Abstract
Compared with our knowledge of senescence in annuals and biennials, little is known about age-related changes in perennials. To get new insights into the mechanisms underlying aging in perennials, we measured oxidative stress markers in leaves and organelles, together with abscisic acid levels in leaves of 2- and 7-year-old Cistus clusii dunal plants grown under Mediterranean field conditions. Recently emerged leaves, which either appeared during autumn or spring, were compared to evaluate the effects of environmental constraints on oxidative stress and abscisic acid accumulation as plants aged. Plant aging led to an enhanced oxidation of α-tocopherol and ascorbate, increased lipid peroxidation and reduced PSII efficiency in leaves during the more stressful conditions of spring and summer, but not during autumn. Analyses of lipid peroxidation in organelles isolated from the same leaves revealed that oxidative stress occurred both in chloroplasts and mitochondria. Although both plant groups showed similar leaf water and nitrogen contents throughout the study, abscisic acid levels were markedly higher (up to 75%) in 7-year-old plants compared to 2-year-old plants throughout the study. It is concluded that (a) meristematic tissues of C. clusii maintain the capacity to make new leaves with no symptoms of oxidative stress for several years, unless these leaves are exposed to environmental constraints, (b) leaves of oldest plants show higher oxidative stress than those of young plants when exposed to adverse climatic conditions, thus supporting the idea that the oxidative stress associated with aging is due at least partly to extrinsic factors, (c) at the subcellular level, age-induced oxidative stress occurs both in chloroplasts and mitochondria, and (d) even in the absence of environmental stress, newly emerged leaves accumulate higher amounts of ABA as plants age.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aging has received considerable attention in biomedicine, but little is known about the regulatory mechanisms responsible for the aging not associated with senescence in plants, particularly in perennials. In plant development, pre-reproductive plants are considered juveniles; the onset of flowering and seed production marks a transition phase, and fully reproductive plants are considered mature (Bond 2000). Perennials maintain the capacity to develop new leaves and grow throughout their life until they enter a developmentally controlled senescing process that inevitably leads to plant death. With increasing age and size, however, growth tends to slow and perennials are more likely to die. Aging has been classically defined as the accumulation of changes in plant development responsible for slow, progressive, and sequential alterations that accompany the plants as they age (Noodén and Guiamet 1996; Dangl et al. 2000). Disentangling the mechanisms by which plants age is still a matter of debate today (Morgan 2003; Peñuelas 2005).
Recent studies indicate that physiological burdens, such as demands on water and nutrient supply, may be responsible for reduced growth as plants age (Mencuccini et al. 2005). Aside from biotic and abiotic stresses limiting plant growth as plant age, it is also possible that intrinsic changes in the shoot meristems could occur through repeated cell divisions and could be fixed during plant development, thereby affecting foliar gas characteristics, such as stomatal conductance and photosynthetic rates in the leaves and shoots that originated from these cells (Day et al. 2002). In both cases, characterization of oxidative stress markers may help better understanding the mechanisms underlying this phenomenon. Aside from measurements on the extent of lipid peroxidation, estimations of the redox state of low-molecular-weight antioxidants may allow better understanding age-related oxidative processes in plants. Although levels of antioxidants indicate the potential extent of antioxidative protection and the balance between their synthesis, oxidation and regeneration, their redox state indicates the extent of oxidative load toward these compounds and provides us with a reliable estimation of oxidative stress in the cell (Munné-Bosch and Alegre 2003).
In animals, age-associated disorders in the cell are believed to be connected with the time-dependent shift in the antioxidant/prooxidant balance in favour of oxidative stress, and it has been suggested that mitochondria are the target organelles for oxidative stress in age-associated changes observed in animals (Ashok and Ali 1999; Rustin et al. 2000). Photosynthesis generally decreases in leaves as plants age (Bond 2000, and references therein), and chloroplasts have been proposed as one of the target organelles of oxidative stress as plants age (Munné-Bosch and Alegre 2002a). However, the relative contribution of mitochondria and chloroplasts in the oxidative stress associated with aging in perennials is still unknown.
The aim of the present study was to characterize (a) the oxidative stress associated with aging in a drought-tolerant shrub, C. clusii grown under Mediterranean field conditions, (b) the contribution of chloroplasts and mitochondria in the aging process, and (c) the mechanism(s) underlying the aging process in perennials. To address these questions, we measured simultaneously oxidative stress markers (levels of photosynthetic pigments, reduced and oxidized forms of α-tocopherol and ascorbate, the F v/F m ratio and the extent of lipid peroxidation in leaves, chloroplasts and mitochondria) in parallel with markers of water and nutrient stress, and abscisic acid (ABA) and sucrose levels in recently emerged leaves of 2- and 7-year-old plants of C. clusii grown under Mediterranean field conditions.
Materials and methods
Plant material, growth conditions and sampling
The study was conducted using two groups of Cistus clusii Dunal plants of different ages: (a) 2-year-old plants, which can be considered young since the formation of flowers was observed once; and (b) 7-year-old plants, which can be considered mature non-senescing plants (the formation of flowers has been observed several times and the plants continue flowering; Bond 2000). Two-year-old plants were obtained from seeds collected from the other plant group as described below, thus studies were conducted on the same population of C. clusii.
