Abstract
Leaf ecophysiological traits are known to change with leaf and tree age. In the present study, we measured the effect of leaf and tree age on leaf ecophysiological and morphological traits of nitrogen-fixing Alnus nepalensis (D. Don) which is a pioneer tree species in degraded lands. Three naturally occurring A. nepalensis forest stands, namely young (5–8 years old), mature (40–55 years old), and old (130–145 years old), were considered in this study. We also investigated the seasonal variations in leaf ecophysiological and morphological traits during leaf flushing, fully expanded, and leaf senescence phenological stages. The ecophysiological and morphological traits were compared between leaf and tree ages using a linear mixed-effect model (LMM) and Tukey’s HSD test. Fully expanded leaves and young trees demonstrate ecophysiological traits consistent with acquisitive resource-use strategies. Our results revealed that net photosynthetic capacity (Aarea and Amass), leaf stomatal conductance (gswarea and gswmass), transpiration rate (Earea and Emass), specific leaf area (SLA), predawn and midday water potential (Ψ), leaf total chlorophyll concentration, photosynthetic N- and P-use efficiency (PNUE and PPUE) were higher in younger trees than mature and old trees. We found lower water-use efficiency (WUE) and intrinsic water-use efficiency (WUEi) in young trees than in mature and old ones. Mass-based net photosynthetic capacity (Amass) was positively correlated with PNUE, PPUE, transpiration rate, stomatal conductance, SLA and chlorophyll concentrations but negatively correlated with WUE and WUEi. However, mass-based leaf nitrogen (N) and phosphorus (P) concentrations were the highest in fully expanded leaves and did not vary with tree age despite N concentration being negatively correlated with SLA. Overall, this study provides valuable insights into the age-related changes in leaf ecophysiological traits of A. nepalensis. The findings underscore the importance of considering tree age when studying plant ecophysiology and highlight the acquisitive resource-use strategies employed by young trees for rapid growth and establishment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Leaf morphological, chemical, and physiological traits and the ability to absorb nutrients may change as trees age. Increase in tree age can cause alterations in leaf morphological traits, vegetative/reproductive resource allocation, nutritional absorption, hormonal control, and environmental adaptability, all of which can lead to variations in the tree structure and functions (Thomas and Winner 2002; Thomas 2011a, b; Damián et al. 2018; Ji et al. 2021). All these traits may induce associated changes in the structure and functions of forests (Thomas 2010; Thomas 2011a, b; Martin and Thomas 2013; Damián et al. 2018).
Leaf ecophysiological traits vary significantly with plant life history, light, and nutrient availabilities (Reich et al. 1992; Ackerly et al. 2000; Han et al. 2020; Kumar et al. 2021; Maharjan et al. 2021; Sigdel et al. 2023). Many studies investigated the physiological traits (i.e., photosynthesis rate) that appear to decline in tall trees because of the limitation of hydraulic transport (Mencuccini and Grace 1996; Ryan and Yoder 1997). High values of leaf photosynthetic rate represent acquisitive strategies (high productivity) for plants, and low values represent conservative strategies (low productivity) (Wright et al. 2005; Gorne et al. 2022). Several studies have indicated that younger trees exhibit greater traits associated with resource acquisition strategies, while mature and older trees tend to display more traits indicative of conservative strategies (Damián et al. 2018; Dayrell et al. 2018). During forest stand development, plants shift from an acquisitive physiological strategy to a conservative physiological strategy (Han et al. 2020) to maintain the plant’s overall productivity at its optimum according to metabolic need.
The leaf phenological cycle provides an interesting opportunity to explore the relationship between leaf gas exchange, water balance, and leaf functional traits in relation to leaf phenology (Fajardo and Siefert 2016). Leaf physiological traits significantly change with phenological phases (Escudero and Mediavilla 2003). Leaf phenological cycle (i.e., leaf initiation, expansion, and senescence) varied among species, among individual plants, and also among leaves on a plant (Chabot and Hicks 1982; Reich et al. 1991; Mediavilla et al. 2014; Bai et al. 2015; Joshi and Garkoti 2023c). Previous studies have revealed that with rise in leaf age and leaf mass per unit area (LMA), the leaf nitrogen (N) and phosphorus (P) decline (Niinemets and Lukjanova 2003; Niinemets et al. 2006; Athokpam and Garkoti 2015). Previous studies also revealed that leaf- and tree age-related decline in photosynthesis traits could be associated with the change in leaf morphology and nutrients over time (Reich et al. 1991). Leaf N is positively correlated with the activity of Rubisco (ribulose-1, 5-biphosphate carboxylase/oxygenase), and its concentration often decreases with age of tree and leaf (Kitajima et al. 2002, Wright et al. 2006; Fajardo and Siefert 2016; Chavana-Bryant et al. 2019). Leaf P is involved in various metabolic activities during photosynthesis and its concentration decreases with leaf and tree age (Wright et al. 2006; Mediavilla et al. 2011; Chavana-Bryant et al. 2017, 2019). Similarly, stomatal conductance and control (i.e., stomatal opening and closing) also decrease with leaf and tree age (Reich and Borchert 1988), which in turn affect photosynthesis.
