Abstract
Stem diameter increments of the broadleaved deciduous tree species Tabebuia chrysantha were measured with high-resolution dendrometers in a tropical lower montane forest and in a dry forest in southern Ecuador, the latter showing a distinct dry season. Those analyses were complemented by wood anatomical studies on regularly collected microcores to determine the season of active cambial growth and the time of formation of annual growth boundaries. The length of the cambial active period varied between 3 and 7 months at the tropical lower montane forest and 2 and 4 months in the dry forest, respectively. During dry days, amplitudes of daily stem diameter variations correlated with vapour pressure deficit. During October and November, inter-annual climate variations may lead to dry and sunny conditions in the tropical lower montane forest, causing water deficit and stem diameter shrinkage in T. chrysantha. The results of the climate–growth analysis show a positive relationship between tree growth and rainfall as well as vapour pressure deficit in certain periods of the year, indicating that rainfall plays a major role for tree growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dendroclimatology is a widely applied technique to reconstruct palaeoclimate in mountain environments of boreal, temperate and subtropical latitudes, where tree growth shows clearly visible annual growth boundaries due to the seasonal occurrence of cold temperatures causing an interruption of cambial activity (e.g. Deslauriers et al. 2003, 2007; Drew and Downes 2009; Hauser 2003). In comparison, little is known about tree-ring formation in tropical mountain regions (Biondi et al. 2005; Bräuning et al. 2008b). Tree-ring-based reconstructions of inner-tropical climate history are often based on teleconnections between the inner tropics and neighbouring regions, where trees with clearly detectable annual rings are found (D’Arrigo et al. 2005, Stahle et al. 1998). Hence, there is a gap of high-resolution palaeoclimate information in the inner tropics, especially concerning mountain areas. Available data on tree rings in tropical mountain regions cover Central and South America (e.g. Anchukaitis et al. 2008; Bräuning et al. 2009; Brienen 2005; Soliz et al. 2009; Stahle 1999; Worbes 2002), Africa (e.g. Gebrekirstos et al. 2008), and southeast Asia (e.g. Poussart et al. 2004).
Due to low seasonal temperature differences in the inner tropics, annual growth cycles generally occur in regions exhibiting pronounced rainfall seasonality or seasonal flooding, causing cambial dormancy due to water shortage or lack of oxygen in the rhizosphere (Lisi et al. 2008; Worbes 2002). Besides seasonal climate variations, phenological phases such as fruiting, flowering and leaf shedding in deciduous species as well as changes in light intensity (solar radiation/ photoperiodic control) may initiate growth ring formation (Borchert et al. 2005; Bräuning et al. 2008a; Deslauriers et al. 2007; Worbes 1999; Verheyden et al. 2004).
In recent years, annual or sub-annual growth boundaries were reported from an increasing number of tropical tree species (e.g. Anchukaitis et al. 2008; Poussart and Schrag 2005; Verheyden et al. 2004). However, detailed knowledge about seasonal growth dynamics and the periodicity of tree-ring formation is often lacking, despite being a prerequisite for a sound ecological interpretation of those visible wood structures for climatological analyses. The use of high-resolution electronic dendrometer measurements has proven suitable to analyse stem diameter variations at various timescales and to record temporal growth dynamics of tropical trees (Bräuning et al. 2008a, 2009; Deslauriers et al. 2007; Downes et al. 1999; Herzog et al. 1995; Krepkowski et al. 2010; Wimmer et al. 2002). Beside long-term and seasonal stem increment, reversible diurnal stem diameter variations can be registered (Deslauriers et al. 2007; Lövdahl and Odin 1992; Offenthaler et al. 2001). These are helpful to understand the dynamics of water depletion and replenishment in tree stems (Deslauriers et al. 2007; Downes et al. 1999; Drew and Downes 2009; Offenthaler et al. 2001; Wimmer et al. 2002), since stem contraction and expansion are related to crown transpiration.
