (Williams et al. 1997; Mayle 2004; Linares-Palomino and Ponce-Alvarez 2005). It is assumed that L. huasango has a deep, extensive root system. Thus, transpiration rates and water supply can better be balanced (Borchert 1994; Zalamea and González 2008; Méndez-Alonso et al. 2013).
Loxopterygium huasango produces very hard and durable wood, characterised by a wood density of 0.73 g cm−3 (Zanne et al. 2009). In case of bark injury, the trees exude an aromatic whitish latex/gum (Heidelberger and Rao 1966). The high economic potential of L. huasango has early been recognized by the furniture and the medical/pharmaceutical industries, causing a strong exploitation of the species. De Elao reported the threatened status of the species (Agrawal 1996a). To counteract these trends, L. huasango has been designated for nature conversation and reforestation projects in Ecuador (Agrawal 1996b).
Dendrometer measurements
High-resolution point dendrometers (Ecomatic, Germany) were installed at breast height (~1.30 m) on three tree individuals growing on a steep slope to study short-term variations as well as long-term trends in stem growth of L. huasango, and to relate them to the local environmental conditions. The installation of the dendrometers was done parallel to the slope. Parts of the outer bark were carefully removed to exclude undesirable shrinking and swelling side effects of the bark (Bräuning et al. 2009a). Since 2009, radial stem variations were measured in 30-min intervals. Calculation methods implemented here are based on the three-phase model of a typical diurnal stem cycle, which has been fully described in previous studies (Wimmer et al. 2002; Deslauriers et al. 2007; Drew and Downes 2009; Volland-Voigt et al. 2011). Daily amplitudes (dA, i.e. the difference between the maximum stem radius and the minimum stem radius of the previous day) of radial stem radius variation, daily radial increments (dR, i.e. the difference between two consecutive daily stem radius maxima), and cumulative radial growth (cum dR, i.e. the accumulation of consecutive daily stem radius maxima) were calculated.
The measuring period of climatic data and dendrometer measurements actually lasted from 2007 to 2013. However, due to longer data gaps of the growth measurements in earlier years we neglect data before 2009. Although cumulative growth trends will be shown until 2013, calculations of increment patterns (e.g. dA) of three tree individuals were only possible until 2011. The time period between 2009 and 2011 is consistent between climate and tree growth data and was therefore used as evaluation period in this study.
Data analysis
Climatic data (temperature, precipitation, wind direction, wind speed and relative air humidity) were analysed with the R package ‘metvurst’ and plots are designed with R ‘windContours’ (Bunn 2008; Appelhans 2013).
To quantify intra- and inter-annual tree growth variability in relation to climatic seasonality (rainy and dry season) we used IBM SPSS STATISTICS 22. Requirements for statistical methods and normal distribution of variables were checked with the Kolmogorov–Smirnov test and also visually (histogram). Since some variables were not normally distributed, difference hypotheses were tested with the Wilcoxon sign rank test and the Mann–Whitney U test. To examine the relation between tree growth patterns and climatic conditions, we calculated Pearson’s correlation coefficients. Due to the lack of normal distribution in the interval-scaled data (monthly radial increment, temperature, relative humidity, irradiation, precipitation, monthly sea surface temperature (SST) in Niño regions 1 + 2, 3, 3.4 and 4 and Southern Oscillation Index Standardized (SOI Std.), we also calculated Spearman rank correlations. Since these results confirmed the Pearson correlations, we do not show Spearman’s rank correlations in the following.
Results
Climate variability
In general, the local climate can be classified as tropical dry, with a strong seasonality of rainfall, occurring between December and May (Valencia et al. 1999). During 2007 to 2013 the overall climatic pattern was characterized by a mean annual temperature of 23.5 °C, whereby an increase of 1 K occurred by comparing the years 2007 and 2013 (Table 1; Fig. 2a). The mean annual relative humidity was 74 %, annual averaged irradiation was 226 W m−2, and mean annual precipitation amount was 550 mm (Table 1). Heavy rainfall events occurred between January and May (Fig. 2b). Over 90 % of the total annual rainfall is restricted to the rainy season.
However, the local climate shows a high inter-annual variability and distinct seasonal climate pattern (Table 1). For example, annual precipitation amounted 792 mm in 2009, but 742 mm fell during the rainy season and only 50 mm during the 7-month dry season. In contrast, only 284 mm of precipitation fell during the rainy season of the extremely dry year 2011, and 30 mm during the dry season. The monthly rainfall ranged between 0.0 mm in July 2007 and 423.5 mm in February 2012. During night-times relative air humidity amounted more than 90 % and dropped below 60 % in the afternoons (Fig. 2c). Within the rainy season, the mean relative humidity amounted approx. 75 % in 2011 and 84 % in 2009. Average minimum daily values of irradiation were 77 W m−2 during the rainy season, when the sky was often covered with clouds. Average maximum daily values of irradiation (309 W m−2) occurred during the dry season. An overview of the hourly values of irradiation is depicted in Fig. 2d. Mean annual vapour pressure deficit amounted 7.5 hPa, whereas minimum vapour pressure deficit amounted 5.7 hPa during the rainy season and the maximum vapour pressure deficit amounted 8.5 hPa during the dry season. During the extremely wet year 2009, vapour pressure deficit reached 9.1 hPa in October and 4.3 hPa in March. In contrast, in the extremely dry year 2011, vapour pressure deficit was 11.2 hPa in March and 5.1 hPa in April (Table 1).