Water, N, and P are essential resources for plant survival, growth, and photosynthesis. Leaf physiological traits such as net photosynthetic capacity, leaf diffusive conductance, and transpiration rate are indicators of CO2 assimilation, resource-use strategies, and water exchange (Rawat et al. 2021; Singh et al. 2023). The water-use efficiency (WUE) and photosynthetic N- and P-use efficiency (PNUE and PPUE) are essential characteristics of plant species that determine leaf physiology, leaf economics, and strategies, and are expected to change with plant and leaf age (Robinson et al. 2001; Wright et al. 2004; Nabeshima and Hiura 2004; Bai et al. 2015). WUE, PNUE, and PPUE are important ecological indicators of species performance in different environmental conditions that are expected to change with plant and leaf age (Funk and Vitousek 2007). PNUE and WUE describe the N concentration per unit leaf area and the amount of water transpired, respectively, for a given rate of photosynthesis. At the leaf level, WUE is the ratio between net CO2 assimilation and water loss via transpiration, and intrinsic water-use efficiency (WUEi) is the ratio between net CO2 assimilation and stomatal conductance. The PNUE, PPUE, and WUE predict how photosynthetic assimilation is optimized per unit of N, P, and water in leaves (e.g., Poorter and Evans 1998; Castellanos et al. 2005; Sheng et al. 2011). Thus, the leaf phenological cycle and resource allocation strategies are physiologically related (Ackerly and Bazzaz 1995; Hikosaka 2005), making leaf age a crucial characteristic controlling plant carbon and nutrient economies, and eventually resulting in an adaptive modification in response to ecological heterogeneity.
Nitrogen-fixing Alnus nepalensis (D. Don) is one of the fast-growing early successional tree species which plays a crucial role in the ecosystem functioning and biogeochemical cycling in the central Himalaya (Joshi and Garkoti 2021b). In this region, A. nepalensis is an important economic and reforestation tree species (Joshi and Garkoti 2023a, b). Previous studies have demonstrated that A. nepalensis is important for soil conservation in degraded forests (Joshi and Garkoti 2021b, 2023a, b). Most studies on A. nepalensis have focused on ecosystem carbon dynamics (Joshi and Garkoti 2021b, 2023a), soil physicochemical properties, below-ground biomass, and litter dynamics (Joshi and Garkoti 2020, 2021a). Understanding the ecophysiology and nutrient dynamics of A. nepalensis may provide insights into its ecological importance and help develop sustainable forest management strategies.
Our study investigated variations in leaf ecophysiological traits, nutrient concentration, and adaptive strategies concerning leaf age and tree age. Leaf ecophysiological traits, such as photosynthetic rate, stomatal conductance, transpiration rate, and leaf nutrient concentrations of nitrogen and phosphorus were measured across different leaf and tree age classes. In this study, we proposed the following questions: (1) How do the leaf ecophysiological and morphological traits change with tree age? We expected a shift from more acquisitive physiological strategies in the young age stand to conservative physiological strategies in the older age A. nepalensis stand. (2) How do the leaf ecophysiological and morphological traits change with leaf age? We believed that these traits varied with leaf phenophase.
2 Materials and methods
2.1 Study site
The study was undertaken at 30°31′36.7″ N and 79°6′42.0″ E, 1612 meters above sea level in the proximity of Kedarnath Wildlife Sanctuary in the western part of the central Himalaya (table 1). The area exhibits a predominantly cool temperate climate and experiences noticeable changes in weather with season. The meteorological station near the study region assessed the annual rainfall, temperatures (maximum and minimum, mean), and precipitation during the study time (data recorded at the nearest meteorological station located at Ukhimath). The mean minimum temperature ranged from −1.1°C in January to 13.4°C in July, and the mean maximum temperature ranged from 11.6°C in January to 24.4°C in June. During the study, annual mean rainfall ranged from 7.3 mm in November to 637.1 mm in July. The cumulative yearly rainfall in the study area was 1983 mm, with over 70–80% of this usually occurring during the monsoon season (July–September) and moderate to heavy snowfall during December–February (Joshi and Garkoti 2020). The soil type in the study area was sandy loam, brown podzolic mixed with pebbles and gravel (Joshi and Garkoti 2021b). The dominant tree species representing forests are A. nepalensis D. Don, Quercus leucotrichophora A. Camus, Myrica esculenta Buch. Ham. ex D. Don, Rhododendron arboreum Smith, Pyrus pashia L., Lyonia ovalifolia (Wall.) Drude, Litsea umbrosa Nees, and Symplocos paniculata Miq (Joshi and Garkoti 2023b).
2.2 Experimental design
Field experiments were conducted in 0.1 ha permanent plots in three age groups of forest sites, i.e., age group I: young stage (5–8 years old), age group II: mature stage (40–55 years old), and group III: old stage (130–145 years old). All study sites were separated by at least 500–800 m. Detailed information for A. nepalensis individuals in the different tree age classes is given in table 1. Since we lacked precise information on the age of the forest, we used the basal area of A. nepalensis (which acts as an indicator of tree age). We corroborated it by interviewing elderly members of the local community who knew of the year when landslides occurred and subsequently when A. nepalensis was established. We further confirmed the data with the Forest Department.
The leaf life span of A. nepalensis was between 9 and 10 months. Leaf flushing starts during spring (March to April), and leaf production completes in 2 to 4 months. Alnus nepalensis retains 15–25% of leaves during winter. During the winter season, herbivory damages the leaves of A. nepalensis that are still retained and affect several leaf traits. Five healthy individuals each of young (5–8 years old), mature (40–55 years old), and old (130–145 years old) A. nepalensis were selected. To assess age-specific ecophysiological traits of both the tree and its leaves, we conducted gas exchange and water potential measurements for all 15 individuals during the three phenophases, each exposed to varying moisture and temperature conditions. The investigation dates for each sampling phenophase were 20–30 March 2021 (spring; leaf flushing), 20–30 June 2021 (early monsoon summer; fully expanded leaf), and 20–30 October 2021 (fall; leaf senescing).