In this study, we investigate tree growth dynamics of Tabebuia chrysantha (Bignoniaceae) using high-resolution point dendrometer measurements. We compare the species’ growth behaviour in two different climatic regimes in southern Ecuador, which are covered by the vegetation types humid tropical lower montane forest and seasonal tropical dry forest, respectively. T. chrysantha is a deciduous tree species which forms annual growth boundaries highlighted by a tangential band of marginal parenchyma and is therefore potentially suitable for dendroclimatic studies. The aims of this study are twofold: first, we want to assess the influence of climate seasonality on cambial activity, growth rates and the time of formation of the annual growth boundary in different climate regimes. In addition, we evaluate the potential of T. chrysantha to serve as a climate proxy for local climate reconstructions in different environments.
Materials and methods
Study areas and local climate
The Ecuadorian Andes form the interface between the subtropical high pressure areas over the Atlantic Ocean and the southeastern Pacific Ocean (Emck 2007). Hence, the coastal area of Ecuador shows a precipitation regime with one single rainfall maximum during summer (January to March) and a well-defined wintry dry season (April to December). In contrast, the eastern Andean slope at elevations between 1,000 and 3,500 m a.s.l. shows a single rainfall maximum in winter (July) without a real dry season during the year (Bendix and Lauer 1992).
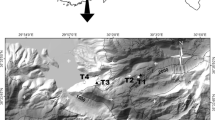
Seasonal growth dynamics of T. chrysantha were studied on adult trees in two tropical forest ecosystems, namely a tropical lower montane forest and a seasonally dry forest. At both study sites, climate stations were installed to register local climatic variables, including air temperature, rainfall, global radiation, wind speed, wind direction, and air humidity. The study site in the tropical lower montane forest ‘Reserva Biológica San Francisco (RBSF)’ is located at the northern slope of the Podocarpus National Park (3°58′S, 79°04′W) at approx. 2,000 m a.s.l. (Bendix et al. 2008; Fig. 1). This part of the Andes is dominated by the inflow of humid air masses from the Amazon lowland (South American summer monsoon) into the eastern part of the research area (Bendix et al. 2008; Bräuning 2009). Local wind systems are controlled by the complex topography of the valley of Rio San Francisco, leading to dominant airflows from SE to SW with some minor influences from NE to SE. Relative air humidity varies strongly between 50 and 99.9% during a day, with an average relative air humidity of 83%. Day and night air temperatures (2 m above ground) are 25 and 10°C, respectively. Highest temperatures occur from September to November; mean annual temperature is 15.5°C. Average annual rainfall amounts to 2,176 mm with an additional input of approx. 121 mm water intake by fog (Bendix et al. 2008; Emck 2007). The region is characterized by a slight rainfall seasonality with a drier season during September to November, when sunny weather situations may prevail for 2–4 weeks (Veranillo del Niño; Richter et al. 2009). During these events, the generally very cloudy area receives higher amounts of solar irradiance which might lead to atmospheric water stress for the vegetation due to the high vapour pressure deficit (Bendix et al. 2008).
The study site in the tropical dry forest “Reserva Laipuna” is located at the southwestern declivity of the Andes (4°12′S, 79°53′W) at an altitude of 1,100 m a.s.l. (Fig. 1). The area is located in the rain shadow of the Andes and is characterized by a single rainy season from January to April (Fig. 1). Precipitation is mostly constricted to nighttime and is basically controlled by wind direction. A local land–sea–wind system causes NE–SE winds during daytime and SW–NW winds at night. From January to April, this system weakens due to the northward shift of the Inter Tropic Convergence Zone (ITCZ). As a consequence, the proportion of SW–NW winds increases, resulting in a flow of moist Pacific air masses into the area. Relative air humidity is very high during the whole rainy season, but even within the dry season it does not drop below 50%. Comparable to RBSF, mean relative air humidity is 83%. Diurnal temperatures range from 11 to 21°C and, since the beginning of our measurements in May 2007, have not shown distinct seasonal variations (Fig. 1).