Plants were obtained as follows. C. clusii seeds were germinated and grown in 0.5-l pots containing a mixture of soil:peat:perlite (1:1:1, by vol). Plants were maintained in a greenhouse with a controlled temperature (24/18°C, day/night) and were watered twice a week, once with tap water and once with Hoagland’s solution. After 1 year of growth, plants were transplanted during the spring of 1999 to the experimental fields of the University of Barcelona (Barcelona, Spain) and 16 plants per plot were distributed homogeneously in a square, 1 m apart, so all plants had the same orientation to sun. After 5 years of growth, seeds were obtained from these plants and grown as described before. After 1 year of growth, plants were transplanted during the spring of 2004 to the same experimental fields and 16 plants per plot were distributed as described for the other plant group. The experimental area consisted of two plots of 4.5 m2 each of calcic Luvisol (FAO) separated by 2 m only. Before the plants were transferred, the soil was ploughed and treated with N: P: K (1:1:1) fertilizer at the rate of 100 kg ha−1 and an analysis of mineral composition of soil was carried out annually to avoid mineral deficiency in plants.
Lipid peroxidation in leaves, chloroplasts and mitochondria, chlorophylls and carotenoids, the levels of reduced and oxidized α-tocopherol and ascorbate, the F v/F m ratio, RWC, C and N contents, and ABA and sucrose contents were analyzed simultaneously in the two age groups during October and November of 2004, and during April, May and June of 2005 from samples collected at midday (at maximum incident diurnal PPFD) in the field. The leaves collected during October–November appeared during mid September, while the leaves collected during April–June appeared during the beginnings of March. Recently emerged leaves (situated up to 15 cm from the apex of branches) from each group of plants were selected for all the measurements. For analyses of MDA, photosynthetic pigments and antioxidants, ABA and sucrose contents in leaves, samples were collected at midday, immediately frozen in liquid nitrogen and stored at −80°C until analyses. For analyses of MDA in chloroplasts and mitochondria, leaves were collected at midday and were immediately subjected to cell fractionation.
Plants received water exclusively from rainfall during the growth and study period. Environmental conditions were monitored at 5-min intervals throughout the day with a weather station (Delta-T Devices, Newmarket, UK). Photosynthetically active photon flux density (PPFD) was measured with a quantum sensor (Li-Cor, Lincoln, Nebraska, USA). Air temperature and relative humidity were measured with a Vaisala thermohygrometer (Vaisala, Helsinki, Finland). The vapor pressure deficit was determined from relative humidity and air temperature data following Nobel (1991). Precipitation was measured with a standard rain gauge. The environmental conditions during the experiment were typical of the Mediterranean climate but characterized by an unexpected dry spring, with very low precipitation (3.2 mm) during April (Table 1).
Estimation of lipid peroxidation in leaves and organelles
The extent of lipid peroxidation was estimated by measuring the amount of MDA in leaves, chloroplasts and mitochondria by the method described by Hodges et al. (1999), which takes into account the possible influence of interfering compounds in the thiobarbituric acid-reactive substances (TBARS) assay.
Chloroplasts were isolated according to Munné-Bosch and Alegre (2001). After grinding the sample in isolation buffer [0.5–1.5 M sorbitol depending on the plant water status, 50 mM tricine, 1 mM dithiothreitol, 1 mM MgCl2, 1 mM citric acid, 1 mM butylated hydroxytoluene (BHT), 0.1% (w/v) bovine serum albumin, pH 7.8], the homogenate was filtered through four layers of cheese cloth and centrifuged at 2,500g for 4 min. The pellet was resuspended in isolation buffer and then centrifuged at 200g for 1 min. The chloroplasts in the supernatant were sedimented by centrifugation at 2,500g for 4 min. Chloroplasts were purified by resuspending the pellets in isolation buffer, layering onto 10 ml of 25% (v/v) percoll (in isolation buffer), and centrifuging at 15,800g for 20 min. The chloroplast pellets were resuspended in isolation buffer, centrifuged at 2,500g for 4 min, and used immediately.
