Abstract
Key message
Age-related declines in photosynthesis and water potentials of revegetated Caragana korshinskii in arid desert regions are likely related to both reduced soil water availability and increased hydraulic resistance in xylem.
Abstract
In addition to soil water depletion, the degradation of revegetated sand-binding shrubs may be attributed to specific biological traits of the plant itself, such as aging. To improve our understanding of the physiological basis of observed age-related changes in photosynthetic capacity and water relations in drought-stressed xerophytic shrubs, we conducted in situ field experiments on juvenile, young-mature and relatively old-growth Caragana korshinskii Kom. during the 2013 growing season. Our results revealed significant age-related changes in gas exchange and water-use characteristics, including decreases in the rates of photosynthesis and transpiration, stomatal conductance, shoot water potential and leaf relative water content and increase in water use efficiency with increasing age. We also found that the gas exchange and water relations of all three age classes of C. korshinskii were similarly and significantly affected by soil and atmospheric water availabilities. We conclude that the age-related changes in photosynthetic capacity and water relations which occur in C. korshinskii are likely related to reduced soil water availability and increased hydraulic resistance in xylem, both of which result in a decline in water potential, stomatal conductance and photosynthetic capacity with increasing age of the shrub.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The establishment of sand-binding vegetation is one of the main strategies adopted for ecological restoration in arid desert regions (Le Houérou 2000; Li et al. 2012). Caragana korshinskii Kom. is a dominant xerophytic shrub which has been widely applied for sand stabilization in arid desert regions of China since 1995 (Li et al. 2004). During the past 60 years since the sand-binding vegetation program was established, long-term monitoring has revealed that the populations of the initially planted sand-binding shrubs have degraded and that between 1956 and 2006 shrub coverage decreased from the highest average of approximately 33 % to approximately 9 % (Li et al. 2007). Xu and Li (2006) reported that a marked fall in the groundwater table during this period, due to the harsh environmental conditions and the aggravating competition for water resources by inter- and intra-species of different plants, had resulted in degradation of the population of sand-binding shrubs and, consequently, decreased vegetation coverage.
In addition to the depletion of soil water by revegetated plants, the aging of the plants may be linked to the process of population degradation and coverage reduction. With increasing age, the growth of these sand-binding shrubs tends to slow, and they are more likely to die (Munné-Bosch and Lalueza 2007), which would lead to increased mortality with increasing with stand age (Ryan et al. 1997). Older plants may be more susceptible to insects and pathogens due to their slower growth (Waring 1987) and may respond differently to environmental change (Ryan et al. 1996), such as water stress; for example, the rates of drought mortality are often relatively high for older plants in arid regions (Donovan and Ehleringer 1992; McDowell et al. 2008).
As knowledge of the age-related changes in gas exchange parameters could provide insight into mechanisms underlying plant growth patterns (Thomas and Winner 2002), and the key physiological processes of photosynthesis and stomatal conductance vary with plant age (Steppe et al. 2011), it is important to explore how photosynthetic traits change with increasing age. The results of previous studies from a variety of experimental situations generally indicate that both photosynthesis and stomatal conductance decline as shrubs and trees age (Donovan and Ehleringer 1992; Franco et al. 1994; Grulke and Miller 1994; Kolb and Stone 2000; Mediavilla and Escudero 2004; Niinemets 2002; Schoettle 1994); however, contrasting results to these general trends have also been reported (de Soyza et al. 1996; Donovan and Ehleringer 1991; Hanson et al. 1994; Hutchison et al. 1990). Since water availability is an important factor affecting photosynthesis and the growth and survival of plants, especially in arid and semi-arid regions (Bacelar et al. 2007), it is useful to gain an understanding of the physiological basis for these age-related changes in photosynthesis and water relations of xerophytic shrubs under drought-stress conditions (Liu et al. 2011). Plant–water relation measures, such as water potential, gas exchange and instantaneous water use efficiency (WUE), are common physiological indicators used to determine water stress and its effects on plants (Vertovec et al. 2001). Data on plant–water relations may provide a useful indication of the capacity of the plant to maintain growth, gas exchange and water use during drought (White et al. 2000).
As shrubs grow older, they increase in size, which causes changes in their surrounding environment. Therefore, when studying the effects of age on photosynthesis and water relations of shrubs, these underlying factors are largely inseparable (Donovan and Ehleringer 1992). Environmental factors may combine with plant age to cause differences in photosynthetic capacity; for example, Cavender-Bares and Bazzaz (2000) found that the responses of seedlings, juveniles and mature trees of Quercus rubra to drought were different. Thus, it is important to assess the age-related differences in response to environmental factors.
Although many studies have investigated the effects of age on water-stressed photosynthesis in trees, few of these have compared photosynthetic performance and response to drought in xerophytic shrubs of different ages (Wang MC et al. 2007). Far less is known about changes in gas exchange characteristics as plants age beyond reproductive maturity to old-growth status, and this transition is not manifest by a rapidly appearing marker, such as the onset of reproductive competence which indicates the change from the juvenile phase to the reproductively mature one (Bond 2000).
In this study, we conducted field experiments to investigate whether revegetated C. korshinskii of different age classes differ in photosynthetic capacity and water relations in their natural environment and concomitantly studied their responses to seasonal changes in environmental factors. In contrast to natural shrubland, the age of revegetated shrubs is known based on records on the planting year, which provides a good model for studying age-related changes in shrubs. The objectives of this study were to compare the differences in photosynthesis and water relations of C. korshinskii among different age classes and to investigate the influence of environmental factors on these variations. We also conducted a preliminary analysis of the factors underlying the age-related changes in photosynthetic performances and water relations. Knowledge of the processes underlying the age-related declines in photosynthetic performances would be useful for maintaining the stability of the revegetated shrublands.