The climate conditions of 2009 and 2012, as well as those of 2011 and 2013 were similar with regard to temperature and precipitation. However, the years 2009 and 2011, in particular differed mostly among each other and showed striking different climatic conditions. Table 2 shows the influence of sea surface temperature (SST) of the four different Niño regions and the Southern Oscillation Index (SOI, pressure difference across the Pacific Ocean, sea surface pressure SSP) on local climate parameters. Highly significant relationships were observed between Niño 1 + 2 region and local relative humidity (r = 0.77, p < 0.01) and local precipitation (r = 0.68, p < 0.01).
Rainfall anomalies of the years 2009 and 2011
In general, precipitation events were concentrated to night-times and were strongly linked to the prevailing wind direction (Fig. 3). We analysed differences of the dominant wind directions and rainfall patterns in the wetter year 2009 (791.6 mm) and the drier La Niña year 2011 (314.1 mm) (Fig. 3; Table 1).
Coloured contour plots of hourly precipitation distribution (mm) and wind frequency of hourly occurrence (%, black isolines) during the rainy season (January–May) in the wet year 2009 (left) and the dry year 2011 (right). Areas with numerous closely spaced wind isolines showed the most occurrence of wind direction during the daily hours in the mentioned month. Blue to red coloured areas showed hourly rainfall distribution within the month. Y axis daily hours, X axis wind direction (°)
Between January and May 2009 (rainy season) northerly winds were most frequent and dominated at noon, night and early morning. During afternoons and evenings winds shifted to a southerly direction. Precipitation was concentrated between afternoon after 3 p.m. and early morning before 9 a.m., whereas no rains occurred between morning and afternoon.
During the dry season (June to December) in 2009, three main wind directions dominated: S and NW winds occurred from midnight to 9 a.m., northerly winds dominated during noon, and during the afternoons easterly winds prevailed. Rainfall occurred only from 2 p.m. until 7 p.m.
Between January and May 2011 (rainy season), the wind mainly breezed from N directions during the nights and mornings, shifting then to SE directions in the afternoons and in the evenings. In particular, a completely different wind distribution occurred during March 2011 compared to March 2009 (Fig. 3). During January and May, rain mostly occurred between 6 p.m. and 9 a.m., whereas in March nearly no rainfall occurred between 1 p.m. and 9 a.m. The wind and precipitation conditions during the dry season (June to December) 2011 were similar as in the dry season 2009.
It is shown (Fig. 3), that during the drier La Niña year 2011 the main dominant northerly wind direction changed into southerly wind direction in the study area, compared to the “normal” year 2009. Therefore, less humid air conditions (less relative humidity and amount of rain) and slightly higher air temperatures occurred (Table 1). In 2009, the amount of precipitation during January to March was much higher than in 2011, whereas April 2011 was wetter than April 2009. In January 2011, precipitation was only around 10 % of the rainfall amount in January 2009. A striking rainfall deficit occurred in March 2011, when only ca. 1 % of the precipitation fell, compared to March 2009.
Seasonal growth variability
Loxopterygium huasango forms diffuse porous wood. Growth boundaries are clearly marked by flattened latewood fibres of up to three rows (Fig. 4). Therefore, L. huasango offers a high potential to analyse climate–growth relationships. Figure 5 illustrates the cumulative radial growth of three studied L. huasango trees in relation to the local climatic conditions. Due to longer data gaps in earlier years, the analysed period lasted from January 2009 to July 2013. All three studied trees showed a strong synchronicity of stem shrinkage, swelling, and increment processes. Despite equal trunk circumferences of 1.1 m at breast height (L. huasango A and L. huasango B) the trees differed considerably in their absolute growth rates (end of measurement July 5, 2013: L. huasango A.: 16.90 mm; L. huasango B: 9.05 mm). The main growing season was consistent with the rainy season, as indicated by a strong increase in radial increment, usually exceeding previous years’ maximum stem diameters. However, growing seasons varied from year to year concerning date of start and duration.
a Transversal thin-section of Loxopterygium huasango with transition zone of sapwood and heartwood with tyloses. An exemplary tree-ring boundary is indicated by white arrows. b Polished surface of L. huasango illustrated by reflected light microscope. Exemplary tree-ring boundary is indicated by white arrows
a Cumulative radial growth of three Loxopterygium huasango and its connection with b daily sums of precipitation (black bars), daily maximum vapour pressure deficit (grey bars), mean daily temperature (light grey line) and 20-smoothed spline of temperature (black line) from January 2009 to July 2013. Dark grey dashed line represents the temperature trend
Shortly after the termination of the rainy season, a slight decrease in stem radii could be found. Minimum stem radius values were reached quite quickly and remained nearly constant until the end of the dry season.
Absolute annual increments strongly varied within the years and were controlled by the amount of precipitation (Fig. 6). In the dry years 2011 and 2013, the maximum stem radii of the previous year (2010 and 2012) were hardly exceeded, indicating almost no growth.