Mitochondria were isolated according to Fan et al. (1999). Leaves were homogenized with a mortar and pestle in prechilled grinding medium containing 0.3 M mannitol, 50 mM Mes, pH 7.2, 1 mM EDTA, 1 mM MgCl2, 0.2% (w/v) defatted BSA, 0.5% (w/v) PVP-400, 4 mM Cys, and 10 mM β-mercaptoethanol. The homogenates were filtered through four layers of cheesecloth and centrifuged at 3,300g for 20 min. The pellet was suspended in resuspension medium I containing 0.3 M mannitol, 20 mM Mes, pH 7.2, 2 mM potassium phosphate, 1 mM EDTA, 0.1% (w/v) defatted BSA, 2 mM MgCl2, and 14 mM β-mercaptoethanol. The suspension was loaded onto a discontinuous gradient composed of 5 ml of 47%, 6 ml of 26%, and 3 ml of 21% (v/v) percoll. The gradients were centrifuged at 58,500g for 45 min in a swinging-bucket rotor. The mitochondrial band, located at the interface between the 26 and 47% percoll layers, was removed and diluted with an equal volume of resuspension medium II containing 0.3 M mannitol, 20 mM Mes, pH 7.2, 1 mM EDTA, 0.1% (w/v) defatted BSA, 2 mM MgCl2, and 2 mM DTT. The diluted mitochondria were loaded onto a second percoll gradient composed of 7.5 ml of 47% and 7.5 ml of 26% (v/v) percoll prepared as in the first gradient. The gradient was centrifuged at 58,500g for 30 min. The mitochondrial band, located at the interface between the 26 and 47% (v/v) percoll layers, was collected and diluted with 10 vol of medium II. The mitochondria were pelleted by centrifugation at 18,800g for 5 min and resuspended in 1 ml of medium II. The purity of chloroplasts and mitochondria was determined by assaying amounts and activities of appropriate markers and confirmed further by microscopic observation as described (Munné-Bosch and Alegre 2001; Keech et al. 2005). Chloroplasts- and mitochondria-enriched fractions did not show detectable activities of NADPH-cytochrome c reductase and vanadate-sensitive ATPase, which are markers of endoplasmic reticulum and plasma membrane, respectively. Furthermore, chloroplasts-enriched fractions did not show detectable activities of cytochrome c oxidase, which is a marker of mitochondrion. Mitochondria showed very small thylakoid contamination as shown by the chlorophyll contents, which averaged 3.2 mg ml−1 for the crude extract and 4.3 μg ml−1 for the purified mitochondria. To give MDA values per dry weight (DW) of organelles, a separate aliquot of chloroplasts- and mitochondria-enriched fractions was used to determine DW by oven-drying the samples to constant weight at 80°C.
For MDA analyses, leaf samples (500 mg FW), chloroplasts- and mitochondria-enriched fractions (50 mg DW) were extracted with 80:20 (v/v) ethanol/water using ultrasonication (Vibra-Cell Ultrasonic Processor, Sonics & Materials Inc., Canbury, CT, USA). After centrifuging at 3000g for 10 min at 4oC, the pellet was re-extracted twice with the same solvent. Supernatants were pooled and an aliquot of appropriately diluted sample was added to a test tube with an equal volume of either (a) −TBA solution comprised of 20% (w/v) trichloroacetic acid and 0.01% (w/v) BHT, or (b) +TBA solution containing the above plus 0.65% (w/v) TBA. Samples were heated at 95oC for 25 min, and after cooling; absorbance was read at 440, 532, and 600 nm. MDA equivalents (nmol ml−1) were calculated as 106 × [(A−B)/15,7000], where A = [(Abs 532 + TBA)−(Abs 600 + TBA)−(Abs 532−TBA−Abs 600−TBA)], and B = [(Abs 440 + TBA−Abs 600 + TBA) × 0.0571)] (Hodges et al. 1999).
Chlorophyll fluorescence measurements
The maximum efficiency of PSII photochemistry (F v/F m) was calculated from chlorophyll fluorescence data obtained from leaves exposed to darkness for 2 h with a portable fluorimeter mini-PAM (Walz, Effeltrich, Germany), by using the equations described by Van Kooten and Snel (1990).
Pigment determination
For determination of photosynthetic pigments, leaf samples (100 mg FW) were ground in a mortar and extracted repeatedly with 80% (w/w) acetone using ultrasonication (Vibra-Cell Ultrasonic Processor). The resulting extracts were immediately assayed spectrophotometrically. Specific absorption coefficients of chlorophylls and total carotenoids reported by Lichtenthaler and Wellburn (1983) were used.
Determination of reduced and oxidized forms of antioxidants
The extraction and analysis of α-tocopherol and its oxidation product, α-tocopherol quinone, was carried out as described (Munné-Bosch and Alegre 2003). In short, leaf samples (300 mg FW) were ground in liquid nitrogen and repeatedly extracted with n-hexane containing 1 μg ml−1 BHT using ultrasonication (Vibra-Cell Ultrasonic Processor). α-Tocopherol and α-tocopherol quinone were analyzed by HPLC using a Partisil-10 ODS column (Sharlau, St Cugat, Spain). The solvents consisted of (a) methanol/water (95:5, v/v) and (b) methanol at a flow rate of 1 ml min−1. The gradient used was: 0–10 min 100% A, 10–15 min decreasing to 0% A, 15–20 min 0% A, 20–23 min increasing to 100% A, and 23–28 min 100% A. α-Tocopherol and α-tocopherol quinone were quantified through their absorbance at 283 and 256 nm, respectively (diode array detector 1000S, Applied Biosystems, Foster City, CA, USA). Compounds were identified by their characteristic spectra and by coelution with authentic standards, which were obtained from Sigma (St Cugat, Spain) and Prof. Kazimierz Strzalka (Jagellonian University, Krakow, Poland).