Materials and methods
Study area
The study was carried out at the Shapotou Desert Research and Experiment Station of the Chinese Academy of Sciences (37°32′N, 105°02′E, approximately 1339 m a.s.l.), which is located on the southeast fringe of the Tengger desert, northern China. Shapotou lies in the steppe desert zone, with an ecotone which ranges between desert and oasis (Li et al. 2004), and is characterized by large and dense reticulate barchan chains of sand dunes (Li et al. 2007). The mean annual air temperature is 9.6 °C, with minimum and maximum temperatures of −25.1 °C in January and 38.1 °C in July, respectively. The mean annual precipitation is 186 mm, approximately 80 % of which falls in the growing season from May to September. The annual sunshine duration is 3264 h. Annual potential evaporation is approximately 2800 mm (Li et al. 2004). The mean volumetric soil water contents in the 0–300 cm depth layer of the revegetated sites ranges from approximately 0.8 to 3.3 % (Li et al. 2014). The soil substrate is loose and impoverished moving sand and consists predominantly of fine sand particles (diameter 0.05–0.25 mm). The clay content is about 0.2 % (Wang et al. 2006). The soil can thus be classified as a Typic Psammaquent (Berndtsson and Chen 1994), with a field capacity of 4.01 % and a wilting point of 0.61 % (Duan et al. 2004). Depth to the groundwater table is >60 m; therefore, the effective water source for plants is primarily precipitation (Berndtsson and Chen 1994). The predominant native plants are Hedysarum scoparium Fisch., Agriophyllum squarrosum Moq. and Psammochloa cillosa Bor., which are scattered throughout the area with approximately 1 % coverage (Shapotou Desert Research and Experiment Station, Chinese Academy of Sciences 1991).
Plant materials
Caragana korshinskii is a perennial drought-resistant shrub species widely used for sand dune fixation and water conservation (Ma et al. 2004). For this study, shrubs planted in 1989, 2000 and 2010 were chosen for the measurements. The shrubs were planted on an artificially stabilized desert dune. The sand dune was first stabilized by placing straw checkerboards (1 × 1 m) over the ground surface of the dune, and then the shrubs were planted within the checkerboard and grown without irrigation (Wang XP et al. 2007). In May 2013, we selected and marked five individuals in each age class at random for physiological measurements. The shrubs were assigned to the following categories based on planting years and shrub sizes (height and canopy diameter) (Table 1): (1) juvenile, 3-year-old shrub, pre-reproductive; (2) young-mature, 13-year-old shrub, reproductive; (3) relatively old-growth (referred to “old-growth” in the following text), 25-year-old shrub, with old-growth morphological characteristics such as low growth rates. The habitats of each age class of shrubs were similar in terms of soil and geographic conditions (e.g., aspect and slope) and of similar climatic characteristics. The selected shrubs were grown in open sunlight so that they were unshaded by adjacent shrubs and the effect of neighboring shrubs on the water relations of the selected plants was minimized, as shown in Fig. 1.
Precipitation and soil water content monitoring
Precipitation was recorded by the meteorological station at Shapotou Desert Research and Experiment Station. In each revegetated stand, three 2.5-m-long neutron access tubes were installed cater-corner to the quadrat before the experiment (Zhang et al. 2008). The volumetric soil water content (SWC) was measured by neutron probe (CNC503DR; Beijing Nucleon Instrument Co., Beijing, China) at 20-cm intervals between 20 and 80 cm soil depth once per month over the study period. The upper SWC (0–20 cm layer) was determined by TDR probe (Trase-TDR; Soilmoisture Equipment Corp., Goleto, CA) which was inserted near each neutron access tube. The mean volumetric SWCs of 0–40 cm and 40–80 cm were calculated as the weighted averages of all volumetric SWCs measured at the corresponding depths (Van Lier et al. 2006). The air temperature (T a), leaf-to-air vapor pressure deficit (VPD), ambient relative humidity (RH) and intercellular carbon dioxide (CO2) concentration (C I) were measured with a portable, open-flow gas exchange system (model LI-6400; LI-COR Inc., Lincoln, NE).
Physiological measurements
In situ field measurements of the shoot water potential (Ψ), leaf relative water content (RWC), leaf gas exchange and WUE were carried out during the growing season in 2013 from June to September only on uniformly clear sunny days.
The water potential of apical shoots (Ψ) was measured monthly using a pressure chamber (model 3005; PMS Instrument Company, Albany, NY). On each sampling date, shoots of five plants (1 shoot per plant to avoid excessive defoliation) from each of the three age classes were measured at predawn (Ψpd; 30 min before sunrise) and midday (Ψmd; 1230–1330 hours, solar time).
For measurements of the leaf RWC, leaf gas exchange rate and WUE, we used the fourth fully expanded intact leaf at the top of the canopy of the selected plants. At least three leaves per plant were used for the gas exchange measurement and five leaves per plant for the leaf RWC measurement. The leaf RWCs were measured at predawn according to the method of Grammatikopoulos (1999). The leaf gas exchange rates were measured every 2 h from 0600 to 1800 hours during 3–5 consecutive days at the end of each month. The light-saturated net CO2 assimilation rates (A), transpiration rates (E) and stomatal conductances (g s) were measured by the LI-6400XT portable photosynthesis system with a 6-cm2 cuvette (LI-COR Inc.). The maximum net CO2 assimilation rate (A max) was determined between 0900 and 1000 hours from diurnal curves. We measured the light and CO2 response curves in advance, and the value of photosynthetically active radiation was controlled at 1400 μmol m−2 s−1 by a red–blue light source of 6400-02B and the ambient CO2 concentration was set at 400 μmol mol−1. Gas flow rate was set at 400 μmol s−1 to maintain the reference RH at 20–40 %, which is close to ambient humidity. Leaf areas were determined with a LI-3100 area meter (LI-COR Inc.). Instantaneous WUE was calculated by dividing instantaneous values of A by E.
Statistical analysis
We used one-way analysis of variance to test the differences in leaf gas exchange, shoot water potential, RWC and WUE among three age classes within the growing season, respectively, followed by Tukey’s post hoc multiple comparisons. The significance level was set at P = 0.05. The correlation among the measured traits was determined with Pearson correlation analysis. All of the statistical analyses were performed with SPSS version 16.0 (IBM Corp., Armonk, NY) and all curves were fitted using Origin version 8.0 (OriginLab Corp., Northampton, MA).