Beside absolute growth differences, also the seasonality of growth increment varied between years. In the wetter years 2009, 2010, and 2012, a steady stem diameter increment occurred, with one maximum at the end of the rainy season. In contrast, the cumulative growth curves of the dry years 2011 and 2013 were characterised by three short peaks of increment occurring in February, April and June. This is in line with the maxima of precipitation and the minima of vapour pressure deficit. In both dry years, increment was initiated considerably later mainly caused by absent precipitation and above average temperature during January. It also applies for the months March and May in 2011 and 2013, respectively.
Daily amplitudes (dA) of radial stem variations were useful to obtain a better understanding of trees’ water balance. We compared seasonal variations of dA during the wettest and driest years 2009 and 2011 (Fig. 7). Both L. huasango individuals (trees A and B) showed considerably higher dA values during the rainy seasons and the first months of the dry season than during the end of the dry season in both years. However, in the wet year 2009, monthly dA declined 2 or 3 months after the beginning of the dry season. Radial stem variations for trees A and B were recorded until August and October, respectively. In the dry year 2011, monthly dA of both individuals dropped rapidly during the transition period (May/June) and remained conspicuously small until December.
Monthly means of daily amplitudes of two individual trees (a, b) of Loxopterygium huasango and their linkage to monthly sums of precipitation in 2009 (left) and 2011 (right). Seasonal and monthly sums of precipitation are depicted in the boxes. Box plots description: the length of the box represents the interquartile range (25th–75th percentile), the dot in the box interior represents the mean, the horizontal line in the box interior represents the median, the vertical lines issuing from the box extend to the minimum and maximum values, outliners are marked with asterisk
To verify the differences between the monthly dAs within the rainy and dry seasons of both years, we averaged the monthly values to dry and wet season means (Fig. 8). Due to data gaps in November and December 2009 for L. huasango B, we restricted dry season values from June until October to calculate a robust mean. For both individuals, dAs did not significantly differ between the rainy seasons of 2009 and 2011 [L. huasango A: Z (N = 151) = −0.005, p = n.s.; L. huasango B: Z (N = 151) = −1.522; p = n.s.]. However, differences of dAs of the dry seasons 2009 and 2011 were significant. Thus, during the strong La Niña year 2011, dAs were significantly smaller than under less dry conditions in 2009 [L. huasango A: Z (N = 165) = −8.750, p < 0.001; L. huasango B: Z (N = 165) = −11.070, p < 0.001].
Daily amplitudes of two individual trees (a on the left and b on the right) of Loxopterygium huasango within the dry and rainy seasons of 2009 and 2011. Seasonal sums of precipitation are depicted in the boxes. Non-significant differences are marked by n.s. significant differences are marked by asterisks (*p < 0.05; ***p < 0.001). Box plots description: the length of the box represents the interquartile range (25th–75th percentile), the dot in the box interior represents the mean, the horizontal line in the box interior represents the median, the vertical lines issuing from the box extend to the minimum and maximum values, outliners are marked with little dots
To verify if intra-annual differences of the dA occur, we calculated the Mann–Whitney U test for both individuals in 2009 and 2011 separately. Daily amplitudes in 2009 and 2011 were significantly higher during the rainy seasons than during the dry season for L. huasango A [L. huasango A 2009: U (n rainy: 151, n dry: 165) = −3.926, p < 0.001; 2011: U (n rainy: 151, n dry: 165) = −13.313, p < 0.001]. However, this could not be confirmed for L. huasango B. While in 2009 the dAs were smaller during the rainy season than dAs during the dry season, the situation reversed in 2011 (L. huasango B 2009: U (n rainy: 151, n dry: 165) = −2.355, p < 0.05; 2011: U (n rainy: 151, n dry: 165) = −11.081, p < 0.001).
Climate–growth relationships
Regarding the monthly radial increment, positive correlations were detected with the monthly sum of precipitation and relative humidity (Table 2). Higher sums of precipitation were accompanied with higher radial increment for all three individuals (p < 0.001). Solar irradiation and monthly radial growth were negatively correlated (although not statistically significant), indicating the negative impact of increased drought stress on tree growth in the dry forest.
Discussion and conclusions
Striking differences were detected between the 2 years 2009 and 2011, regarding both, climatic conditions (changes in wind direction and precipitation distribution) and increment patterns of the trees (increased annual tree growth vs. almost no annual tree growth). Consequently, a comparison of these 2 years seemed particularly useful to answer our research questions.
Climatic teleconnections
The annual cycle of the measured climate parameters precipitation, temperature, irradiation, wind direction and speed, and also relative humidity is similar every year, although absolute values vary strongly between years. Total precipitation amounts ranged between 296 mm (2013) and 850 mm (2012) and the rainy season strongly varied in length, like 2013 it ended in May whereas in 2012 it lasted until July. This in turn primarily affected the mean relative humidity (higher during wetter years), and average temperature and mean irradiation. Eamus (1999) described very similar annual climatic cycles in other tropical dry woodland ecosystems (savannahs).