The extraction and HPLC analysis of reduced and oxidized ascorbate was performed as described (Munné-Bosch and Alegre 2003). In short, leaf samples (100 mg FW) were ground in liquid nitrogen and repeatedly extracted with ice-cold extraction buffer (40% [v/v] methanol, 0.75% [w/v] m-phosphoric acid, 16.7 mM oxalic acid, 0.127 mM diethylenetriaminepentaacetic acid) using ultrasonication (Vibra-Cell Ultrasonic Processor). After centrifugation, 0.1 ml of the supernatant was transferred to 0.9 ml of the mobile phase (24.25 mM Na-acetate/ acetic acid, pH 4.8; 0.1 mM diethylenetriaminepentaacetic acid; 0.015% [w/v] m-phosphoric acid; 0.04% [w/v] octylamine; 15% [v/v] methanol) for determination of reduced ascorbate. For determination of total ascorbate (reduced plus oxidized) 0.1 ml of the supernatant was incubated for 10 min at room temperature in darkness with 0.25 ml of 2% (w/v) dithiothreitol and 0.5 ml of 200 mM NaHCO3. The reaction was stopped by adding 0.25 ml of 2% (v/v) sulfuric acid and 0.8 ml of the mobile phase. Ascorbic acid was isocratically separated on a Spherisorb ODS C8 column (Teknokroma, St Cugat, Spain) at a flow rate of 0.8 ml min−1. Detection was carried out at 255 nm (Diode array detector 1000S, Applied Biosystems). Ascorbic acid was identified by its characteristic spectrum and by coelution with an authentic standard from Sigma (Steinheim, Germany).
Plant water status and elemental analyses
Relative leaf water content (RWC) was determined as 100 × (FW-DW)/(TW-DW), where FW is the fresh matter, TW is the turgid matter after re-hydrating the leaves for 24 h at 4oC in darkness, and DW is the dry matter after oven-drying the leaves for 24 h at 80oC. Total C and N concentrations in leaves were measured by the Dumas elemental analysis method by using a protein nitrogen analyzer NA2100 (Thermo, Milan, Italy).
ABA and sucrose levels
The extraction and analysis of ABA was carried out essentially as described (López-Carbonell and Jáuregui 2005). Leaf samples (100 mg FW) were ground in liquid nitrogen and repeatedly extracted with acetone/water/acetic acid (80:19:1, by vol) using ultrasonication (Vibra-Cell Ultrasonic Processor). The supernatants were combined and dried in a rotavapor until the aqueous fractions were obtained; these fractions were dried completely under a nitrogen stream. The extracts were immediately reconstituted in solvent A, filtered through a 0.45 μm PTFE filter (Waters, Milford, MA, USA) and injected into the LC–MS/MS system. The HPLC system consisted of a Perkin Elmer Series 200 (Norwalk, CT, USA) quaternary pump equipped with an autosampler and an UV detector. For the analysis of the extracts, a Luna C18 Phenomenex (Torrance, CA, USA) column (50 × 2.1 mm, 3.5 μm) equipped with a Securityguard C18 Phenomenex (4 × 3 mm, i.d.) was used. Gradient elution was done with water with 0.05% acetic acid (solvent A) and acetonitrile (solvent B) at a constant flow rate of 0.4 ml min−1. A linear gradient profile with the following proportions of solvent A was applied (t (min), %A): (0, 85), (5, 0), (5.2, 0), (6, 85), (10, 85). MS/MS experiments were performed on an API 3000 triple quadrupole mass spectrometer (PE Sciex, Concord, Ont, Canada). All the analyses were performed using the Turbo Ionspray source in negative ion mode with the following settings: capillary voltage −3500 V, nebulizer gas (N2) 10 (arbitrary units), curtain gas (N2) 12 (arbitrary units), collision gas (N2) 4 (arbitrary units), declustering potential (DP) −55 V, focusing potential −250 V, entrance potential 10 V, collision energy (CE) −30 V; drying gas (N2) was heated to 400°C and introduced at a flow-rate of 5,000 cm3 min−1. In negative mode, the spectrum for ABA gave the deprotonated molecule [M–H]−. MRM acquisition was done monitoring the 263/153 transition with a dwell time of 2,000 ms. Quantification used MS/MS (MRM method, MRM). The MRM mode was required because many compounds could present the same nominal molecular mass, but the combination of the parent mass and unique fragment ions was used to selectively monitor ABA (López-Carbonell and Jáuregui 2005).
The extraction and HPLC analysis of sucrose was essentially performed as described (Gerrits et al. 2001). Leaf samples (50 mg FW) were ground in liquid nitrogen and repeatedly extracted with 80% [v/v] ethanol at 80°C. After centrifugation, the supernatants were pooled and dried completely under a nitrogen stream. The extracts were immediately reconstituted in 1 ml of water and passed through a Sep-Pak Plus cartridge (Waters, Milford, MA, USA). Sucrose was isocratically separated on an Aminex HPX-87C column (Bio-Rad, Hercules, CA, USA) using water as a solvent at a flow rate of 0.6 ml min−1. Detection was carried out with a differential refractometer (Knauer, Berlin, Germany) with the cell at 40°C. Sucrose from Sigma (Steinheim, Germany) was used for quantification.