Tree species
T. chrysantha (Jacq.) Nicholson (Bignoniaceae) is a tropical broadleaved tree with a distribution range from Northern Mexico and Central America, Columbia, Venezuela, to the south of Ecuador to the Peruvian Amazon and Bolivia. The tree reaches up to 20-m height and 60-cm breast height diameter (BHD). T. chrysantha produces very hard, durable and valuable wood and a thick bark (Gonzales Estrella et al. 2005; Günter 2009, Sire 2001). T. chrysantha is a common species in both study sites. Three subspecies (spp. chrysantha, meridionalis and pluvicola) can be distinguished (Günter 2009). T. chrysantha shows a very synchronous flowering pattern (E. Cueva, personal communication 2009; Homeier 2004). From July to September, most individuals are leafless and in bloom. Afterwards, fruiting occurs simultaneously with the formation of new shoots. The phenology of T. chrysantha (short leafless period with a fast bud break at the end) resembles the ‘leaf-exchanging’ type as defined by Borchert et al. (2005) in the sense that the leafless period is only short. Long-term low-resolution band dendrometer measurements reveal a pronounced seasonality of growth in T. chrysantha, with highest growth rates from October to April and a cambial dormancy period from July to September (Homeier 2004). In general, growth rates decrease with increasing tree age and trunk diameter.
Dendrometer measurements and wood anatomy
To study short-term stem diameter variations and to relate them to environmental conditions, high-resolution point dendrometers (Ecomatik, Germany) were installed at breast height (ca. 1.3 m) on four individuals in each study area. Stem diameters were automatically registered in 30-min intervals since April 2006. To reduce the influence of expansion and shrinkage processes in the bark, parts of the outer bark were removed without wounding the cambial zone. The range of the tree diameter varied between 25 and 52 cm at breast height. The total height of the sampled trees is about 20 m.
A typical diurnal stem diameter cycle is characterized by three distinct phases (Deslauriers et al. 2007; Downes et al. 1999; Drew and Downes 2009; Wimmer et al. 2002): After a morning maximum, stem diameters contract until mid-day, since crown transpiration and water transport through the trunk cannot be compensated by root water uptake, except during very wet periods. After the mid-day to early afternoon minimum, water status recovers until the previous maximum is reached. The difference between the tree diameter maximum and the following minimum is called daily amplitude (dA), whereas the difference between two consecutive tree diameter maxima is described as daily radial increment (dR). In case of cambial dormancy and net water loss, the stem diameters can show a net decrease and dR becomes negative. If the previous diameter maximum is exceeded, the tree shows positive increment which may indicate the formation of new cells. However, a positive dR is not automatically related to cambial activity. After longer drought phases, the stem resaturates over several days which causes positive dR without cambial activity. Due to the occurrence of data gaps in some data series, we only show data for one selected individual from each studied forest type.
To evaluate the interrelation of stem diameter increment, cambial activity and wood formation, microcores were collected with an increment puncher (Forster et al. 2000) in monthly intervals. From these cores, thin sections of ca. 20-μm thicknesses were cut with a microtome. These thin sections were stained with solutions of safranin red and astra blue to indicate the distribution of ligneous and living wood anatomical tissues.
Data analyses
To quantify different phases of tree growth behaviour during the year, we separated one complete annual cycle into phases defined by the phenological status of Tabebuia (foliated, leafless) and by the prevailing climatological conditions (dry period, humid period) (Table 1). Thus, three climate–growth phases in the tropical lower montane forest and four phases in the dry forest were outlined, respectively. Within these phases, averages of important climatic parameters were calculated. Besides, the spread of daily stem diameter variations (dA values) and cumulative radial increment (dR) within these phases were calculated as measures of tree growth dynamics and tested for significant differences by a Tukey test.