Therefore, it is necessary to specify the influence of supra-regional climate forcing factors like SSTs and SSPs. We found significant relations between SSTs in the tropical Pacific and SOI with climate parameters at our study site Laipuna, confirming the great influence of La Niña/El Niño events on local climatic conditions. This is in line with results of Bendix et al. (2011) who found teleconnections between climate in the Andean highlands in South Ecuador and the tropical Pacific. Local relative humidity was significantly correlated with SOI and SSTs in all Niño regions (Table 2). Contrary to the Niño regions 3, 3.4 and 4, the Niño 1 + 2 regions show high positive correlation coefficients with relative humidity and precipitation. Negative correlations were found for irradiation. This signifies that tropical Pacific SSTs strongly impact the local climate at Laipuna. Also for the neighbouring region NW Peru, strong relations between Niño 1 + 2 region SSTs and wet season precipitation were found (Bazo et al. 2013; Lagos et al. 2008). Rodríguez et al. (2005) proved the sensitive reaction of trees in the Sechura dessert of north Peru (Prosopis juliflora and Bursera graveolens) to El Niño events, especially during the extreme ENSO event 1997–1998, when the region profited from abundant rainfall. In our study, the monthly means of relative humidity show a negative correlation with the SOI. The differences among the atmospheric pressure between Tahiti and Darwin affect the trade winds and therefore air humidity (Table 2). Likewise, Vuille et al. (2000) found a close influence of SST anomalies and ENSO on precipitation and temperature along a gradient from the Pacific coast to the western Andean slope (1° to 3° S), whereby the coastal regions of Peru were mostly affected. During extreme rainfall events associated with the strong El Niños 1997–1998 and 2002, the easterly wind component in Piura (NW Peru, near to our study area) was weak and west winds dominated during rainy days (Takahashi 2004).
For 2011, Cotrill (2012) reported about a La Niña ‘return’, because the moderate La Niña in 2010 turned into a strong one during the first quarter of 2011. Tobin and Skinner (2012) noted that the SOI in April 2011 was the second highest since November SOI 1973 and the second-highest April SOI on record (since 1904). The coldest SSTs were measured between autumn 2010 and winter 2011 with a peak in January (Tobin and Skinner 2012). In summary, the La Niña 2010–2011 was one of the longest events with ca. 18 months duration.
Due to the very fast change of SSTs between 2009–2010 (moderate El Niño) and 2010–2011, Hoyos et al. (2013) appointed the La Niña 2010–2011 as “one of the most intense” events and Boening et al. (2012) measured a (ocean) sea level fall during the extreme cooling of the Pacific. These adverse weather conditions during LN10-11 caused the ‘growth collapse’ of L. huasango in 2011, indicating a strong impact of such climate extremes on carbon sequestration rates of the dry tropical forest ecosystem.
Seasonal growth variability of L. huasango
High-resolution dendrometer measurements have been applied to detect seasonal tree growth variability in different climate zones and sites. Especially for the tropics, where seasonal tree growth is limited by water availability (Rozendaal and Zuidema 2011; Wagner et al. 2014) and not by a seasonally induced cambial dormancy during a cold winter (cf. temperate or boreal zones), measurements of radial stem increase and shrinkage over a season provide a suitable technique to record intra-annual growth dynamics (Worbes 1999; Deslauriers et al. 2007; Bräuning et al. 2009a, b; Biondi and Hartsough 2010; Krepkowski et al. 2011; Volland-Voigt et al. 2011; Wils et al. 2011; Biondi and Rossi 2014; Drew et al. 2014; Gebrekirstos et al. 2014).
Our results showed that the seasonal radial stem variations of the three studied L. huasango individuals were strongly coherent over the five studied years, triggered by climatic forcing and phenological phases. We are aware that the number of tested trees is rather small. However, there are publications with the same context and an equal number of studied individuals, e.g. Jiang et al. (2015), Biondi and Rossi (2014), Wang et al. (2014). Very homogenous daily radial stem movement patterns have been reported from other broadleaved tree species (Tabebuia chrysantha) within the research area Reserva Laipuna (Volland-Voigt et al. 2011) and even from different tree life forms in Ethiopia (Krepkowski et al. 2011).
We found strongly different absolute growth rates between individuals of L. huasango (Fig. 6). Differences in total growth are often explained by the trees’ age or the trees’ location within the population (Bräuning et al. 2008, 2009a, b; Volland-Voigt et al. 2011). However, we found no significant differences in the stem circumferences of our studied trees (L. huasango A.: 1.1 m; L. huasango B: 1.1 m, L. huasango C: 1.4 m), and hence a conclusion regarding their age cannot be made. We rather assume that the competitive status and the location on the slope determined increments rates due to local differences in soil water availability (Worbes 1999; Cardoso et al. 2012).