Area-based physiological traits, e.g., photosynthetic rate (Aarea; µmol CO2 m−2 s−1 ), stomatal conductance (gswarea; mol H2O m−2 s−1), transpiration rate (Earea; mol H2O m−2 s−1) were measured using an open-flow, portable measurement infrared gas analyzer (IRGA) (Li-6800, Li-Cor, Lincoln, NE, USA) (Evans and Santiago 2014) between 9:30 and 11:00 h local solar time (to minimize sources of diurnal heterogeneity and avoid mid-day depression) under ambient conditions and air temperature (T air,°C). The leaf temperature (Tleaf,°C) and photosynthetic photon flux density (PPFD, µmol−2s−1) were recorded at each measurement by the IRGA using a 6 cm2 chamber with red–blue light-emitting diodes on normal cloudless days. To avoid influence of fluctuating environmental conditions, photosynthetically active radiation (PAR) was set to 1200 µmol m−2s–1, while the concentration of CO2, temperature, and humidity was set according to the ambient conditions of the study site. The cuvette’s vapor pressure deficit (VPD) was maintained at 1 kPa. Specific leaf area (SLA; cm2g−1) represents the inverse of leaf mass area (LMA) and was calculated as the ratio of dry leaf area and leaf mass (Poorter et al. 2009). Leaf area was measured by leaf area meter (LI 3000C, LI-COR, Lincoln, Nebraska, USA). Mass-based assimilation rate (Amass; µmol CO2 kg−2s–1), mass-based stomatal conductance (gswmass; mol H2O kg−1s−1), and mass-based transpiration rate (Emass; mol H2O kg−1 s−1) were calculated as Amass = A area × SLA; gsw mass = gsw area × SLA, and Emass = Earea × SLA, respectively. Leaf functional traits measured included specific leaf area (SLA; cm2g−1), total nitrogen (leaf N; g kg−1), total phosphorus (leaf P; g kg−1) concentrations, and total chlorophyll (Chl; mg g−1) concentrations. To estimate the mass-based leaf nitrogen (Nm) and phosphorus (Pm), eight to ten leaf discs of definite area (1.60 cm2) were excised from the leaf (leaf without petiole), dried at 64°C to constant weight, and weighed. During the analysis, all samples were triplicated and averaged. Mass-based leaf nitrogen (Nm) and phosphorus (Pm) concentrations were calculated by K2Cr2O7-H2SO4 oxidation, the Kjeldahl method, and the modified H2O2-H2SO4 method (Rapp et al. 1999), respectively. The concentration of P was determined at 725 nm using a spectrophotometer (UV-1800; Shimadzu Corp., Kyoto, Japan). Mass-based leaf chlorophyll concentration (mg g−1) was measured on fresh leaf discs, extracted using 5 mL of dimethylsulfoxide (DMSO), with three replicates for each tree and leaf age. After the sample test, the tube was preheated to 64°C in the water bath for 4 h and sample tissues were decolorized and cooled at room temperature; the absorbance of the supernatant was measured using a spectrophotometer (Shimadzu UV-1201, Kyoto, Japan). Chlorophyll a and b concentrations (mg g−1) were calculated using 665 and 645 nm readings. Area-based leaf nitrogen (Na) and phosphorus (Pa) concentrations were calculated as mass-based leaf nitrogen (Nm) and phosphorus (Pm) concentrations each divided by the specific leaf area (i.e., Na and Pa = Nm/SLA and Pm/SLA, respectively). The photosynthetic N- and P-use efficiency were measured by calculating nitrogen- and phosphorus-use efficiency (PNUE or PPUE = Aarea/Narea or Aarea/Parea µmol CO2 N and P s−1 g−1). Intrinsic water-use efficiency (WUEi; µmol CO2 µmol−1 H2O) was measured as the ratio of Aarea/gsw area, and water-use efficiency (WUE; µmol CO2 µmol−1 H2O) was derived as the ratio of Aarea /Earea (Farquhar and Sharkey 1982). We measured mid-day water potential on the same branch on which leaf gas exchange experiments were conducted using a pressure chamber (Model 1000, PMS Instrument, Corvallis, OR). We also measured pre-dawn water potential on each tree before the leaf physiological traits measurement.
2.3 Statistical analysis
All statistical analysis in this study was performed using the R programming language, version 4.0 (R Core Team 2021). Data sets of physiological, leaf morphological, and chemical traits were tested for normality and homoscedasticity by the Shapiro–Wilk and Levene tests, respectively. For leaf ecophysiological, morphological, and chemical traits, tree age (young, mature, and old) and leaf age (flushing, fully expanded, and senescing) were considered fixed effects for the mixed-model ANOVA, where interactions among fixed effects were also assessed. In order to analyze results of the mixed models, we employed the R package ‘lme4’. Additionally, we utilized the ‘car’ package to determine the significance of individual fixed factors as well as their interactions. Moreover, after conducting an ANOVA to detect significant variations, a post hoc test (Tukey’s HSD) was employed utilizing the ‘emmeans’ package. This test was carried out for pairwise comparisons of means to determine significant differences. Linear regression was performed to observe the correlations between physiological, leaf morphological, and chemical traits with regression equations. All analyzed correlations were considered significantly different when p<0.05.