Results
Precipitation and soil water content
In 2013, total precipitation was 127 mm, which was lower than the mean annual precipitation of 186 mm (Li et al. 2003), and the maximum monthly rainfall occurred in July (57.9 mm, about 45 % of the annual precipitation) (Fig. 2). Total rainfall between June and September was 104 mm, accounting for approximately 80 % of the annual precipitation in that year. However, there were relatively distinct moist (June–July) and dry periods (August–September) during the growing season.
Similar values of volumetric water content in the topsoil (soil depth 0–40 cm) were observed for all three age classes of C. korshinskii (range 1.05–2.28 %). With respect to water content in the deeper soil layer (soil depth 40–80 cm), young-mature and juvenile shrubs had similar values (range 1.49–1.99 %), but old-growth shrubs had significantly lower values of SWC, ranging from 2.1 % in topsoil to approximately 1.3 % in the deeper layer in July. For all age classes, the SWC in the upper soil layer (0–40 cm) showed seasonal fluctuations, whereas they remained fairly constant in the deeper soil layer (40–80 cm) (Fig. 3).
Seasonal changes in volumetric soil water content at a soil depth of 0–40 (a) and 40–80 cm (b) for old-growth, young-mature and juvenile C. korshinskii. Error bars Standard errors of the mean (n = 5), asterisks significantly higher soil water content value for young-mature and juvenile shrubs compared to old-growth shrubs (P < 0.05). § With the exception of soil water content at 40–80 cm soil depth in September, no significant differences in soil water content were observed between young-mature and juvenile shrubs
Shoot water potential and leaf relative water content
Old-growth shrubs exhibited both significantly lower Ψpd and Ψmd than the other two age classes of C. korshinskii (P < 0.05) throughout the entire growing season, with the exception of September. While values of Ψpd and Ψmd were similar between young-mature and juvenile shrubs, higher values were observed in young-mature shrubs on all sample dates, except for Ψmd in July and August, indicating that in general the young-mature shrubs had a superior water status. The three age classes of C. korshinskii showed similar seasonal patterns of Ψpd, with the highest values (−3.2, −1.52 and −1.92 MPa for old-growth, young-mature and juvenile shrubs, respectively) in July and August and the lowest values (−5.52, −4.92 and −5.13 MPa for old-growth, young-mature and juvenile shrubs, respectively) in September. During the dry period from the middle of August to late September, Ψpd rapidly decreased to a minimum of less than −5 MPa for all three age classes, and the Ψmd reached −5.5 MPa for young-mature shrubs and −6 MPa for old-growth and juvenile shrubs. Ψpd and Ψmd showed similar patterns over the growing season (Fig. 4a, b).
Leaf RWC showed the same trend as Ψpd (Fig. 4c), with the highest values monitored in July (0.704, 0.741 and 0.835 for old-growth, young-mature and juvenile shrubs, respectively), followed by marked decrease, and the lowest values (0.479, 0.554 and 0.518, respectively) recorded at the end of the growing season (reduction of 32, 25 and 38 % relative to the July values, respectively). With respect to seasonal changes, although all three age classes of C. korshinskii had similar patterns in leaf RWC, there were significant differences (P < 0.05) between old-growth and juvenile shrubs in each month except for September. However, the differences in RWC between young-mature and juvenile shrubs were only significant (P < 0.05) in July.
Leaf gas exchange
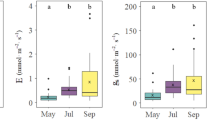
During the growing season, maximum rates of net CO2 assimilation (A max), transpiration (E) and stomatal conductance (g s) occurred at mid-morning for the different age classes of shrubs as determined by the diurnal measurements of these parameters (data not shown). With respect to seasonal changes, there was an apparent trend in leaf gas exchange measurements of C. korshinskii. With the exception of the highest A max value for young-mature shrubs being recorded in August, the A max, g s and E of the three age classes of shrubs were found to be higher in June and July during the period of high precipitation than during the dry period of August and September. At the end of the growing season, A max of old-growth, young-mature and juvenile shrubs reached 4.18, 5.94 and 6.58 µmol m−2 s−1, which is a decline of 61.8, 54.6 and 58.2 %, respectively, compared with their respective maximum (Fig. 5). Except for E in July, young-mature and juvenile showed similar values of A max, g s and E throughout the study period, and their values were significantly higher than those for old-growth shrubs (P < 0.05), while there were no significant differences in g s and E in September between old-growth and young-mature or juvenile shrubs. Both young-mature and juvenile shrubs, however, had lower instantaneous WUE than old-growth shrubs due to the differences in A max and E. For all three age classes, A max and E followed seasonal patterns similar to g s , whereas the seasonal pattern of instantaneous WUE showed the opposite trend. The differences in gas exchange parameters and WUE between old-growth and young-mature or juvenile shrubs were smaller at the end of the growing season than during the middle of the growing season.