Results
Intra-annual growth dynamics of T. chrysantha
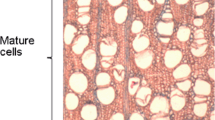
In Fig. 2, stem diameter changes from one individual T. chrysantha tree in the RBSF tropical lower montane forest during the 3.5-year long period from April 2006 to August 2009 are shown. The dendrometer curve demonstrates a seasonality of cambial activity, as indicated by the steplike appearance of stem increments. The stem diameter remained rather constant or even decreased during the leafless period and the short drier period ‘Veranillo del Niño’ from July to December. Afterwards, the tree needs several weeks or even months to reach the pre-leafless stem diameter. Wood anatomical characteristics were used as an additional source of information on seasonal growth. Annual growth boundaries of T. chrysantha consist of a marginal parenchyma band less than three cell rows wide which seems to be formed at the beginning of the growing period (Fig. 3). However, in some of the microsections, the parenchyma bands are difficult to detect, which might indicate the formation of a missing or partly missing ring (see below). Vessels of T. chrysantha are solitary or arranged in clusters or tangential bands with encircled aliform, confluent axial parenchyma. As the alignment of the microsections demonstrates, T. chrysantha at RBSF shows cambial activity and the formation of new xylem cells during April and August (Fig. 3). During climate–growth phase I (July 2007 to September 2007), Tabebuia was leafless despite rather cool and humid climatic conditions with increased cloudiness and rather low incoming radiation levels. The ranges of dA vary between 0.016 and 0.11 mm. Phase II is marked by less humid conditions, a higher portion of westerly winds, causing less cloudiness and a higher amount of incoming radiation at the study site. As a result, the dA values of the re-foliated tree vary between 0.02 and 0.09 mm. In the cloudy and humid phase III, mean temperature was 16.2°C, average relative humidity was 83.5% and average vapour pressure deficit reached 16.5%. In this phase, dA varied between 0.02 and 0.13 mm. Net growth of Tabebuia was restricted to phase III, when cumulative radial change (dR) was positive (1.9 mm for tree 554) (Fig. 4).
a Stem diameter changes of T. chrysantha no. 554 in the tropical lower montane forest. Phase I (dark grey) shows the leafless period, phase II (light grey) short drier period ‘Veranillo del Niño’ and phase III (white) the foliate season. Interruptions of the curve are caused by data gaps due to temporal failure of the data logger. b Daily sums of precipitation (black bars) and vapour pressure deficit (grey bars)
In the dry forest (bosque seco deciduo), growth of Tabebuia closely follows the external climatic forcing which is dominated by the strong seasonality of precipitation (Fig. 5) (Balselv and Øllgaard 2002). The first heavy rains at the onset of the wet season in January caused rehydration of the stem of the studied tree to compensate for the shrinkage that occurred during the previous dry season (May to December). This phase lasted for around 4 weeks. After rehydration was accomplished and leaf flushing had occurred (E. Cueva, personal communication 2009, Figs. 5, 6), the resumption of cambial activity started around mid-February, when the tree exceeded its maximum diameter of the previous year. The phase of stem increment continued after the end of the rainy period and extended into the following dry season. Unfortunately, a data gap occurred during the transition from the rainy to the dry season in 2008, so we were not able to exactly date the end of the stem increment phase (Fig. 5). During the late dry season (starting May/June), the cambium was dormant and stem diameters decreased or remained on a ‘plateau’ until the onset of the next rainy season. However, stem diameters did not shrink beyond the diameter reached in previous May, indicating that the irreversible production of new cells had resulted in net increment. We separated the period May 2007 to April 2008 into four climate–growth phases (Table 2). During phase I (May 2007 to August 2007), Tabebuia was foliated and dry climatic conditions prevailed. The range of dA varies between 0.03 and 0.3 mm. Phase II (September 2007 to December 2007) was marked by leafless Tabebuia trees and dry conditions with high incoming radiation. Single rainfall events during the dry season caused short-term tree diameter expansions due to rehydration. As a result, dA of the leafless tree varied between 0.0 and 0.2 mm. In the cloudy and humid phase III (January 2008), ranges of dA varied between −0.02 and 0.1 mm. Phase IV (February 2008 to April 2008) was marked by re-foliated Tabebuia trees and ongoing wet conditions. Ranges of dA varied between 0.0 and 0.15 mm. Net growth of T. chrysantha was restricted to phases III and IV, when cumulative radial change (dR) was positive, whereas phases I and II were characterized by stem shrinkage or almost constant stem diameter values, respectively (Table 2).