3 Results
3.1 Variation in leaf ecophysiological traits with leaf and tree age
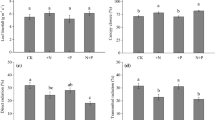
Although influence of tree age and leaf age manifested significant physiological trait differences in A. nepalensis, fully expended leaves had the highest values of all the physiological traits at the flushing and senescing stages regardless of tree age (figure 1). Most of the leaf physiological trait values were higher in young trees compared with mature and old trees (figure 1). Most of the leaf trait values followed a descending order from summer (fully expanded) to spring (flushing) to autumn (senescing). Fully expanded leaves showed greater capacity for photosynthesis compared with leaves either at the flushing or senescing stage. Like the physiological traits, SLA, total chlorophyll concentrations, and N and P concentration per unit leaf mass followed a similar leaf age-related pattern (higher for the fully expanded stage than flushing and senescing) (figure 2).
Seasonal variation in different leaf physiological traits in the young, mature, and old stages of A. nepalensis trees in the central Himalaya. Lower and upper box boundaries represent the 25% and 75% quantiles, respectively; the solid lines across each box are the median. Different lowercase letters indicate significant differences at p<0.05.
Aarea and Amass, Earea and Emass, gswarea and gswmass, PNUE, and PPUE tend to decrease with tree age. WUEi and WUE tend to increase with tree age. Despite high physiological traits in the fully expanded leaves, pre-dawn (Ψpd) and mid-day (Ψmd) water potential were more negative during the fully expanded stage and leaf senescing stage in old trees (figure 3). However, SLA, N, and P per unit area did not change significantly with tree age. Total chlorophyll concentrations peaked when leaves were fully expanded, which decreased with tree age.
3.2 Relationship between leaf traits
The present study found a positive correlation between Emass, gswmass, PNUE, and PPUE with Amass; Earea, gswarea with Aarea; Earea with gswarea, and a negative correlation between WUEi and WUE with Aarea; gws area with WUEi, and Earea with WUE across the tree age classes (figure 4). Intrinsic water-use efficiency and water-use efficiency were negatively correlated with PNUE in all three tree age stages. Midday water potential (Ψmd) was positively correlated with Aarea, Earea, and gswarea (figure 5). Across tree age stages, SLA was positively correlated with Amass, Emass, PNUE, and PPUE, but negatively related to N concentration per unit leaf mass. Tree age exhibited a significant negative relationship between Narea and PNUE, Parea, and PPUE (figure 6).
4 Discussion
4.1 Variation in leaf ecophysiological traits with tree age
Our first objective was to understand how key ecophysiological tree traits change from resource acquisition to resource conservation with plant age and leaf age. As expected, we noticed a shift toward resource-conservative features with tree age. Thus, our results reinforce the finding that older trees have more conservative traits (Martin and Thomas 2013; Damián et al. 2018; Dayrell et al. 2018; Funk et al. 2021). The older trees had lower SLA, lower leaf Nmass, and reduced photosynthetic traits than young trees. Lower SLA values indicate a more conservative strategy, while higher SLA values indicate a more resource-acquisitive strategy, where plants invest more in leaf area to capture resources (Khan et al. 2022).
Modifications in photosynthetic rate were strongly dependent on E, gsw, PNUE, PPUE, WUE, and SLA (figure 4). Our results demonstrated that leaf age and tree age had a significant effect on gsw, which may be due to the leaf age- and tree age-related changes in hydraulic conductance. The lower gsw of the old trees than young and mature trees would be consistent with the hydraulic limitation hypothesis and the influence of the gravitational hydrostatic gradient (Ryan and Yoder 1997). The hydraulic limitation hypothesis proposes that as plants grow taller, the hydraulic resistance to water flow in the vascular system increases, making it increasingly difficult to transport water from roots to leaves (Ryan et al. 2006). This can lead to a situation where the leaves at the top of the plant experience water stress and cannot perform photosynthesis effectively, hence limiting a plant’s overall growth and productivity (Ryan and Yoder 1997). Stomatal closure is well recognized as linked to decreased soil-to-leaf hydraulic conductance and variations in Ψleaf (Hubbard et al. 1999; Kolb and Stone 2000). In the present study, young trees generally have less negative water potential, indicating that water is more readily available to them compared to old trees. This can be attributed to their higher water absorption capacity, efficient water transport systems, and generally healthier root systems. Additionally, their higher transpiration rates can help maintain favorable water potential by effectively drawing up water from roots (McDowell 2011). As a result, plant strategies gradually shift from resource acquisition to resource conservation (Guariguata and Ostertag 2001). Similarly, shifts in plant strategies were also reflected through higher leaf chlorophyll concentrations and greater PNUE and PPUE in young trees which declined as trees grow old. Higher leaf chlorophyll concentration and greater PNUE and PPUE in young trees reflect leaf traits with faster growth strategies involving greater photosynthesis and light capture.
Differences in chemical and morphological traits, driven by leaf and tree age, significantly shape physiological traits observed at the leaf scale. In the present study, the total chlorophyll, nitrogen mass (Nmass), and phosphorus mass (Pmass) values were higher in younger trees compared with older ones, suggesting a higher growth rate and productivity in young trees. These traits may have positively influenced the cycling of nutrients and water within the young trees.