Seasonal changes in maximum net CO2 assimilation rate (A max ) (a), transpiration rate (E) (b), stomatal conductance (g s ) (c) and instantaneous water use efficiency (WUE) (d) for old-growth, young-mature and juvenile C. korshinskii. Error bars Standard errors of the mean (n = 5). Different lowercase letters indicate significant differences between the three age classes at P < 0.05
Relationships among gas exchange, plant water potential and environmental factors
The relationships among gas exchange, plant water potential and environmental factors are shown in Tables 2 and 3. For the gas exchange parameters of C. korshinskii, we found a strong relationship between A and E, between A and g s and between E and g s within the three age classes. Although A was significantly correlated to Ψ (r = 0.602, 0.657 and 0.685 for old-growth, young-mature and juvenile C. korshinskii, respectively; P < 0.001) in all three age classes, a relatively weaker but significant correlation was found between g s and Ψ in young-mature and juvenile shrubs (r = 0.326, P < 0.05; r = 0.347, P < 0.05, respectively), and even in old-growth g s did not correlate with Ψ (r = 0.318, P > 0.05). Intercellular CO2 concentration (C I ) was significantly related to g s (P < 0.001) but had relatively weaker relationship with A (r = 0.341, 0.292 and 0.334 for old-growth, young-mature and juvenile C. korshinskii, respectively; P < 0.05), indicating that photosynthesis is regulated through both stomatal control and non-stomatal control in this shrub (Table 2). In terms of the soil water available to the shrubs, A, g s and Ψ were positively correlated with the water content of the topsoil (depth 0–40 cm) (P < 0.05). However, water content in the deeper layer of the soil water (depth 40–80 cm) showed no correlation with A, g s and Ψ within the three age classes (Table 3). As for atmospheric water availability, A, g s and Ψ were significantly (P < 0.001) positively correlated with RH (r = 0.692–0.877) and negatively correlated with VPD (r = −0.816 to −0.465) in all three age classes. T a was strongly related to both A and g s (P < 0.001), but not to Ψ (Table 3). Figure 6 shows the relationships between gas exchange and water status in C. korshinskii: A can be seen to be linearly positively correlated to g s (R 2 = 0.62), and the relationship between A and Ψ is fitted by a reciprocal curve (R 2 = 0.50). In comparison, g s can be fitted by an exponential curve with Ψ (R 2 = 0.48) and by a reciprocal curve with VPD (R 2 = 0.55).
Relationships between net assimilation rate (A) and stomatal conductance (g s) (a), between A and shoot water potential (Ψ) (b), between g s and vapor pressure deficit (VPD) (c) and between g s and Ψ (d) for old-growth, young-mature and juvenile C. korshinskii. Data include both morning and afternoon measurements over all measurement dates (n = 45)
Discussion
Differences in SWCs and water relations in the three age classes of C. korshinskii
The root systems of sand-binding shrubs tend to be shallowly distributed to maximize the efficient use of rainfall (Liu et al. 1991). According to Zhang et al. (2009), the coarse roots of 20-year-old C. korshinskii are concentrated in the topsoil (soil depth 40 cm) in our study area, even though the root system of C. korshinskii can extend to 6 m in depth (Fang et al. 2013). We therefore compared volumetric SWC between topsoil and the deeper soil layer (soil depth 40–80 cm). Our results show that there were seasonal variations in SWC in upper layer, while the SWC remained constant in the deeper layer for all three age classes. One explanation for these findings is that SWC in upper layer is closely linked to precipitation (Li et al. 2007), whereas that in the deeper soil layer is only rarely influenced by precipitation (Li et al. 2004). In our study, shrubs of different age had similar SWCs in the upper soil layer, while old-growth shrubs were associated with a significant lower SWC than the other two classes in the deeper soil layer. This result is in accordance with the suggestion by Li et al. (2007) that at our study site the water content of the topsoil does not change with increasing age of C. korshinskii while that of the deeper soil layer decreases significantly with increasing age of the shrub. In the Shapotou area, rainfall is mainly characterized as a small rainfall pulse, with rain events of 0–10 mm precipitation accounting for 76 % of the total precipitation (Huang and Zhang 2015). In our study, ten small rain events accounted for the 57.9 mm of rain which fell during July (data not shown). According to Kidron (2015), a 60-mm rain event can be expected to infiltrate to soil to a depth of approximately 60–70 cm; in comparison, the small rainfalls noted during our study period cannot be expected to infiltrate down to the deep soil layers (below 40 cm) and will be limited to the shallow soil layers (Li et al. 2004). As fine roots are the primary pathway for water uptake (George and Marschner 1996) and fine-root biomass increases to a maximum when a juvenile plant becomes an adult one (Claus and George 2005), the roots of C. korshinskii may extend downward to extract soil water from the deep layers (Zhang et al. 2009). However, due to plant water uptake in the upper soil layer, minimal infiltration of rainwater into the deeper soil layers and lack of groundwater recharging, the water content of deep soil layers in the areas of older vegetation declines significantly compared to that in areas of younger vegetation (Li et al. 2013). Variations in the vertical distribution of SWC may also indicate that different-aged C. korshinskii shrubs are dependant on different sources of soil water (Su et al. 2014), with young-mature and juvenile shrubs mainly consuming infiltrated precipitation and old-growth shrubs consuming water from both the shallow and deeper (water storage) soil layers. With regard to the areas of older vegetation, SWC has been reported to decrease continuously with soil depth until maintained at a lower level ranging from 1 to 1.5 % (Li et al. 2007), which means that the availability of deep soil water extracted by the older shrubs is limited.
As Ψpd is a good indicator of plant internal water status (Franco et al. 1994), and significant difference in Ψ among the three age classes that occurred at predawn were generally consistent throughout the day, we used Ψpd values to compare the age classes in terms of plant internal water status. Old-growth shrubs maintained a lower Ψ than young-mature and juvenile shrubs both at predawn and midday throughout the study period. This result combined with the lower SWC observed at the deeper soil depth (40–80 cm) in the older vegetation areas indicated that old-growth shrubs in our study were more water stressed under conditions of lower soil water availability (Donovan and Ehleringer 1992), possibly due to progressive consumption of water in the deeper soil layers. However, no significant differences in water potential were observed between young-mature and juvenile shrubs, implying the absence of any substantial difference in soil water availability between these two age classes. Among the three age classes, old-growth shrubs showed the least seasonal changes in Ψpd and RWC, also suggesting that older C. korshinskii plants may partially rely on the lower and more stable water content found in the deeper soil layers less affected by precipitation (Li et al. 2004). Juvenile plants may be exposed to greater spatial and temporal variations in soil water availability and, consequently, experience the greatest seasonal changes in water status, photosynthesis and WUE. Differences in Ψpd and RWC indicated the differential use of water sources among the age classes of C. korshinskii. Seasonally, the decline in Ψpd and RWC was linked to the gradual depletion of soil moisture as the study progressed, with replenishment only by low-intensity rainfall events which occurred at a low frequency.