Statistical analyses
Statistical differences between age groups were analyzed by the Student t-test using the SPSS package (Chicago, IL). Differences were considered significant when P < 0.05.
Results
Plant aging increases oxidative stress under adverse climatic conditions
Plants were exposed to adverse climatic conditions during spring and summer, including exposure to drought and high light stress, as indicated by the low precipitation and high diurnal PPFD values during this period (Table 1). Chlorophyll and carotenoid levels changed in parallel throughout the study and were always significantly smaller in 7-year-old plants than in 2-year-old plants, both during autumn and the more stressful period of spring and summer (Fig. 1). 7-year-old plants displayed significantly smaller α-tocopherol levels in leaves throughout the study, which were between 22 and 34% smaller than those observed in 2-year-old plants (Fig. 2). This age-related reduction in α-tocopherol was associated with an enhanced oxidation of this antioxidant, but only during spring and summer. The amount of α-tocopherol quinone per total tocopherol (reduced plus oxidized forms) was higher in 7-year-old plants than in 2-year-old plants, the former showing values 31–97% higher than the latter between April and June. Seven-year-old plants also showed significantly increased proportion of oxidized ascorbate in leaves during this period, since the amount of dehydroascorbate per total ascorbate, was between 1.7 and 3-fold, higher in 7-year-old plants than in 2-year-old plants. In contrast, no consistent differences in the amounts of reduced ascorbate were observed throughout the study between both plant groups (Fig. 3).
Chlorophyll a + b (Chl) and carotenoid contents in leaves of 2 (black bars) and 7-year-old (white bars) C. clusii plants grown under Mediterranean field conditions. Data correspond to the mean ± SE of four measurements. Statistical differences (P ≤ 0.05, Student’s t-test) between 2- and 7-year-old plants are indicated by an asterisk
α-Tocopherol (α-T) contents and α-TQ/α-Tt ratio, where α-TQ is α-tocopherol quinone and α-Tt = α-T + α-TQ, in leaves of 2- (black bars) and 7-year-old (white bars) C. clusii plants grown under Mediterranean field conditions. Data correspond to the mean ± SE of four measurements. Statistical differences (P ≤ 0.05, Student’s t-test) between 2- and 7-year-old plants are indicated by an asterisk
Ascorbate (Asc) contents and Dha/Asct ratio, where Dha is dehydroascorbate and Asct = Asc + Dha, in leaves of 2- (black bars) and 7-year-old (white bars) C. clusii plants grown under Mediterranean field conditions. Data correspond to the mean ± SE of four measurements. Statistical differences (P ≤ 0.05, Student’s t-test) between 2- and 7-year-old plants are indicated by an asterisk
The maximum efficiency of PSII photochemistry, estimated as the F v/F m ratio, was also slightly smaller during spring and summer in 7-year-old plants, which displayed reductions in this ratio up to 6% compared to 2-year-old plants (Fig. 4). Seven-year-old plants showed enhanced lipid peroxidation, estimated as malondialdehyde (MDA) accumulation, in leaves during spring and summer, which were between 10 and 50 % higher than those observed in 2-year-old plants during the same period. No significant differences in the F v/F m ratio and the extent of lipid peroxidation were observed between both age groups during autumn (Fig. 4).
Maximum efficiency of PSII photochemistry (F v/F m ratio) and malondialdehyde (MDA) levels, an indicator of lipid peroxidation, in leaves of 2 (black bars) and 7-year-old (white bars) C. clusii plants grown under Mediterranean field conditions. Data correspond to the mean ± SE of eight measurements. Statistical differences (P ≤ 0.05, Student’s t-test) between 2 and 7-year-old plants are indicated by an asterisk
Plant aging increases lipid peroxidation in chloroplasts and mitochondria
To evaluate the effects of plant aging on lipid peroxidation at the subcellular level, MDA levels were measured in isolated organelles obtained from leaves collected between April and June (Fig. 5). These studies revealed that enhanced age-dependent lipid peroxidation in leaves was, at least partly, due to increased lipid peroxidation in chloroplasts and mitochondria. The amount of MDA in chloroplasts and mitochondria increased with plant aging, showing 7-year-old plants levels between 2 and 2.8-fold and between 1.6 and 3.6-fold higher, respectively, than those of 2-year-old plants (Fig. 5).