a Stem diameter changes of T. chrysantha no. 9 in the dry forest. Phase I (black shaded) shows the foliated dry period, phase II (dark grey) the leafless dry period, phase III (light grey) the leafless wet season and phase IV (white) the foliated wet period. Interruptions of the curve are caused by data gaps due to temporal failure of the data logger. b Daily sums of precipitation (black bars) and vapour pressure deficit (grey bars)
Inter-annual variation of tree growth related to climate variability
Figure 7 demonstrates the inter-annual variability of seasonal T. chrysantha growth in response to precipitation and vapour pressure deficit during October and November 2006 and 2007. During the ‘Veranillo del Niño’ situation in 2006, mean precipitation per day was 1.6 mm, whereas during the same season in 2007, when no ‘Veranillo del Niño’ occurred, mean precipitation was 5.5 mm/day. In the middle of October 2006, a series of nine consecutive rainless and sunny days occurred. Due to high vapour pressure deficit, T. chrysantha reacted with drastic stem shrinkage and needed several rainy days to recover to its stem diameter from the beginning of the dry event. In comparison, the stem diameter only slightly decreased during October/November 2007, when no more than four consecutive rainless days were registered.
Discussion and conclusions
As shown by the wood anatomical sample series, T. chrysantha forms annual growth rings in both studied environments. By combining high-resolution dendrometer measurements and wood anatomical studies, it was possible to time the seasonality of cambial dormancy, stem increment and the formation of characteristic wood tissues that demarcate the annual growth boundary. While the length of the active growth period varied in different years between 3 and 7 months at the RBSF, it was only 2–4 months in the dry forest. Interestingly, T. chrysantha showed no net growth or even stem shrinkage during the seasonal phases I and II (Table 1) in the tropical lower montane forest. This is a clear indication for seasonal cambial activity even in such a humid environment. A similar behaviour was found for another deciduous tree species, Cedrela montana (Meliaceae) in the RBSF tropical lower montane forest (Bräuning et al. 2009).
In the dry forest, daily amplitudes were largest during phase I (dry, foliated) and smallest during phase II (dry, leafless) and not significantly different during phases III and IV (dry/humid, leafless) (Fig. 8). During the dry season, potential transpiration is very high due to high temperatures and vapour pressure deficit; whereas transpiration is strongly reduced during the humid period and when T. chrysantha is leafless. During phase III, even days without shrinkage phases occur since the stems are in a water saturated state (Deslauriers et al. 2007).
Fluctuation of daily amplitudes of T. chrysantha no. 9 in the four growth phases May 2007 to April 2008. The box extends the 25th percentile to 75th percentile, with a line at the Median (50th percentile). The circles are outliers, the stars are extreme values. Letters on top of the graph indicate statistically significant (p < 0.05) differences between the daily amplitudes as indicated by a Tukey test
Although the seasonal phases of cambial activity were well synchronized among individuals within one forest type, absolute growth rates varied remarkably between almost 0 and 2.0 mm/year at RBSF and 1.8 and 4.8 mm/year in the dry forest. Thus, the length of the period of cambial activity is not the only factor determining absolute growth amounts. However, it has to be taken into account that the diameters at breast height of our two sample trees were 52.8 cm for tree 554 (RBSF site) and only 19.8 cm for tree 9 at the dry forest site, respectively. Thus, a part of the higher growth rates of the dry forest individual might also be a result of its younger age. Beside age, social status can influence growth rates (Bräuning et al. 2008b; Volland-Voigt et al. 2009). Although our results do not provide yet enough data about absolute growth rates, they document first findings on seasonal tree growth behaviour in two different tropical environments. However, they have to be substantiated by longer observation periods and by inclusion of additional trees of different diameter classes. As already reported by Kozlowski and Winget (1964), several trees of the same species may respond differently to the same drought event. Nevertheless, our first results indicate that in both environments, available moisture strongly influences tree growth in both forest types, which was not expected for the humid RBSF environment.