The 5-year long growth record of L. huasango confirmed the hypotheses that the radial increment of tropical tree species is strongly controlled by climatic conditions (Worbes 1999; Wagner et al. 2014). Extreme growth variations between separate years are mainly affected by changes in rainwater input (Clark and Clark 1994). However, tree species growing in seasonally tropical dry forests may respond differently to rainfall events (Rozendaal and Zuidema 2011). Radial increment can respond either positively or negatively to the total annual rainfall (Schöngart et al. 2004; Worbes 1999; Gebrekirstos et al. 2008; Rozendaal and Zuidema 2011; Grogan and Schulze 2012), to rainfall events during the dry season (Worbes 1999; Sass-Klaassen et al. 2008) or during the rainy season, exclusively (Fichtler et al. 2004; Trouet et al. 2006; Brienen et al. 2010; Volland-Voigt et al. 2011; Pompa-García et al. 2015). We suggest that L. huasango belongs to the latter group, because positive increments were only measurable during the rainy season and stem shrinkage immediately set in due to water loss with the beginning of the dry season. Phenological cyclicality corroborates these findings, since length and duration of foliation is related to moisture conditions (‘unpublished data’ E. Cueva 2007; Fig. 9). Leaves flush at the onset of the rainy season (December/January) and leave shedding starts with the beginning of the dry season in summer (May/June). Consequently, photosynthesis and radial increment rapidly ceased. Other deciduous species within the dry forest behave similarly, e.g. B. graveolens, Maclura tinctoria and T. chrysantha (Linares-Palomino and Ponce-Alvarez 2005; Volland-Voigt et al. 2011; Pucha Cofrep et al. 2015). These findings are also described by Estrada-Medina et al. (2013) and Valdez-Hernández et al. (2010) in the tropical dry forest in Yucatán. Leaf shedding is initiated by low soil water potential and vapour pressure deficit, leading to slight inter-annual variations in the phenological years (Do et al. 2005; Valdez-Hernández et al. 2010; Estrada-Medina et al. 2013).
Absolute radial increment strongly differed between years, correlating strongly with the total amount of precipitation within each rainy season (Fig. 5). During the dry La Niña year 2011 no radial increment occurred, demonstrating the high impact of extreme climate conditions on carbon sequestration of the tropical dry forest ecosystem. Worbes (1999) stated that “Growth periodicity in tropical regions is triggered when there is a dry season with less than 50 mm precipitation per month (Worbes 1995).” Accordingly, we found that the growth of L. huasango is initiated when monthly mean precipitation during the rainy season exceeded ca. 60 mm. Accordingly, cambial activity was initiated by prolonged rainfall and extreme rain events exceeding 20 mm of precipitation at the onset of the rainy season (January and February). While in 2009 six rain events with more than 20 mm per day occurred, there was just one such extreme event in 2011 (Fig. 5). As a consequence, a sufficient water saturation of the trunk to start cambial growth could not be reached in 2011.
The analysis of daily amplitudes of L. huasango validated trees’ growth dependence on precipitation and revealed large intra-annual, inter-annual and inter-species differences (Figs. 7, 8). In this context it must be considered that every La Niña year is a dry year but not vice versa. Unfortunately, studies about the duration pattern of the daily amplitudes of tropical trees for further comparison are still sparse.
Our study on L. huasango is in agreement with a recent pan-tropical overview, stating that “precipitation and solar radiation are the main seasonal drivers of tree growth, within the (pan) tropics” (Wagner et al. 2014). Our results provide a baseline for further studies, including wood anatomical and dendrochronological approaches, to differentiate effects of rehydration processes from true cambial activity within the stem. Including additional tree species and wood parameters like tree-ring width, and stable carbon and oxygen isotope analysis may help to indicate the influence of ENSO events on tree water relationships, changes in water use efficiency, and drought risk of vessel cavitation for different tree species in this endangered and highly sensitive forest ecosystem at longer time scales.
Author contribution statement
Susanne Spannl and Franziska Volland analysed the data, designed the study and wrote the first version of the manuscript together. Susanne Spannl analysed the dendrometer data and created the corresponding graphs. Franziska Volland analysed the climate and phenology data. Darwin Pucha created the climate graphics. Achim Bräuning initiated the project, and Thorsten Peters contributed to the writing of the manuscript and supported the interpretation of data and results. Eduardo Cueva collected and organized phenology data. All authors reviewed the paper.
References