Differences in photosynthesis and WUE in the three age classes of C. korshinskii
We found significant differences in the photosynthetic parameters and WUE of C. korshinskii among the different age classes. In this study, young-mature and juvenile shrubs had higher A max and g s than old-growth ones, thereby indicating that the younger C. korshinskii plants had higher photosynthetic capacities than the older ones. These results are consistent with earlier studies on other species which generally indicate that both photosynthesis and stomatal conductance declines with increasing plant age (Cavender-Bares and Bazzaz 2000; Day et al. 2001; Donovan and Ehleringer 1991, 1992; Fredericksen et al. 1996; Yoder et al. 1994). Higher A max and g s in young-mature C. korshinskii may be related to its higher water potential, as reflected by the nonlinear relationship of A and g s with Ψ shown in Fig. 6b, d. A higher SWC should be a contributing factor to a higher water potential in young-mature shrubs, as reflected by the significant correlation between SWC and Ψ in Table 3. The differences in photosynthetic parameters found in C. korshinskii among the age classes were more significant during the mid-growing season (P < 0.01) than at the end of growing season (Fig. 5). Young-mature and juvenile shrubs experienced pronounced seasonal fluctuations in photosynthetic values, whereas those of old-growth shrubs did not show much seasonal change. This difference is due partly to the seasonal variations in SWC in the upper soil layer.
The study of WUE may be of particular interest in situations where growth is affected by limitations in water availability (Anyia and Herzog 2004). In our study plant C. korshinskii, WUE had an opposite seasonal trend to that of photosynthetic pattern, with the values increasing with drought progression. Our data suggest that the reduction in g s led to more of a reduction in E than in A, enabling the increase in WUE under dry conditions and indicating that g s controlled E to a greater extent that it did A. The WUE of plants rises with decreasing soil moisture within a certain scope, as has been reported in many experiments on other plant species (Damesin et al. 1998; Dickmann et al. 1992; Korol et al. 1999; Seiler and Johnson 1988; Smit and van den Driessche 1992). A few studies have shown an increase in WUE with increasing plant age, based on either carbon isotope analysis or gas exchange measurements (Donovan and Ehleringer 1992; Mille et al. 1995; Yoder et al. 1994). In general, smaller plants in the juvenile life-history stage are less efficient in water use (Franco et al. 1994), which is supported by our observation that the WUE values of juvenile plants were significant lower than those of the old-growth shrubs (P < 0.05; Fig. 5d). This difference between old-growth and juvenile plants may represent a shift from low WUE during establishment to high WUE as an older adult (Sandquist et al. 1993). Donovan and Ehleringer (1991) suggested that a lower WUE may be favorable for juvenile shrubs during establishment, as they found it was always accompanied by increased growth and proportional allocation to root biomass. Increased allocation to roots would increase the probability of the plant have quicker access to the deeper water stores, thereby increasing the chances of survival during drought. In contrast, older plants might develop a more conservative water-use strategy to cope with soil water limitations (Su et al. 2014). In our study, old-growth C. korshinskii showed a higher WUE than young-mature and juvenile shrubs, indicating that they were well adapted to the dry habitats. Given the low soil water availability, a lower Ψ and higher WUE would be favorable to older shrubs, facilitating the extraction of deep soil water and maximizing water use.
Relationships among gas exchange and plant water potential in C. korshinskii
Our results show that g s was highly correlated with A and E. Accordingly, the observed decrease in the A and E of C. korshinskii followed similar patterns to that in g s for all the age classes. It is well known that g s can be a major rate-determining component of photosynthetic gas exchange (Franco et al. 1994) and that lower values for A are caused primarily by stomatal control to water loss during drought (Llorens et al. 2003), as also shown in the linear relationship between A and g s in Fig. 6a. Cornic (2000) reported that reductions in g s may limit A due to a direct effect on CO2 availability to chloroplasts by limiting diffusion through stomata. Indeed, we found that C I also decreased during the drought period (data not shown). According to Yoder et al. (1994), stomatal control increases with plant age. Ryan and Yoder (1997) proposed that increased stomatal limitation to gas exchange in old trees results from decreased hydraulic conductivity in their longer (or more complex) hydraulic pathways. Along a similar line, we observed that the leaf-specific apparent hydraulic conductance (K L) of old-growth C. korshinskii was significantly lower than that of young-mature or juvenile shrubs (K L = 0.98, 1.63 and 1.72 mmol m−2 s−1 MPa−1 for old-growth, young-mature and juvenile shrubs, respectively; our unpublished data). When shrubs grow older and taller, K L declines, causing stomata to close earlier under drought conditions, and this closure lowers stomatal conductance and photosynthesis (Hubbard et al. 2001). In most cases, both stomatal and metabolic components are responsible for the observed decrease in photosynthetic rates (Ehleringer and Cook 1984). However, the stomatal control of water losses has been identified as an early event in plant response to water deficit (Chaves 1991; Cornic and Massacci 1996). As drought progresses, non-stomatal controls, such as metabolic impairments to photosynthetic apparatus, play an important role (Flexas et al. 2004; Medrano et al. 2009). Our results that older C. korshinskii with lower Ψ had a lower A than younger plants supports this mechanism, with the effects of both increased stomatal control and non-stomatal control on photosynthesis of older shrubs accounting for the difference. In addition, reduced plant water potential itself also contributes to the decrease of A (Brodribb and Field 2000), as indicated in our study by the positive association between A and Ψ (Table 2; Fig. 6b). Another factor that may contribute to increased non-stomatal control is the shrinkage of tissue with decreasing RWC (Hassiotou et al. 2009), and lower RWC in older C. korshinskii plants may lead to reduced plant metabolism (Galmés et al. 2007), as reflected by our results showing that photosynthesis of old-growth plants declined with the sharp decrease in leaf RWC. Figure 6d shows that in C. korshinskii, g s declined nonlinearly as Ψ decreased. This nonlinear relationship may be attributed to the occurrence of cavitation in the xylem (Sperry et al. 2003; Tyree and Ewers 1991). In fact, the reduced Ψ values play a key role in maintaining the plant water status within a security range in order to avoid xylem cavitation (Cochard et al. 1996).