4.2 Variation in leaf ecophysiological traits with leaf age
The results supported optimum productivity during summer when the leaf was fully expanded (summer) then leaf flushing (spring) and leaf senescing stages (autumn). Previous studies also suggested similar leaf age trends of physiological traits in that they showed peak values during the fully expanded leaves (Wilson et al. 2000, 2001; Bauerle et al. 2004; Grassi and Magnani 2005). In the present study, physiological traits showed peak values during the fully expanded leaf stage. During summer, various factors such as longer daylight hours, increased photosynthetically active radiation, ideal air temperature, and higher leaf nitrogen and phosphorus content contribute to the optimal physiological traits of plants (Grassi and Magnano 2005; Wright et al. 2006; Hikosaka et al. 2007). However, as leaves enter the senescence stage, the levels of nitrogen and chlorophyll in the leaf reach their lowest point due to a nutrient remobilization process (Maillard et al. 2015). This leads to reduced physiological traits following leaf senescence. Such leaf age- and tree age-dependent decline in leaf morphological and chemical traits are significantly associated with changes in leaf physiological traits (i.e., photosynthetic rate, stomatal conductance, and transpiration rate) (Reich et al. 1991). In addition, the nutrient resorption process during leaf senescence negatively affected the overall productivity during the leaf senescing stage (Crous et al. 2019). The photosynthetic rate in a fully expanded leaf was significantly associated with other physiological traits, including transpiration rate, stomatal conductance, water potential, and photosynthetic nutrient-use efficiency (Reich et al. 1998; Wright et al. 2005). The tight connection between levels of photosynthesis, chlorophyll, and Nmass reflected the contribution of nitrogen to the Calvin–Benson cycle enzyme (in particular, ribulose-1,5-biphosphate carboxylase/oxygenase, Rubisco) and chlorophyll for better light harvesting (Whitmarsh 1999). The high chlorophyll concentrations in the fully expanded leaves supported high light-harvesting and higher carbon assimilation per unit leaf area (Li et al. 2018).
4.3 Acquisitive vs. conservative resource-use strategy
The present study found a significant PNUE–WUE trade-off in young, mature, and old trees. Young trees achieved higher PNUE and lower WUE, whereas mature and old trees followed the reverse trend. The trade-off between WUE and PNUE may explain the greater rates of physiological traits in the young trees. As trees mature and grow old, they may become more resource-limited and face environmental stressors such as competition for resources, drought, or nutrient limitations. In response, mature and old trees adapt by reducing their water requirements and increasing water-use efficiency, potentially sacrificing some of their photosynthetic efficiency (Niinemets et al. 2006; Niinemets 2010). PNUE was 12% higher for young trees than for mature trees and 18% higher than for old trees. Young trees exhibited higher water potential, suggesting a trade-off between leaves with greater photosynthetic rates in young trees and leaves that are water-stressed (low water potential) in old trees. Young trees may have a higher capacity for water uptake and transport, allowing them to maintain higher water potentials despite their higher rates of water loss through transpiration. This may be due to various differences including root architecture, root morphology, physiology and anatomy of young and old tree leaves, and environmental stress (Freschet et al. 2021). PNUE and PPUE were higher in the young trees, favoring low investment and quick resource return strategy than in mature and old trees, which favored a slow resource return strategy (Wright et al. 2004; Reich and Flores-Moreno 2017). In the present study, the young trees showed greater N assimilation efficiency and more allocation of N to photosynthetic chlorophyll tissue than other non-photosynthetic tissues to ensure optimum growth. This was supported by the comparatively higher SLA values in young trees than mature and older trees (Abdul-Hamid and Mencuccini 2009), to reduce resource investment in non-photosynthetic tissues of young trees. Previous studies have also reported the trade-off in N partitioning between photosynthetic and non-photosynthetic tissues in varying tree ages (Hikosaka and Hirose 2000; Hikosaka 2004).
In the present study, the majority of mass based-physiological traits (i.e., Amass, Emass, and gswmass) and photosynthetic nutrient-use efficiency showed positive correlation with SLA, which is similar to several previous reports (Wright et al. 2005; Crous et al. 2017). Onoda et al. (2017) also revealed that leaves with greater SLA tend to enhance Amass, Emass, and PNUE to support fast growth. Old trees had higher WUE than young and mature trees, leading to significantly lower physiological traits (Forrester 2015). In general, high WUE indicates a more conservative resource-use pattern (Lambers et al. 2008). Mid-day water potential (Ψmd) was more negative during the summer in old trees, which may be due to a combination of factors, including differences in leaf morphology, increasing temperature, vapor pressure deficit (VPD), biochemical characteristics, larger overall biomass, root distribution, and physiological regulation of stomatal conductance (Rodriguez-Calcerrada et al. 2008; Abdul-Hamid and Mencuccini 2009; Schönbeck et al. 2022). Consequently, to adapt to the pressure and balance of demand and supply, old trees acquire a more conservative approach to shift toward higher WUE, which indicates that at a given photosynthetic rate, the transpiration rate, stomatal conductance, and photosynthetic nutrient-use efficiency were higher in young rather than mature and old trees (Abdul-Hamid and Mencuccini 2009).