Malondialdehyde (MDA) levels in chloroplasts and mitochondria obtained from leaves of 2- (black bars) and 7-year-old (white bars) C. clusii plants grown under Mediterranean field conditions. Data correspond to the mean ± SE of four measurements. Statistical differences (P ≤ 0.05, Student’s t-test) between 2- and 7-year-old plants are indicated by an asterisk
Plant aging increases ABA levels in leaves
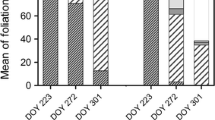
Plants kept midday relative leaf water content (RWC) above 74% during autumn, while they decreased to ca. 55% when plants were subject to drought during spring and summer (Fig. 6). Besides drought, plants were exposed to high light stress, as indicated by the maximum diurnal PPFD values ranging between 1,841 and 1,940 μmol m−2s−1 during the measurement days of spring and summer. April was unexpectedly dry (Table 1), which explains the low RWC values obtained during this period. No differences in RWC between 2- and 7-year-old plants were observed throughout the experiment. Neither carbon nor nitrogen contents differed in leaves of both plant groups (Fig. 1). Although no differences were observed in these parameters, ABA levels were markedly higher (up to 75%) in 7-year-old plants compared to 2-year-old plants. Interestingly, age-induced differences in ABA levels were observed both during autumn and during the more stressful period of spring and summer (Fig. 7). Furthermore, ABA levels were significantly higher (up to 30 pmol [g dry wt]−1) during spring and summer than during autumn in both plant groups. Sucrose contents were also (up to four-fold) higher during spring and summer compared to autumn, but although this increase, both plant groups showed similar sucrose levels per DW throughout the study. However, when given per unit of chlorophyll, sucrose contents were significantly higher (up to 57%) in the oldest plants, and this was consistent throughout the study (Fig. 8).
Relative leaf water content (RWC), total N content and the C/N ratio in leaves of 2 (black bars) and 7-year-old (white bars) C. clusii plants grown under Mediterranean field conditions. Data correspond to the mean ± SEM of eight measurements. No statistical differences (P ≤ 0.05, Student’s t-test) were observed between 2- and 7-year-old plants
Sucrose levels, given per DW and per chlorophyll unit, in leaves of 2- (black bars) and 7-year-old (white bars) C. clusii plants grown under Mediterranean field conditions. Data correspond to the mean ± SE of four measurements. Statistical differences (P ≤ 0.05, Student’s t-test) between 2- and 7-year-old plants are indicated by an asterisk
Discussion
Although studies on aging in perennials have been limited in part by the challenges of working with long-lived organisms, such studies are essential if we are to understand the phenomenon of aging in plants. In the present study, the use of C. clusii as an experimental model allowed studying the effects of environmental stress on the aging process in a drought-tolerant shrub. This species is a branched sclerophyllous shrub with a lifespan of 15–20 years that is naturally found in littoral brushwood growing in calcareous and dry soils of the Mediterranean coast, but it can also inhabit sandy and perturbed areas, being therefore characterized by its resistance to drought stress. The study was performed by comparing the physiology of recently emerged leaves of plants of different ages, the youngest ones being obtained from seeds of the oldest ones, so both plant groups had the same genetic background, and they grew under the same environmental conditions.
In the present study, plant aging increased oxidative stress during the more stressful period of spring and summer, as indicated by an enhanced oxidation of α-tocopherol and ascorbate. α-Tocopherol, in co-operation with other antioxidants, contributes to preserve an adequate cellular redox state in chloroplasts and to maintain thylakoid membrane structure and function. α-Tocopherol can physically quench, and therefore deactivate singlet oxygen in photosynthetic membranes (Trebst et al. 2002; Trebst 2003). In addition, α-tocopherol can chemically scavenge singlet oxygen and lipid peroxyl radicals. The chemical scavenging of the former by α-tocopherol irreversibly leads to their quinones and epoxides, while the scavenging of lipid peroxyl radicals results in the formation of tocopheroxyl radicals, which can be recycled back to α-tocopherol by the ascorbate-glutathione cycle (Munné-Bosch and Alegre 2002b). Thus, age-dependent enhanced oxidation of α-tocopherol to α-tocopherol quinone indicates that enhanced lipid peroxidation in chloroplasts in 7-year-old plants was caused at least in part by an enhanced production of reactive oxygen species (ROS) in photosynthetic membranes. Ascorbate is also essential for photosynthetic activity in the chloroplasts and for a variety of processes in other cellular compartments via the detoxification of hydrogen peroxide (Smirnoff 1996; Asada 1999; Smirnoff and Wheeler 2000), thus age-dependent enhanced oxidation of this antioxidant indicates an overall enhanced production of ROS within the cell.
Age-induced oxidation of antioxidants was accompanied by reductions in the F v/F m ratio and increases in the extent of lipid peroxidation, which is indicative of increased inhibition of PSII and oxidation to lipids in the oldest plants. It is interesting to note that age-induced differences in these oxidative stress markers were only noticeable during spring and summer, when plants were exposed to periods of drought and high light stress, which can lead to excess energy in chloroplasts, and therefore to the formation of ROS by increasing stomatal closure and consequently decreasing the activity of the Calvin cycle as a sink for ATP and reducing equivalents produced in photosynthetic electron transport. This contention is supported by the results, which demonstrate an enhanced accumulation of endogenous ABA levels in leaves as plants age. ABA functions primarily in plant responses to drought stress by inducing stomatal closure, but it also regulates the production of desiccation protectants and limits cell division and expansion (reviewed by Filkelstein et al. 2002; Himmelbach et al. 2003). Age-induced reductions in photosynthesis due to stomatal closure have already been reported in a previous study using the same species (Munné-Bosch and Alegre 2002a), as it has been reported in many other woody perennials (reviewed by Bond 2000). It is therefore likely that an age-related enhanced production of ABA levels in leaves favour the photo-oxidative stress associated with aging in plants.