Agrawal AA (1996a) Seed germination of Loxopterygium guasango, a threatened tree of coastal Northwestern South America. Trop Ecol 37:273–276
Agrawal AA (1996b) Reforestation in Ecuador’s dry forest. Desert Plants 12:12–14
Appelhans T (2013) Metvurst: METeorological visualisation utilities using R for science and teaching. https://metvurst.wordpress.com/. Accessed 31 Aug 2015
Bazo J, de las Nieves Lorenzo M, Porfirio da Rocha R (2013) Relationship between monthly rainfall in NW Peru and tropical sea surface temperature. Adv Meteorol. doi:10.1155/2013/152875
Bendix J, Trachte K, Palacopis E, Rollenbeck R, Göttlicher D, Nauss T, Bendix A (2011) El Niño meets La Niña—anomalous rainfall patterns in the “Traditional” El Niño region of Southern Ecuador. Erdkd 65:151–167
Biondi F, Hartsough P (2010) Using automated point dendrometers to analyze tropical treeline stem growth at Nevado de Colima, Mexico. Sens 10(6):5827–5844
Biondi F, Rossi S (2014) Plant-water relationships in the Great Basin Desert of North America derived from Pinus monophylla hourly dendrometer records. Intern J Biometeorol. doi:10.1007/s00484-0-014-0907-4
Biondi F, Hartsough P, Estrada I (2005) Daily weather and tree growth at the tropical treeline of North America. Arctic Antarct Alp Res 37:16–24
Boening C, Willis JK, Landerer FW, Nerem RS, Fasullo J (2012) The 2011 La Niña: so strong, the oceans fell. Geophys Res Lett 39:L19602
Boninsegna J, Argollo J, Aravena J, Brichivich J, Christie D, Ferrero M, Lara A, Le Quesne C, Luckmann B, Masiokas M, Morales M, Oliveiera J, Roig F, Srur A, Villalba R (2009) Dendroclimatological reconstructions in South America: a review. Paleogeogr Paleoclimatol Paleoecol 281:210–228
Borchert R (1994) Water status and development of tropical trees during seasonal drought. Trees 8:115–125
Borchert R, Renner SR, Calle Z, Navarrete D, Tye A, Gautier L, Spichiger R, von Hildebrand P (2005) Photoperiodic induction of synchronous flowering near the Equator. Nature 433:627–629
Bräuning A, von Schnakenburg P, Volland-Voigt F, Peters T (2008) Seasonal growth dynamics and its climate forcing in a tropical mountain rain forest in southern Ecuador. Tree Rings Archaeol Climatol Ecol 6:27–30
Bräuning A, Volland-Voigt F, Burchardt I, Ganzhi O, Nauss T, Peters T (2009a) Climatic control of radial growth of Cedrela montana in a humid mountain rain forest in southern Ecuador. Erdkd 63:337–345
Bräuning A, Volland-Voigt F, von Schnakenburg P (2009b) Jahrringe als Ausdruck von Klimabedingungen und Lebensform: wie wachsen Tropenbäume? Biol unserer Zeit 39:124–132
Brienen R, Lebrija-Trejos E, Zuidema P, Marinez-Ramos M (2010) Climate–growth analysis for Mexican dry forest tree shows strong impact of sea surface temperatures and predicts future growth declines. Glob Change Biol 16:2001–2012
Bullock SH, Mooney HA, Medina E (1995) Seasonally dry tropical forests, 1st edn. Cambridge University Press, Cambridge Books, Cambridge. doi:10.1017/CBO9780511753398
Bunn A (2008) A dendrochronology program library in R. Dendrochronologia 26:115–124
Burnham RJ, Carranco N (2004) Miocene winged fruits of Loxopterygium (Anacardiaceae) from the ecuadorian Andes. Am J Bot 91:1767–1773
Cardoso F, Marques R, Botosso P, Marques M (2012) Stem growth and phenology of two tropical trees in contrasting soil conditions. Plant Soil 354:269–281
Clark DA, Clark DB (1994) Climate-induced annual variation in canopy tree growth in a Costa Rican tropical rain forest. J Ecol 82(4):865–872
Cotrill DA (2012) Seasonal climate summery southern hemisphere (spring 2011): La Niña returns. Aust Meteorol Oceanograph J 62:179–192
Deslauriers A, Rossi S, Anfodillo T (2007) Dendrometer and intraannual tree growth: what kind of information can be inferred? Dendrochronologia 25:113–124
Do FC, Goudiaby VA, Gimenez O, Diagne AL, Diouf M, Rocheteau A, Akpo LE (2005) Environmental influence on canopy phenology in the dry tropics. Forest Ecol Manag 215:319–328
Drew DM, Downes GD (2009) The use of precision dendrometers in research on daily stem size and wood property variation: a review. Denrochronologia 27:159–172
Drew DM, Richards AE, Cook GD, Downes GM, Gill W, Baker PJ (2014) The number of days on which increment occurs is the primary determinant of annual ring width in Callitris intratropica. Trees 28:31–40
Eamus D (1999) Ecophysiological traits of deciduous and evergreen woody species in the seasonally dry tropics. Trees 14:11–16
Estrada-Medina H, Santiago LS, Graham RC, Allan MF, Jimenez-Osornio JJ (2013) Source water, phenology and growth of two tropical dry forest tree species growing on shallow karst soils. Trees 27:1297–1307
Fichtler E, Trouet V, Beeckman H, Coppin P, Worbes M (2004) Climatic signals in tree rings of Burkea africana and Pterocarpus angolensis from semiarid forests in Namibia. Trees 18:442–451
Garreaud RD, Vuille M, Compagnucci R, Marengo J (2009) Present-day South American climate. Palaeogeogr Palaeoclimatol Palaeoecol 281:180–195