4.4 Ecosystem implication
Leaf and tree age lead to adaptive changes in the ecophysiological processes, such as variation in water relation, gas exchange, and growth rate among varying tree age stages (Sala et al. 2010). Physiological responses, such as evolution and adaptation to changing environments, are influenced by phenotypic plasticity, which is considered the primary underlying process with implications for ecological processes (Hovenden and Vander Schoor 2003; Thomas 2011a, b). Better comprehension of physiological traits of major native trees is required for introducing resilient forest management strategies to minimize expected effects of climatic change on plant development and water stress. Age-specific physiological traits of A. nepalensis appear important for ecosystem processes. WUE, PNUE, and PPUE play an essential regulatory role in functioning of A. nepalensis-dominated ecosystems. PNUE and PPUE were significantly greater in the young stage, providing additional evidence that A. nepalensis has developed a mechanism for the efficient use of N and P nutrients and modifications in physiological traits. Ishida et al. (2005) have also explained similar ontogenetic morphological, anatomical, and chemical modifications leading to evolution and adaptation in leaf physiology along the age gradient for the pioneer tree species Macaranga gigantea. Furthermore, different-aged stands of A. nepalensis may exhibit different adaptive strategies in response to soil resource availability (soil water and nutrient availability), which may potentially affect growth, survival, and competitive ability. Further studies are needed to test these hypotheses and to better understand the underlying mechanisms and ecological implications of these relationships.
5 Conclusion
In conclusion, our findings indicate that most of the physiological traits of A. nepalensis decreased with tree age, indicating that the ecological strategy of A. nepalensis changed from a resource-acquisitive approach to a resource-conservative approach along the tree age gradient. The relationship between leaf traits and structural and chemical traits changed through tree age, indicating different trade-off strategies across the age gradient. High photosynthetic nitrogen- and phosphorus-use efficiency in young trees could support rapid growth of A. nepalensis. Specific leaf area and total chlorophyll concentration strongly influence many physiological traits and serve as vital regulators. Our results contributed to a more dynamic understanding of the relationship between leaf physiological traits and their interaction with leaf morphological and chemical traits. Additional studies are needed to understand the interaction with soil physicochemical properties and soil moisture concentration along the age gradient in A. nepalensis forest stands in the central Himalaya.
References
Abdul-Hamid H and Mencuccini M 2009 Age-and size-related changes in physiological characteristics and chemical composition of Acer pseudoplatanus and Fraxinus excelsior trees. Tree Physiol. 29 27–38
Athokpam FD and Garkoti SC 2015 Dynamics of foliar nitrogen of evergreen and deciduous plant species in a wet tropical forest, South Assam, India. Plant Ecol. 216 1117–1135
Ackerly DD and Bazzaz FA 1995 Seedling crown orientation and interception of diffuse radiation in tropical forest gaps. Ecology 76 1134–1146.
Ackerly DD, Dudley SA, Sultan SE, et al. 2000 The evolution of plant ecophysiological traits: recent advances and future directions: new research addresses natural selection, genetic constraints, and the adaptive evolution of plant ecophysiological traits. Bioscience 50 979–995.
Bauerle WL, Weston DJ, Bowden JD, et al. 2004 Leaf absorptance of photosynthetically active radiation in relation to chlorophyll meter estimates among woody plant species. Sci. Hortic. 101 169–178.
Bai K, He C, Wan X, et al. 2015 Leaf economics of evergreen and deciduous tree species along an elevational gradient in a subtropical mountain. AoB Plants 7 plv064.
Castellanos AE, Martinez MJ, Llano JM, et al. 2005 Successional trends in Sonoran Desert abandoned agricultural fields in northern Mexico. J. Arid Environ. 60 437–455.
Chavana-Bryant C, Malhi Y, Wu J, et al. 2017 Leaf aging of Amazonian canopy trees as revealed by spectral and physiochemical measurements. New Phytol. 214 1049–1063.
Chavana-Bryant C, Malhi Y, Anastasiou A, et al. 2019 Leaf age effects on the spectral predictability of leaf traits in Amazonian canopy trees. Sci. Total Environ. 666 1301–1315.
Chabot BF and Hicks DJ 1982 The ecology of leaf life spans. Annu. Rev. Ecol. Evol. Syst. 13 229–259.
Crous KY, Wujeska-Klause A, Jiang M, et al. 2019 Nitrogen and phosphorus retranslocation of leaves and stemwood in a mature Eucalyptus forest exposed to 5 years of elevated CO2. Front. Plant Sci. 10 664.
Crous KY, Wallin G, Atkin OK, et al. 2017 Acclimation of light and dark respiration to experimental and seasonal warming are mediated by changes in leaf nitrogen in Eucalyptus globulus. Tree Physiol. 37 1069–1083.
Dayrell RL, Arruda AJ, Pierce S, et al. 2018 Ontogenetic shifts in plant ecological strategies. Funct. Ecol. 32 2730–2741.
Damián X, Fornoni J, Domínguez CA, et al. 2018. Ontogenetic changes in the phenotypic integration and modularity of leaf functional traits. Funct. Ecol. 32 234–246.
Evans JR and Santiago LS 2014 PrometheusWiki gold leaf protocol: gas exchange using LI-COR 6400. Funct. Plant Biol. 41 223–226.
Escudero A and Mediavilla S 2003 Decline in photosynthetic nitrogen use efficiency with leaf age and nitrogen resorption as determinants of leaf life span. J. Ecol. 91 880–889.
Funk JL and Vitousek PM 2007 Resource-use efficiency and plant invasion in low-resource systems. Nature 446 1079–1081.
Funk JL, Larson JE and Vose G 2021 Leaf traits and performance vary with plant age and water availability in Artemisia californica. Ann. Bot. 127 495–503.
Forrester DI 2015 Transpiration and water-use efficiency in mixed-species forests versus monocultures: effects of tree size, stand density and season. Tree Physiol. 35 289–304.