Influence of environmental factors on photosynthesis and plant water potential
Based on our analyses, it would appear that the three age classes of C. korshinskii were similarly and significantly influenced by seasonally changing environmental factors. Our results showed that A, g s and Ψ had a close relationship with SWC (soil depth 0–40 cm), RH and VPD—but no relationship with SWC (soil depth 40–80 cm)—suggesting that the photosynthetic activity and water potential of C. korshinskii shrubs were affected by both soil and atmospheric water availability, but not by the water content in the deeper soil layers. It is therefore possible that during the growing season in the Shapotou area, the main source of water tapped by C. korshinskii was that available in the upper soil layers (Huang and Zhang 2015).
Conclusions
In this study, we found significant differences in photosynthesis and water relations among the three age classes of C. korshinskii Compared with young-mature and juvenile shrubs, old-growth C. korshinskii displayed significantly a lower water potential and lower photosynthetic activity, but exhibited higher WUE. These differences between age classes may be due to older shrubs having a lower availability to soil water and higher hydraulic resistance in the xylem, which resulted in reduced photosynthesis. Higher WUE in old-growth may be attributed to long-term adaptation to drought. Although there were few significant differences in water relations and photosynthesis between young-mature and juvenile shrubs, the former had relatively higher values for most of these measurements. Moreover, water relationship and gas exchange were similarly and significantly affected by the availabilities of soil and atmospheric water in all three age classes of shrubs. We conclude that the age-related changes in photosynthetic capacities and water relations for C. korshinskii are probably related to reduced soil water availability and increased hydraulic resistance in xylem. With lower water availability and reduced carbon assimilation, older C. korshinskii may be more prone to wither and die in our study site. The interpretation of the underlying process in age-related changes of C. korshinskii could provide some insights for the maintaining stability of revegetated shrublands.
Author contribution statement
Jing-Ting Bao, Jin Wang and Xin-Rong Li conceived and designed the experiments; Jing-Ting Bao and Jin Wang performed the experiments, analyzed the data and wrote the paper; Xin-Rong Li and Zhi-Shan Zhang contributed to the writing of the manuscript; Jie-Qiong Su contributed analysis tools. All authors reviewed the paper.
References
Anyia AO, Herzog H (2004) Water use efficiency, leaf area and leaf gas exchange of cowpeas under mid-season drought. Eur J Agron 20:327–339
Bacelar EA, Moutinho-Pereira JM, Gonçalves BC, Ferreira HF, Correia CM (2007) Changes in growth, gas exchange, xylem hydraulic properties and water use efficiency of three olive cultivars under contrasting water availability regimes. Environ Exp Bot 60:183–192
Berndtsson R, Chen HS (1994) Variability of soil water content along a transect in a desert area. J Arid Environ 27:127–139
Bond BJ (2000) Age-related changes in photosynthesis of woody plants. Trends Plant Sci 5:349–353
Brodribb TJ, Field TS (2000) Stem hydraulic supply is linked to leaf photosynthetic capacity: evidence from New Caledonian and Tasmanian rainforests. Plant Cell Environ 23:1381–1388
Cavender-Bares J, Bazzaz FA (2000) Changes in drought response strategies with ontogeny in Quercus rubra: implications for scaling from seedlings to mature trees. Oecologia 124:8–18
Chaves MM (1991) Effects of water deficits on carbon assimilation. J Exp Bot 42:1–16
Claus A, George E (2005) Effect of stand age on fine-root biomass and biomass distribution in three European forest chronosequences. Can J For Res 35:1617–1625
Cochard H, Bréda N, Granier A (1996) Whole tree hydraulic conductance and water loss regulation in Quercus during drought: evidence for stomatal control of embolism? Ann Sci For 53:197–206
Cornic G (2000) Drought stress inhibits photosynthesis by decreasing stomatal aperture—not by affecting ATP synthesis. Trends Plant Sci 5:187–188
Cornic G, Massacci A (1996) Leaf photosynthesis under drought stress. In: Baker NR (ed) Photosynthesis and the Environment. Kluwer Academic Publishers, Dordrecht, pp 347–366
Damesin C, Rambal S, Joffre R (1998) Seasonal and annual changes in leaf δ13C in two co-occurring Mediterranean oaks: relations to leaf growth and drought progression. Funct Ecol 12:778–785
Day ME, Greenwood MS, White AS (2001) Age-related changes in foliar morphology and physiology in red spruce and their influence on declining photosynthetic rates and productivity with tree age. Tree Physiol 21:1195–1204
de Soyza AG, Franco AC, Virginia RA, Reynolds JF, Whitford WG (1996) Effects of plant size on photosynthesis and water relations in the desert shrub Prosopis glandulosa (Fabaceae). Am J Bot 83:99–105
Dickmann DI, Liu Z, Nguyen PV, Pregitzer KS (1992) Photosynthesis, water relations and growth of two hybrid Populus genotypes during a severe drought. Can J Forest Res 22:1094–1106
Donovan LA, Ehleringer JR (1991) Ecophysiological differences among juvenile and reproductive plants of several woody species. Oecologia 86:594–597
Donovan LA, Ehleringer JR (1992) Contrasting water-use patterns among size and life-history classes of a semi-arid shrub. Funct Ecol 6:482–488
Duan ZH, Xiao HL, Li XR, Dong ZB, Wang G (2004) Evolution of soil properties on stabilized sands in the Tengger Desert, China. Geomorphology 59:237–246