Gebrekirstos A, Mitlöhner R, Teketay D, Worbes M (2008) Climate-growth relationships of the dominant tree species from semi-arid savanna woodland in Ethiopia. Trees 22:631–641
Gebrekirstos A, Bräuning A, Sass-Klaassen U, Mbow C (2014) Opportunities and applications of dendrochronology in Africa. Curr Opin Env Sustain 6(1):48–53
Gonzáles Estrella JE, Garcia Riofrio JC, Correa Conde J (2005) Especies forestales del bosqoue seco “Cerro Negro-Cazaderos” Zapotillo—Puyango—Loja Ecuador. Fundación Ecológica Arcoiris, Loja
Grogan J, Schulze M (2012) The impact of annual and seasonal rainfall patterns on growth and phenology of emergent tree species in Southeastern Amazonia, Brazil. Biotropica 44(3):331–340
Heidelberger M, Rao C (1966) Immunchemical properties of hualtaco gum. Immunology 10(6):543–548
Hoyos N, Escobar J, Restrepo JC, Arango AM, Ortiz JC (2013) Impact of the 2010–2011 La Niña phenomenon in Columbia, South America: the human toll of an extreme weather event. Appl Geogr 39:16–25
Jiang Y, Wang B-Q, Dong M-U, Huang Y-M, Wang M-C, Wang B (2015) Response of daily stem radial growth of Platycladus orientalis to environmental factors in a semi-arid area of North China. Trees 29:87–96
Krepkowski J, Bräuning A, Gebrekirstos A, Strobl S (2011) Cambial growth dynamics and climatic control of different tree life forms in tropical mountain forest in Ethiopia. Trees 25:59–70
Krepkowski J, Gebrekirstos A, Shibistova O, Bräuning A (2013) Stable carbon isotope labeling reveals different carry-over effects between functional types of tropical trees in an Ethiopian mountain forest. New Phytol 199:431–440
Lagos P, Silva Y, Nickl E, Mosquera K (2008) El Niño-related precipitation variability in Perú. Adv Geosci 14:231–237
Linares-Palomino R, Ponce-Alvarez SI (2005) Tree community patterns on seasonally dry forests in the Cerros de Amotape Cordillera, Tumbes, Peru. Forest Ecol Manag 209:261–272
Linares-Palomino R, Ponce-Alvarez SI (2009) Structural patterns and floristics of a seasonally dry forest in Reserva Ecológica Chaparri, Lambayeque, Peru. Tropic Ecol 50:305–314
Linares-Palomino R, Kvist LP, Aguirre-Mendoza Z, Gonzales-Inca C (2010) Diversity and endemism of woody plant species in the Equatorial Pacific seasonally dry forest. Biodivers Conserv 19:169–185
Maestre F, Quero J, Gotelli N, Escudero A, Ochoa V, Delgado-Baquerizo M, García Gómez M, Bowker M, Soliveres S, Escolar C, García-Palacios P, Berdugo M, Valencia E, Gozalo B, Gallardo A, Aguilera L, Arredondo T, Blones J, Boeken B, Bran D, Conceição A, Cabrera O, Chaieb M, Derak M, Eldridge D, Espinosa C, Florentino A, Gaitán J, Gatica G, Ghiloufi W, Gómez-González S, Gutiérrez R, Hernández R, Huang X, Huber-Sannwald E, Jankju M, Miriti M, Monerris J, Mau R, Morici E, Naseri K, Ospina A, Polo V, Prina A, Pucheta E, Ramírez-Collantes D, Romão R, Tighe M, Torres-Díaz C, Val J, Veiga J, Wang D, Zaady E (2012) Plant species richness and ecosystem multifunctionality in global drylands. Science 335:214–218
Mayle FE (2004) Assessment of the Neotropical dry forest refugia hypothesis in the light of palaeoecological data and vegetation model simulations. J Q Sci 19:713–720
Méndez-Alonso R, Pineda-García F, Paz H, Rosell JA, Olson JC (2013) Leaf phenology is associated with soil water availability and xylem traits in a tropical dry forest. Trees 27:745–754
Ministerio de Agricultura y Ganaderia (1984) Macara—Mapa de Suelos, Quito
Ministerio de Recursos Naturales y Energeticos (1982) National geological map of the republic of Ecuador, Quito
Pennington RT, Lewis G, Ratter JA (2006) Neotropical savannas and dry forests: plant diversity. Biogeography and observation. CRC Press, Florida
Pompa-García M, Miranda-Aragón L, Aguirre-Salado CA (2015) Tree growth response to ENSO in Durango, Mexico. Int J Biometerol 59:89–97
Pucha Cofrep D, Peters T, Bräuning A (2015) Wet season precipitation during the past 120 years reconstructed from tree rings of a tropical dry forest in Southern Ecuador. Global Planet Change 133:65–78
Reich P, Borchert R (1982) Phenology and ecophysiology of the tropical tree, Tabebuia neochrysantha (Bignoniaceae). Ecology 63:294–299
Rodríguez R, Mabres A, Luckman B, Evans M, Masiokas M, Ektvedt TM (2005) “El Niño” events recorded in dry-forest species of the lowlands of northwest Peru. Dendrochronologia 22:181–186
Rozendaal DMA, Zuidema PA (2011) Dendroecology in the tropics: a review. Trees 25:3–16
Särkinen T, Pennington RT, Lavin M, Simon MF, Hughes CE (2012) Evolutionary islands in the Andes: persistence and isolation explain high endemism in Andes dry tropical forests. J Biogeogr 39:884–900
Sass-Klaassen U, Couralet C, Sahle Y, Sterck F (2008) Juniper from Ethiopia contains a large-scale precipitation signal. Int J Plant Sci 169(8):1057–1065
Sayer EJ, Newbery DM (2003) The role of tree size in the leafing phenology of seasonally dry tropical forest in Belize, Central America. J Trop Ecol 19:539–548
Schöngart J, Junk W, Piedade M, Ayres J, Hüttermann A, Worbes M (2004) Teleconnection between tree growth in the Amazonian floodplains and El-Niño-Southern Oscillation effect. Glob Change Biol 10:683–692