Freschet GT, Roumet C, Comas LH, et al. 2021 Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytol. 232 1123–1158.
Fajardo A and Siefert A 2016 Temperate rain forest species partition fine-scale gradients in light availability based on their leaf mass per area (LMA). Ann. Bot. 118 1307–1315.
Farquhar GD and Sharkey TD 1982 Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 33 317–345
Grassi G and Magnani F 2005 Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell & Environ. 28 834–849.
Guariguata MR and Ostertag R 2001 Neotropical secondary forest succession: changes in structural and functional characteristics. For. Ecol. Manag. 148 185–206.
Gorné LD, Díaz S, Minden V, et al. 2022. The acquisitive–conservative axis of leaf trait variation emerges even in homogeneous environments. Ann. Bot. 129 709–722.
Han T, Ren H, Wang J, et al. 2020 Variations of leaf ecophysiological traits in relation to environmental factors during forest succession. Ecol. Indic. 117 106511
Hikosaka K 2004 Interspecific difference in the photosynthesis–nitrogen relationship: patterns, physiological causes, and ecological importance. J. Plant. Res. 117 481–494
Hikosaka K 2005 Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Ann. Bot. 95 521–533.
Hikosaka K and Hirose T 2000 Photosynthetic nitrogen-use efficiency in evergreen broadleaved woody species coexisting in a warm-temperate forest. Tree Physiol. 20 1249–1254
Hikosaka K, Nabeshima E and Hiura T 2007 Leaf age changes in the temperature response of photosynthesis in canopy leaves of Quercus crispula in a cool-temperate forest. Tree Physiol. 27 1035–1041
Hovenden MJ and Vander Schoor JK 2003 Nature vs nurture in the leaf morphology of Southern beech, Nothofagus cunninghamii (Nothofagaceae). New Phytol. 161 585–594
Hubbard RM, Bond BJ and Ryan MG 1999 Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa trees. Tree Physiol. 19 165–172
Ishida A, Yazaki K and Hoe AL 2005 Ontogenetic transition of leaf physiology and anatomy from seedlings to mature trees of a rain forest pioneer tree Macaranga gigantea. Tree Physiol. 25 513–522
Ji M, Jin G and Liu Z 2021 Effects of ontogenetic stage and leaf age on leaf functional traits and the relationships between traits in Pinus koraiensis. J. For. Res. 32 2459–2471
Joshi RK and Garkoti SC 2020 Litter dynamics, leaf area index and forest floor respiration as indicators for understanding the role of Nepalese alder in white oak forests in central Himalaya, India. Ecol. Indic. 111 106065
Joshi RK and Garkoti SC 2021a Influence of Nepalese alder on soil physico-chemical properties and fine root dynamics in white oak forests in the central Himalaya, India. Catena 200 105140
Joshi RK and Garkoti SC 2021b Dynamics of ecosystem carbon stocks in a chronosequence of nitrogen-fixing Nepalese alder (Alnus nepalensis D. Don.) forest stands in the central Himalayas. Land Degrad. Dev. 32 4067–4086
Joshi RK and Garkoti SC 2023a Effect of forest chronosequence on ecological stoichiometry and nitrogen and phosphorus stocks in Alnus nepalensis forest stands, central Himalaya. Land Degrad. Dev. 12 3769–3789
Joshi RK and Garkoti SC 2023b Ecosystem carbon stocks and sequestration rates in white oak forests in the central Himalaya: Role of nitrogen-fixing Nepalese alder. J. Biosci. 48 13
Joshi RK and Garkoti SC 2023c Seasonal patterns of leaf physiological traits, nutrient and adaptive strategies of co-occurring Alnus nepalensis and Quercus leucotrichophora tree species in the central Himalaya. Perspect. Plant Ecol. Evol. Syst. 61 125761.
Khan A, Yan L, Hasan MM, et al. 2022 Leaf traits and leaf nitrogen shift photosynthesis adaptive strategies among functional groups and diverse biomes. Ecol. Indic. 1 109098
Kitajima K, Mulkey SS, Samaniego M, et al. 2002 Decline of Vcmax with leaf age and position in two tropical pioneer tree species. Am. J. Bot. 89 1925–1932
Kolb TE and Stone JE 2000 Differences in leaf gas exchange and water relations among species and tree sizes in an Arizona pine–oak forest. Tree Physiol. 20 1–12
Kumar A, Kumar P and Singh H 2021 Modulation of plant functional traits under essential plant nutrients during seasonal regime in natural forests of Garhwal Himalayas. Plant Soil 465 197–212
Li Y, He N, Hou J, et al. 2018 Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 6 64
Lambers H, Chapin FS and Pons TL 2008 Plant physiological ecology, Vol. 2. (Springer, New York) PP. 11–99
Maharjan SK, Sterck FJ, Dhakal BP, et al. 2021 Functional traits shape tree species distribution in the Himalayas. J. Ecol. 109 3818–3834
Martin AR and Thomas SC 2013 Size-dependent changes in leaf and wood chemical traits in two Caribbean rainforest trees. Tree Physiol. 33 1338–1153
McDowell NG 2011 Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 155 1051–1059
Mencuccini M and Grace J 1996 Hydraulic conductance, light interception and needle nutrient concentration in Scots pine stands and their relations with net primary productivity. Tree Physiol. 16 459–468.