What is, in turn, inducing an enhanced production of ABA in recently emerged leaves as plants age? Size is probably the determining factor responsible for an age-induced production of ABA in leaves. As plants age and increase in size, plants tend to increase their total biomass and leaf area. It is therefore essential that plants increase ABA levels to reduce water loss by transpiration. ABA reduces transpiration of the plant by inducing stomatal closure, but also by decreasing leaf area (through reductions in cell division and expansion), while maintaining leaf turgor. In the present study, RWC values were not affected by aging, while ABA levels increased, which supports this contention.
Although not statistically significant, 7-year-old plants showed slightly higher C/N ratios than 2-year-old plants throughout the experiment, thus indicating a possible feed back inhibition of photosynthesis by sugars. While sucrose contents per DW were similar in both plant groups, sucrose levels per unit of chlorophyll were higher in 7-year-old plants compared to 2-year-old plants throughout the study. Sucrose accumulation per photosynthetic unit appeared therefore to be higher in the oldest plants. Increased sucrose contents could indicate a higher general availability of sugars, including hexoses, although hexose contents were not determined in the present study. Sugars, in a process that appears to be hexose-dependent, repress the transcription of photosynthetic genes, thus leading to a feed back inhibition of photosynthesis (Sheen 1990; Wingler et al. 1998). It is therefore likely that age-induced reductions in photosynthesis are not only caused by ABA-induced stomatal closure, but also by a feed back inhibition of photosynthesis by sugars. Further research is however needed to support this contention.
In plants, chloroplasts are one the organelles most exposed to oxygen toxicity because they function at high oxygen and in the light (Halliwell and Gutteridge 1999) and it has been shown that they may be responsible for the oxidative stress associated with aging in plants (Munné-Bosch and Alegre 2002a). Furthermore, the metabolism of chloroplasts and mitochondria is interconnected and mitochondria play a crucial role in protecting chloroplasts from photoinhibition (Igamberdiev et al. 2001; Padmasree et al. 2002), thus we wondered about the relative contribution chloroplasts and mitochondria play in plant age-induced oxidative stress. Plant aging not only increased lipid peroxidation in chloroplasts, as previously shown (Munné-Bosch and Alegre 2002a), but also in mitochondria. Mitochondria showed between 1.8 and 6.8-fold higher MDA levels than chloroplasts in 7-year-old plants, thus it appears that age-induced oxidative stress in leaves during periods of environmental stress may result from an increased production of ROS not only within chloroplasts, but also within mitochondria. In fact, oxidation of lipids may also contribute to decreasing leaf growth rates during periods of drought as plants age. Moreover, we could see during the experiment that the leaves emerged in autumn senesced and dropped during the next summer in 7-year-old plants, but not in 2-year-old plants. This observation supports further the role of ABA and oxidative stress in the regulation of aging in perennials, since both ABA and oxidative stress are known to accelerate leaf senescence in water-stressed plants (Munné-Bosch et al. 2001; Yang et al. 2003).
First, it is concluded that leaves of oldest plants showed higher oxidative stress than those of young plants when exposed to adverse climatic conditions, thus supporting the idea that the oxidative stress associated with aging is due to extrinsic factors. Second, at the subcellular level, age-induced oxidative stress occurred both in chloroplasts and mitochondria, thus indicating that not only chloroplasts but also the overall cellular oxidative metabolism may be affected by plant aging. Third, meristematic tissues of C. clusii maintained the capacity to make new leaves with no symptoms of oxidative stress for several years, unless these leaves were exposed to environmental constraints. However, even in the absence of environmental stress, newly emerged leaves accumulated higher amounts of ABA as plants aged. Thus, it appears that intrinsic factors, such as plant size (and probably others), may also influence plant response to the environment by inducing ABA increases, thus modulating plant responses to extrinsic factors. Enhanced ABA levels may contribute to maintain leaf turgor by inducing stomatal closure and by reducing leaf area, but they may also accelerate leaf senescence, thus leading to a shortening of the lifespan of leaves as plants age.