Beside environmental conditions, phenological status and water-storage capacity are important aspects for tree growth (Borchert and Pockmann 2005; Lüttge and Hertel 2009). Both dehydration and rehydration in deciduous hardwood species such as T. chrysantha indicate a low water-storage capacity under dry conditions and a higher water-storage capacity during humid times. Species with a wood anatomy showing paratracheal and extensive wood parenchyma are drought avoiders (Borchert and Pockmann 2005). However, deciduous trees such as T. chrysantha are potentially drought tolerant. T. chrysantha is able to replenish its water reserves quickly under humid conditions and even during single rainfall events in dry periods (Borchert et al. 2005). The range of dA correlates to the daily transpiration during dry periods (Borchert and Pockmann 2005), when incoming radiation increases water pressure deficit and hence tree transpiration (Bräuning et al. 2008b).
Our results correspond to observations of Reich and Borchert (1982). Obviously, T. chrysantha is able to reduce the impact of seasonal drought by adaptive mechanisms such as leaf shedding and utilization of soil water reserves. Tabebuia trees survive 8 months of drought in the tropical dry forest and also grow under almost perhumid wet conditions in the tropical montane rain forest. The species, therefore, has a wide ecologic potential and competitive basis and a wood anatomical structure that enables it to survive under such different climate conditions. Our results about the seasonal dynamics of growth and ring boundary formation provide a basis for the interpretation of larger wood samples like increment cores or wood disks, provided that individual trees of the same species show synchronized growth patterns that can be merged to site chronologies. A recent study on Cedrela montana at RBSF (Bräuning et al. 2009) has shown that cross-dating of increment curves and chronology development in south Ecuadorian tropical forests are possible. Finally, we intend to reconstruct rainfall history in two different climatic regimes along a humidity gradient in southern Ecuador to analyse the variability of the interplay between two atmospheric circulation patterns. This effort is justified by the highly sensitive reactions of T. chrysantha to short-term drought events (longer than ca. 5–7 days). Thus, inter-annual climate variability like the occurrence of ‘Veranillo del Niño’ has a strong effect on growth dynamics even in the humid environment of a tropical lower montane forest.