Ehleringer J, Cook C (1984) Photosynthesis in Encelia farinosa Gray in response to decreasing leaf water potential. Plant Physiol 75:688–693
Fang XW, Turner NC, Xu DH, Jin Y, He J, Li FM (2013) Limits to the height growth of Caragana korshinskii resprouts. Tree Physiol 33:275–284
Flexas J, Bota J, Cifre J, Escalona JM, Galmés J, Gulías J, Lefi EK, Martínez-Cañellas SF, Moreno MT, Ribas-Carbó M, Riera D, Sampol B, Medrano H (2004) Understanding down-regulation of photosynthesis under drought stress: future prospects and searching for physiological tools for irrigation management. Ann Appl Biol 144:273–283
Franco AC, de Soyza AG, Virginia RA, Reynolds JF, Whitford WG (1994) Effects of plant size and water relations on gas exchange and growth of the desert shrub Larrea tridentata. Oecologia 97:171–178
Fredericksen TS, Steiner KC, Skelly JM, Joyce BJ, Kolb TE, Kouterick KB, Ferdinand JA (1996) Diel and seasonal patterns of leaf gas exchange and xylem water potentials of different-sized Prunus serotina Ehrh. trees. Forest Sci 42:359–365
Galmés J, Flexas J, Savé R, Medrano Hipólito (2007) Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant Soil 290:139–155
George E, Marschner H (1996) Nutrient uptake by roots of forest trees. Z Pflanz Bodenkunde 159:11–21
Grammatikopoulos G (1999) Mechanisms for drought tolerance in two Mediterranean seasonal dimorphic shrubs. Funct Plant Biol 26:587–593
Grulke NE, Miller PR (1994) Changes in gas exchange characteristics during the life span of giant sequoia: implications for response to current and future concentrations of atmospheric ozone. Tree Physiol 14:659–668
Hanson PJ, Samuelson LJ, Wullschleger SD, Tabberer TA, Edwards GS (1994) Seasonal patterns of light-saturated photosynthesis and leaf conductance for mature and seedling Quercus rubra L. foliage: differential sensitivity to ozone exposure. Tree Physiol 14:1351–1366
Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR (2009) Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. J Exp Bot 60:2303–2314
Huang L, Zhang Z (2015) Stable isotopic analysis on water utilization of two xerophytic shrubs in a revegetated desert area: Tengger Desert, China. Water 7:1030–1045
Hubbard RM, Ryan MG, Stiller V, Sperry JS (2001) Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ 24:113–121
Hutchison KW, Sherman CD, Weber J, Smith SS, Singer PB, Greenwood MS (1990) Maturation in larch, II. Effects of age on photosynthesis and gene expression in developing foliage. Plant Physiol 94:1308–1315
Kidron GJ (2015) Dune crests serve as preferential habitats for perennial plants during frequent drought years. J Hydrol 522:295–304
Kolb TE, Stone JE (2000) Differences in leaf gas exchange and water relations among species and tree sizes in an Arizona pine-oak forest. Tree Physiol 20:1–12
Korol RL, Kirschbaum MUF, Farquhar GD, Jeffreys M (1999) Effects of water status and soil fertility on the C-isotope signature in Pinus radiate. Tree Physiol 19:551–562
Le Houérou HN (2000) Restoration and rehabilitation of arid and semiarid Mediterranean ecosystems in North Africa and West Asia: a review. Arid Soil Res Rehab 14:3–14
Li XR, Zhou HY, Wang XP, Zhu YG, O’Conner PJ (2003) The effects of sand stabilization and revegetation on cryptogam species diversity and soil fertility in the Tengger desert, Northern China. Plant Soil 251:237–245
Li XR, Ma FY, Xiao HL, Wang XP, Kim KC (2004) Long-term effects of revegetation on soil water content of sand dunes in arid region of Northern China. J Arid Environ 57:1–16
Li XR, Kong DS, Tan HJ, Wang XP (2007) Changes in soil and vegetation following stabilisation of dunes in the southeastern fringe of the Tengger Desert, China. Plant Soil 300:221–231
Li XR, Zhang P, Su YG, Jia RL (2012) Carbon fixation by biological soil crusts following revegetation of sand dunes in arid desert regions of China: a four-year field study. Catena 97:119–126
Li X, Zhang Z, Huang I, Wang X (2013) Review of the ecohydrological processes and feedback mechanisms controlling sand-binding vegetation systems in sandy desert regions of China. Chin Sci Bull 58:1483–1496
Li XR, Zhang ZS, Tan HJ, Gao YH, Liu LC, Wang XP (2014) Ecological restoration and recovery in the wind-blown sand hazard areas of northern China: relationship between soil water and carrying capacity for vegetation in the Tengger Desert. Sci China Life Sci 57:539–548
Liu YX, Li YJ, Yang XL (1991) Root system of psammophytes. In: Studies of sand dune control in Shapotou area of Tengger Desert (in collection no. 2) (in Chinese with English abstract). Ningxia People’s Publishing House, Yinchuan, pp 185–209
Liu CC, Liu YG, Guo K, Li GQ, Zheng YR, Yu LF, Yang R (2011) Comparative ecophysiological responses to drought of two shrub and four tree species from karst habitats of southwestern China. Trees 25:537–549
Llorens L, Penuelas J, Filella I (2003) Diurnal and seasonal variations in the photosynthetic performance and water relations of two co-occurring Mediterranean shrubs, Erica multiflora and Globularia alypum. Physiol Plantarum 118:84–95
Ma CC, Gao YB, Guo HY, Wang JL (2004) Photosynthesis, transpiration, and water use efficiency of Caragana microphylla, C. intermedia, and C. korshinskii. Photosynthetica 42:65–70
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
Mediavilla S, Escudero A (2004) Stomatal responses to drought of mature trees and seedlings of two co-occurring Mediterranean oaks. Forest Ecol Manag 187:281–294
Medrano H, Flexas J, Galmés J (2009) Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 317:17–29
Mille PM, Eddleman LE, Miller JM (1995) Juniperusoccidentalis juvenile foliage: advantages and disadvantages for a stress-tolerant, invasive conifer. Can J Forest Res 25:470–479