Steppe K, Sterck F, Deslauries A (2015) Diel growth dynamics in tree stems: linking anatomy and ecophysiology. Trends Plant Sci. doi:10.1016/j.tplants.2015.03.015
Takahashi K (2004) The atmospheric circulation associated with extreme rainfall events in Piura, Peru, during the 1997–1998 and 2002 El Niño events. Ann Geophys 22:3917–3926
Tobin S (2012) Seasonal climate summary southern hemisphere (winter 2011): a dry season in the lull of La Niña events. Aust Meteorol Oceanograph J 62:97–110
Tobin S, Skinner TCL (2012) Seasonal climate summary southern hemisphere (autumn 2011): one of the strongest La Niña events on record begins to decline. Aust Meteorol Oceanograph J 62:39–50
Trenberth KE (1997) The definition of El Niño. B Am Meteorol Soc 78:2771–2777
Trouet V, Coppin P, Beeckman H (2006) Annual growth ring patterns in Brachystegia spiciformis reveal influence of precipitation on tree growth. Biotropica 38:375–382
United States Department of Agriculture, Natural Resources Conservation Service (1999) Soil Taxonomy: a basic system of soil classification for making and interpreting soil surveys. Government Printing Office Washington, Washington
Valdez-Hernández M, Andrade J, Jackson P, Rebolledo-Vieyra M (2010) Phenology of five tree species of a tropical forest in Yucatan, Mexico: effects of environmental and physiological factors. Plant Soil 329:144–171
Valencia R, Cerón C, Palacios W, Sierra R. (1999) Las Formaciones naturales de la Sierra del Ecuador. In: Sierra R (edn) Propuesta preliminar de un sistema de clasificación de vegetación para el Ecuador Continental. Proyecto INEFAN/GEF y Ecociencia Quito-Ecuador, pp 79–108
Volland-Voigt F, Bräuning A, Ganzhi O, Peters T, Maza H (2011) Radial stem variations of Tabebuia chrysantha (Bignoniaceae) in different tropical forest ecosystems of southern Ecuador. Trees 25:39–48
Vuille M, Bradley RS (2000) Mean annual temperature trends and their vertical structure in the tropical Andes. Geophys Res Lett 27:3885–3888
Vuille M, Bradley RS, Keimig F (2000) Climate variability in the Andes of Ecuador and its relation to tropical Pacific and Atlantic sea surface temperature anomalies. J Climate 13:2520–2535
Wagner F, Rossi V, Aubry-Kientz M, Bonal D, Dalitz H (2014) Pan-tropical analysis of climate effects on seasonal tree growth. PLoS ONE 9(3):e92337. doi:10.1371/journal.pone.0092337
Wang Z, Yang B, Deslauriers A, Bräuning A (2014) Intra-annual stem radial increment response of Qilian juniper to temperature and precipitation along an altitudinal gradient in northwestern China. Trees 29:25–34
Williams RJ, Myers BA, Muller WJ, Duff GA, Eamus D (1997) Leaf phenology of woody species in a north Australian tropical savanna. Ecology 78:2542–2558
Wils G, Sass-Klassen U, Eshetu Z, Bräuning A, Gebrekirstos A, Couralet C, Robertson I, Touchan R, Koprowski M, Conway D, Briffa K, Beeckman H (2011) Dendrochronology in the dry tropics: the Ethiopian case. Trees 25:345–354
Wimmer R, Downes GM, Evans R (2002) High resolution analysis of radial growth and wood density in Eucalyptus nitens, grown under different irrigation regimes. Ann For Sci 59:519–524
Worbes M (1999) Annual growth rings, rainfall-dependent growth and long-term growth pattern of tropical trees from Caparo Forest Reserve in Venezuela. J Ecol 87:391–403
Worbes M (2002) One hundred years of tree-ring research in the tropics: a brief history and an outlook to future challenges. Dendrochronologia 20:217–231
Zalamea M, González G (2008) Leaffall phenology in a subtropical wet forest in Puerto Rico: from species to community patterns. Biotropica 40:295–304
Zanne AE, Lopez-Gonzalez G, Coomes DA, Ilic J, Jansen S, Lewis SL, Miller RB, Swenson NG, Wiemann MC, Chave J (2009) Global wood density database. Dryad. Identifier: http://hdl.handle.net/10255/dryad.235.2014.06.3
Acknowledgments
SSp and FV acknowledge Oswaldo Ganzhi and Volker Raffelsbauer for field work support. SSp and FV also thank Cathrin Meinardus for constructive and fruitful discussions. We also thank Stephan Adler for preparing the map. This study was supported by the German Research Foundation (DFG) by funding the project BR 1895/14 (FOR 816) and BR 1895/23 (PAK 823). DP acknowledges support by the German Academic Exchange Service (DAAD). We also thank Naturaleza y Cultura Internacional (NCI, Loja, Ecuador) for their help in accessing the Laipuna Nature Reserve. The authors would like to thank the reviewers for their invaluable suggestions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. Buckeridge.
Rights and permissions
About this article
Cite this article
Spannl, S., Volland, F., Pucha, D. et al. Climate variability, tree increment patterns and ENSO-related carbon sequestration reduction of the tropical dry forest species Loxopterygium huasango of Southern Ecuador. Trees 30, 1245–1258 (2016). https://doi.org/10.1007/s00468-016-1362-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1362-0