References
Anchukaitis KJ, Evans MN, Wheelswright NT, Schrag DP (2008) Stable isotope chronology and climate signal calibration in neotropical montane cloud forest trees. J Geophys Res 113:G03030. doi:10.1029/2007JG000613
Balselv H, Øllgaard B (2002) Mapa de vegetaciónes del sur de Ecuador. Botánica height Austroecuatoriana. Abya Yala, Quito, pp 51–64
Bendix J, Lauer W (1992) Klimatologie. Westermann, Braunschweig
Bendix J, Rollenbeck R, Fabian P, Emck P, Richter M, Beck E (2008) Climatic variability. Ecological studies, vol 198. Springer, Berlin, pp 281–290
Biondi F, Hartsough PC, Estrada IG (2005) Daily weather and tree growth at the tropical treeline of North America. Arct Antarct Alp Res 37:16–24
Borchert R, Pockmann W (2005) Water storage capacitance and xylem tension in isolated branches of temperate and tropical trees. Tree Physiol 25:457–466
Borchert R, Robertson K, Schwartz MD, Williams-Linera G (2005) Phenology of temperate trees in tropical climates. Int J Biometerol 50:57–65
Bräuning A (2009) Climate variability of the tropical Andes since the late Pleistocene. Adv Geosci 7:1–13
Bräuning A, Homeier J, Cueva E, Beck E, Günter S (2008a) Growth dynamics of trees in tropical mountain ecosystems. Ecological studies, vol 198. Springer, Berlin, pp 291–302
Bräuning A, von Schnakenburg P, Volland-Voigt F, Peters T (2008b) Seasonal growth dynamics and its climate forcing in a tropical mountain rain forest in southern Ecuador. Tree Rings Archaeol Climatol Ecol 6:27–30
Bräuning A, Volland-Voigt F, Burchardt I, Ganzhi O, Nauss T, Peters T (2009) Climatic control of radial growth of Cedrela montana in a humid mountain rain forest in southern Ecuador. Erdkunde 63:337–345
Brienen RJW (2005) Tree rings in the tropics: a study on growth and ages of Bolivian rain forest trees. PROMAB Scientific Series 10
D’Arrigo R, Cook ER, Wilson RJ, Allan R, Mann ME (2005) On the variability of ENSO over the past six centuries. Geophys Res Lett 32:L03711
Deslauriers A, Morin H, Urbinati C, Carrer M (2003) Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada). Trees 17:477–484
Deslauriers A, Rossi S, Anfodillo T (2007) Dendrometer and intra-annual tree growth: what kind of information can be inferred? Dendrochronologia 25:113–124
Downes G, Beadle C, Worledge D (1999) Daily stem growth patterns in irrigated Eucalyptus blobulus and E. nitens in relation to climate. Trees 14:102–111
Drew DM, Downes GM (2009) The use of precision dendrometers in research on daily stem size and wood property variation: a review. Dendrochronologia 27:159–172
Emck P (2007) A climatology of South Ecuador. Diss. Uni. Erlangen
Forster T, Schweingruber FH, Denneler B (2000) Increment puncher: a tool for extracting small cores of wood and bark from living trees. IAWA 21:169–180
Gebrekirstos A, Mitlöhner R, Teketay D, Worbes M (2008) Climate–growth relationships of the dominant tree species from semi-arid savanna woodland in Ethiopia. Trees 22:631–641
Gonzales Estrella JE, Garcia Riofrio JC, Correa Conde J (2005) Especies forestales del bosque seco ‘Cerro Negro-Cazaderos’ Zapotillo-Puyango-Loja Ecuador. Fundación Ecológica Arcoiris, Loja, Ecuador, p 39
Günter S (2009) Tabebuia chrysantha (Jacq.) Nichols., 1887. Enzyklopädie der Holzgewächse. Wiley VCH, Weinheim
Hauser S (2003) Dynamik hochaufgelöster radialer Schaftveränderungen und des Dickenwachstums bei Buchen (Fagus sylvatica L.) der Schwäbischen Alb unter dem Einfluss von Witterung und Bewirtschaftung. Diss. Uni. Freiburg. http://www.freidok.uni-freiburg.de//volltexte/1121
Herzog KM, Häsler R, Thum R (1995) Diurnal changes in the radius of a subalpine Norway spruce stem: their relation to the sap flow and their use to estimate transpiration. Trees 10:94–101
Homeier J (2004) Baumdiversität, Waldstruktur und Wachstumsdynamik zweier tropischer Bergregenwälder in Ecuador und Costa Rica. Diss. Uni. Bielefeld, p 207