Abbreviations
- ABA:
-
Abscisic acid
- DW:
-
Dry weight
- F v/F m :
-
Maximum efficiency of PSII
- FW:
-
Fresh weight
- MDA:
-
Malondialdehyde
- PPFD:
-
Photosynthetically-active photon flux density
- PSII:
-
Photosystem II
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water content
References
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Ashok B, Ali R (1999) The aging paradox: free radical theory of aging. Exp Gerontol 34:293–303
Bond BJ (2000) Age-related changes in photosynthesis of woody plants. Trends Plant Sci 5:349–353
Dangl JL, Dietrich RA, Thomas H (2000) Senescence and programmed cell death. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. ASPP, Rockville, pp 1044–1100
Day ME, Greenwood MS, Díaz-Sala C (2002) Age- and size-related trends in woody plant shoot development: regulatory pathways and evidence for genetic control. Tree Physiol 22:507–513
Fan L, Zheng S, Cui D, Wang X (1999) Subcellular distribution and tissue expression of phospholipase Dα, Dβ, and Dγ in Arabidopsis. Plant Physiol 119:1371–1378
Finkelstein R, Gampala S, Rock C (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14:S15–S45
Gerrits N, Turk SCHJ, van Dun KPM, Hulleman SHD, Visser RGF, Weisbeek PJ, Smeekens SCK (2001) Sucrose metabolism in plastids. Plant Physiol 125:926–934
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press (Clarendon), Oxford
Himmelbach A, Yang Y, Grill E (2003) Relay and control of abscisic acid signaling. Curr Opin Plant Biol 6:470–479
Hodges MD, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Igamberdiev AU, Bykova NV, Lea PJ, Gardeström P (2001) The role of photorespiration in redox and energy balance of photosynthetic plant cells: a study with a barley mutant deficient in glycine decarboxylase. Physiol. Plant 111:427–438
Keech O, Dizengremel P, Gardeström P (2005) Preparation of leaf mitochondria from Arabidopsis thaliana. Physiol Plant 124:403–409
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
López-Carbonell M, Jáuregui O (2005) A rapid method for analysis of abscisic acid (ABA) in crude extracts of water stressed Arabidopsis thaliana plants by liquid chromatography-mass spectrometry in tandem mode. Plant Physiol Biochem 43:407–411
Mencuccini M, Martínez-Vilalta J, Vanderklein D, Hamid HA, Korakaki E, Lee S, Michiels B (2005) Size-mediated ageing reduces vigour in trees. Ecol Lett 8:1183–1190
Morgan K (2002) Perennially young? SAGE KE DOI: 10.1126/sageke.2002.46.ns9
Munné-Bosch S, Alegre L (2001) Subcellular compartmentation of the diterpene carnosic acid and its derivatives in the leaves of rosemary. Plant Physiol 125:1094–1102
Munné-Bosch S, Jubany-Marí T, Alegre L (2001) Drought-induced senescence is characterized by a loss of antioxidant defences in chloroplasts. Plant Cell Environ 24:1319–1327
Munné-Bosch S, Alegre L (2002a) Plant aging increases oxidative stress in chloroplasts. Planta 214:608–615
Munné-Bosch S, Alegre L (2002b) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21:31–57
Munné-Bosch S, Alegre L (2003) Drought-induced changes in the redox state of α-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiol 131:1816–1825
Nobel PS (1991) Physicochemical and environmental plant physiology. Academic Press, San Diego
Noodén LD, Guiamet JJ (1996) Genetic control of senescence and aging in plants. In: Schneider El, Rowe JW (eds) Handbook of the biology of aging. Academic Press, San Diego, pp 94–118
Padmasree K, Padmavathi L, Raghavendra AS (2002) Essentiality of mitochondrial oxidative metabolism for photosynthesis: optimization of carbon assimilation and protection against photoinhibition. Crit Rev Biochem Mol Biol 37:71–119
Peñuelas J (2005) A big issue for trees. Nature 437:965–966
Rustin P, Kleist-Retzow J, Vajo Z, Rotig A, Munnich A (2000) For debate: defective mitochondria, free radicals, cell death, aging-reality or myth-ochondria? Mech Aging Devel 114:201–206
Sheen J (1990) Metabolic repression of transcription in higher plants. Plant Cell 2:1027–1038
Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Plant Sci 19:267–290
Trebst A (2003) Function of β-carotene and tocopherol in photosystem II. Z Naturforsch 58c:609–620
Trebst A, Depka B, Holländer-Czytko H (2002) A specific role for tocopherol and of chemical singlet oxygen quenchers in the maintenance of photosystem II structure and function in Chlamydomonas reinhardtii. FEBS Lett 43:2157–2162
Van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Wingler A, von Schaewen A, Leegood RC, Lea PJ, Quick WP (1998) Regulation of leaf senescence by cytokinin, sugars, and light. Plant Physiol 116:329–335
Yang JC, Zhang JH, Wang ZQ, Zhu QS, Liu LJ (2003) Involvement of abscisic acid and cytokinins in the senescence and remobilization of carbon reserves in wheat subjected to water stress during grain filling. Plant Cell Environ 26:1621–1631
Acknowledgments
We are indebted to Maria Reixach, Olga Jáuregui and Pilar Fernández (Serveis Científico-Tècnics, Universitat de Barcelona), and Josep Matas (Serveis dels Camps Experimentals, Universitat de Barcelona) for technical assistance. We also thank Tana Jubany and Leonor Alegre for their help in obtaining the seeds and growing the plants. We are very grateful to Prof. Kazimierz Strzalka (Jagellonian University, Krakow) for providing α-tocopherol quinone standard. This work was supported by the Ministry of Education and Science of the Spanish government (project no. BFU2006–01127).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munné-Bosch, S., Lalueza, P. Age-related changes in oxidative stress markers and abscisic acid levels in a drought-tolerant shrub, Cistus clusii grown under Mediterranean field conditions. Planta 225, 1039–1049 (2007). https://doi.org/10.1007/s00425-006-0412-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0412-z