Munné-Bosch S, Lalueza P (2007) Age-related changes in oxidative stress markers and abscisic acid levels in a drought-tolerant shrub, Cistus clusii grown under Mediterranean field conditions. Planta 225:1039–1049
Niinemets Ü (2002) Stomatal conductance alone does not explain the decline in foliar photosynthetic rates with increasing tree age and size in Picea abies and Pinus sylvestris. Tree Physiol 22:515–535
Ryan MG, Yoder BJ (1997) Hydraulic limits to tree height and tree growth. Bioscience 47:235–242
Ryan MG, McMurtrie RM, Ågren GI, Hunt ER, Aber JD Jr, Friend AD, Rastetter EB, Pulliam WM (1996) Comparing models of ecosystem function for temperate conifer forests. II. Simulations of the effect of climate change. In: Melillo JM, Ågren GI, Breymeyer A (eds) Effects of climate change on production and decomposition in coniferous forests and grasslands (SCOPE). John Wiley and Sons, London, pp 363–387
Ryan MG, Binkley D, Fownes JH (1997) Age-related decline in forest productivity: pattern and process. Adv Ecol Res 27:213–262
Sandquist DR, Schuster WSF, Donovan LA, Phillips SL, Ehleringer JR (1993) Differences in carbon isotope discrimination between seedlings and adults of southwestern desert perennial plants. Southwest Nat 38:212–217
Schoettle AW (1994) Influence of tree size on shoot structure and physiology of Pinus contorta and Pinus aristata. Tree Physiol 14:1055–1068
Seiler JR, Johnson JD (1988) Physiological morphological responses of three half-sib families of loblolly pine to water-stress conditioning. Forest Sci 34:487–495
Shapotou Desert Research and Experiment Station, Chinese Academy of Sciences (1991) Study on shifting sand control in Shapotou region of Tengger Desert (2). Ningxia People’s Publishing House, Yingchuan (in Chinese, English abstract)
Smit J, van den Driessche R (1992) Root growth and water use efficiency of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) and Lodgepole pine (Pinus contorta Dougl.) seedlings. Tree Physiol 11:401–410
Sperry JS, Stiller V, Hacke UG (2003) Xylem hydraulics and the soil–plant–atmosphere continuum: opportunities and unresolved issues. Agron J 95:1362–1370
Steppe K, Niinemets Ü, Teskey RO (2011) Tree size-and age-related changes in leaf physiology and their influence on carbon gain. In: Meinzer FC, Lachenbruch B, Dawson TE (eds) Size-and age-related changes in tree structure and function. Springer SBM, Dordrecht, pp 235–253
Su H, Li Y, Liu W, Xu H, Sun OJ (2014) Changes in water use with growth in Ulmus pumila in semiarid sandy land of northern China. Trees 28:41–52
Thomas SC, Winner WE (2002) Photosynthetic differences between saplings and adult trees: an integration of field results by meta-analysis. Tree Physiol 22:117–127
Tyree MT, Ewers FW (1991) The hydraulic architecture of trees and other woody plants. New Phytol 119:345–360
Van Lier QJ, Metselaar K, Van Dam JC (2006) Root water extraction and limiting soil hydraulic conditions estimated by numerical simulation. Vadose Zone J 5:1264–1277
Vertovec M, Sakçali S, Ozturk M, Salleo S, Giacomich P, Feoli E, Nardini A (2001) Diagnosing plant water status as a tool for quantifying water stress on a regional basis in Mediterranean drylands. Ann For Sci 58:113–125
Wang MC, Wang JX, Shi QH, Zhang JS (2007) Photosynthesis and water use efficiency of Platycladus Orientalis and Robinia Pseudoacacia saplings under steady soil water stress during different stages of their annual growth period. J Integr Plant Biol 49:1470–1477
Wang XP, Li XR, Xiao HL, Pan YX (2006) Evolutionary characteristics of the artificially revegetated shrub ecosystem in the Tengger Desert, Northern China. Ecol Res 21:415–424
Wang XP, Li XR, Xiao HL, Berndtsson R, Pan YX (2007) Effects of surface characteristics on infiltration patterns in an arid shrub desert. Hydrol Process 21:72–79
Waring RH (1987) Characteristics of trees predisposed to die. Bioscience 37:569–574
White DA, Turner NC, Galbraith JH (2000) Leaf water relations and stomatal behavior of four allopatric Eucalyptus species planted in Mediterranean southwestern Australia. Tree Physiol 20:1157–1165
Xu H, Li Y (2006) Water-use strategy of three central Asian desert shrubs and their responses to rain pulse events. Plant Soil 285:5–17
Yoder BJ, Ryan MG, Waring RH, Schoettle AW, Kaufmann MR (1994) Evidence of reduced photosynthetic rates in old trees. Forest Sci 40:513–527
Zhang ZS, Li XR, Wang T, Wang XP, Xue QW, Liu LC (2008) Distribution and seasonal dynamics of roots in a revegetated stand of Artemisia ordosica Kracsh. in the Tengger desert (North China). Arid Land Res Manag 22:195–211
Zhang ZS, Li XR, Liu LC, Jia RL, Zhang JG, Wang T (2009) Distribution, biomass, and dynamics of roots in a revegetated stand of Caragana korshinskii in the Tengger Desert, northwestern China. J Plant Res 122:109–119
Acknowledgments
We would like to thank two anonymous reviewers whose suggestions greatly improved the manuscript. This study was jointly supported by the State Key Development Program for Basic Research of China (Grant No. 2013CB429906), the National Natural Science Foundation of China (41201250, 41401334) and the Natural Science Foundation of Gansu Province, China (1107RJYA068 and 145RJZA168).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by E. Liang.
Rights and permissions
About this article
Cite this article
Bao, JT., Wang, J., Li, XR. et al. Age-related changes in photosynthesis and water relations of revegetated Caragana korshinskii in the Tengger desert, Northern China. Trees 29, 1749–1760 (2015). https://doi.org/10.1007/s00468-015-1255-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1255-7