Kozlowski TT, Winget CH (1964) Diurnal and seasonal variations in radii of tree stems. Ecology 45:149–155
Krepkowski J, Bräuning A, Gebrekirstos A, Strobl S (2010) Seasonal growth dynamics and climatic control of different tree life forms in Munessa Forest (Ethiopia). Trees (this volume)
Lisi CS, Tomazello F, Botosso PC, Roig A, Maria VRB, Ferreira-Fedele L, Voigt ARA (2008) Tree-ring formation, radial increment periodicity, and phenology of tree species from a seasonal semi-deciduous forest in southeast Brazil. IAWA 29:189–207
Lövdahl L, Odin H (1992) Diurnal changes in the stem diameter of Norway spruce in relation to relative humidity and air temperature. Trees 6:245–251
Lüttge U, Hertel B (2009) Diurnal and annual rhythms in trees. Trees 23:683–700
Offenthaler I, Hietz P, Richter H (2001) Wood diameter indicates diurnal and long-term patterns of xylem water potential in Norway spruce. Trees 15:215–221
Poussart PF, Schrag DP (2005) Seasonally resolved stable isotope chronologies from northern Thailand deciduous trees. Earth Planet Sci Lett 235:752–765
Poussart PF, Evans MN, Schrag DP (2004) Resolving seasonality in tropical trees: multi-decade, high-resolution oxygen and carbon records from Indonesia and Thailand. Earth Planet Sci Lett 218:301–316
Reich PB, Borchert R (1982) Phenology and ecophysiology of the tropical tree, Tabebuia neochrysantha (Bignoniaceae). Ecology 63:294–299
Richter M, Diertl KH, Emck P, Peters T, Beck E (2009) Reasons for an outstanding plant diversity in the tropical Andes of Southern Ecuador. Landsc Online 12:1–35
Sire (2001) Tabebuia chrysantha. Sire-paquetes Tecnologicos
Soliz C, Villalba R, Argollo J, Christie D, Morales MS, Moya J, Pacajes J (2009) Spatial and temporal variations in Polylepis tarapacana growth across the Bolivian Altiplano during the 20th century. Palaeogeogr Palaeoclimatol Palaeoecol 281:296–308
Stahle DW (1999) Useful strategies for the development of tropical tree-ring chronologies. IAWA 20:249–253
Stahle DW, D’Arrigo RD, Krusic PJ, Cleaveland MK, Cook ER, Allan RJ, Cole J (1998) Experimental dendroclimatic reconstruction of the southern oscillation. Bull Am Meteorol Soc 79:2137–2152
Verheyden A, Helle G, Schleser GH, Dehairs F, Beeckman H, Koedam N (2004) Annual cyclicity in high-resolution stable carbon and oxygen isotope ratios in the wood of the mangrove tree Rhizophora mucronata. Plant Cell Environ 27:1525–1536
Volland-Voigt F, Bräuning A, Ganzhi O (2009) High-resolution dendrometer measurements in a tropical lower montane forest and a dry forest in South Ecuador. Tree Rings Archaeol Climatol Ecol 7:85–88
Wimmer R, Downes GM, Evans R (2002) High-resolution analysis of radial growth and wood density in Eucalyptus nitens, grown under different irrigation regimes. Ann For Sci 59:519–524
Worbes M (1999) Annual growth rings, rainfall-dependent growth and long-term growth patterns of tropical trees from the Caparo Forest Reserve in Venezuela. Ecology 87:391–403
Worbes M (2002) One hundred years of tree-ring research in the tropics—a brief history and a outlook to the future challenges. Dendrochronologia 20:217–231
Acknowledgments
This study was funded within the project BR 1895/14-1 (FOR 816) by the German Research Foundation (DFG). We also thank Ing. Eduardo Cueva for providing phenology data of Tabebuia in the dry forest of Laipuna.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Bräuning.
Contribution to the special issue “Tropical Dendroecology”.
Rights and permissions
About this article
Cite this article
Volland-Voigt, F., Bräuning, A., Ganzhi, O. et al. Radial stem variations of Tabebuia chrysantha (Bignoniaceae) in different tropical forest ecosystems of southern Ecuador. Trees 25, 39–48 (2011). https://doi.org/10.1007/s00468-010-0461-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-010-0461-6