Mediavilla S, González-Zurdo P, García-Ciudad A, et al. 2011 Morphological and chemical leaf composition of Mediterranean evergreen tree species according to leaf age. Trees 25 669–677
Mediavilla S, Herranz M, González-Zurdo P, et al. 2014 Ontogenetic transition in leaf traits: a new cost associated with the increase in leaf longevity. J. Plant Ecol. 7 567–575
Maillard A, Diquélou S, Billard V, et al. 2015 Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 6 317
Nabeshima E and Hiura T 2004 Size dependency of photosynthetic water- and nitrogen-use efficiency and hydraulic limitation in Acer mono. Tree Physiol. 24 745–752
Niinemets Ü 2010 Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 260 1623–1639
Niinemets Ü and Lukjanova A 2003 Needle longevity, shoot growth and branching frequency in relation to site fertility and within-canopy light conditions in Pinus sylvestris. Ann. For. Sci. 60 195–208
Niinemets U, Cescatti A, Rodeghiero M, et al. 2006 Complex adjustments of photosynthetic potentials and internal difusion conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species Quercus ilex. Plant Cell Environ. 29 1159–1178
Onoda Y, Wright IJ, Evans JR, et al. 2017 Physiological and structural trade offs underlying the leaf economics spectrum. New Phytol. 214 1447–1463
Poorter H and Evans JR 1998 Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116 26–37
Poorter H, Niinemets Ü and Poorter L 2009 Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 182 565–588
R Core Team 2021 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rawat M, Arunachalam K, Arunachalam A, et al. 2021 Assessment of leaf morphological, physiological, chemical and stoichiometry functional traits for understanding the functioning of Himalayan temperate forest ecosystem. Sci. Rep. 11 23807
Rapp M, Santa Regina I, Rico M, et al. 1999 Biomass, nutrient content, litterfall and nutrient return to the soil in Mediterranean oak forests. For. Ecol. Manag. 119 39–49
Reich PB and Borchert R 1988 Changes with leaf age in stomatal function and water status of several tropical tree species. Biotropica 60–69
Reich PB and Flores-Moreno H 2017 Peeking beneath the hood of the leaf economics spectrum. New Phytol. 214 1395–1397
Reich PB, Walters M and Ellsworth DS 1991 Leaf age and leaf age inuence the relationships between leaf nitrogen, leaf mass per area and photosynthesis in maple and oak trees. Plant Cell Environ. 14 25
Reich PB, Walters MB and Ellsworth DS 1992 Leaf lifespan in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 62 365–392
Reich PB, Walters MB, Ellsworth DS, et al. 1998 Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf lifespan: a test across biomes and functional groups. Oecologia 114 471–482
Ryan MG and Yoder BJ 1997 Hydraulic limits to tree height and tree growth. Bioscience 47 235–242
Ryan MG, Phillips N and Bond BJ 2006 The hydraulic limitation hypothesis revisited. Plant Cell Environ. 29 367–381
Robinson DE, Wagner RG, Bell FW, et al. 2001 Photosynthesis, nitrogen-use efficiency, and water-use efficiency of jack pine seedlings in competition with four boreal forest plant species. Can. J. For. Res. 31 2014–2025
Rodriguez-Calcerrada J, Reich PB, Rosenqvist E, et al. 2008 Leaf physiological versus morphological acclimation to high-light exposure at different stages of foliar development in oak. Tree Physiol. 28 761–771
Sala A, Piper F and Hoch G 2010 Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 186 274–281
Schönbeck LC, Schuler P, Lehmann MM, et al. 2022 Increasing temperature and vapour pressure deficit lead to hydraulic damages in the absence of soil drought. Plant Cell Environ. 45 3275–3289
Sheng W, Ren S, Yu G, et al. 2011 Patterns and driving factors of WUE and NUE in natural forest ecosystems along the North-South Transect of Eastern China. J. Geogr. Sci. 21 651–665
Sigdel SR, Liang E, Rokaya MB, et al. 2023 Functional traits of a plant species fingerprint ecosystem productivity along broad elevational gradients in the Himalayas. Funct. Ecol. 37 383–394
Singh R, Rawat M and Pandey R 2023 Quantifying leaf-trait co-variation and strategies for ecosystem functioning of Quercus leucotrichophora (Ban Oak) forest in Himalaya. Ecol. Indic. 150 110212
Thomas SC 2010 Photosynthetic capacity peaks at intermediate size in temperate deciduous trees. Tree Physiol. 30 555–573
Thomas SC 2011a Age-related changes in tree growth and functional biology: the role of reproduction; in Size- and age-related changes in tree structure and function (Eds) Meinzer FC, Lachenbruch B and Dawson TE (Springer) pp 33–64
Thomas SC 2011b Genetic vs. phenotypic responses of trees to altitude. Tree Physiol. 31 1161–1163
Thomas SC and Winner WE 2002 Photosynthetic differences between saplings and adult trees: an integration of field results by meta-analysis. Tree Physiol. 22 117–127
Whitmarsh J 1999 The photosynthetic process; in Concepts in photobiology: photosynthesis and photomorphogenesis (Springer) pp 11–51
Wilson KB, Baldocchi DD and Hanson PJ 2000 Quantifying stomatal and non-stomatal limitations to carbon assimilation resulting from leaf aging and drought in mature deciduous tree species. Tree Physiol. 20 787–797
Wilson KB, Baldocchi DD and Hanson PJ 2001 Leaf age affects the leaf ageal pattern of photosynthetic capacity and net ecosystem exchange of carbon in a deciduous forest. Plant Cell Environ. 24 571–583
Wright IJ, Leishman MR, Read C, et al. 2006 Gradients of light availability and leaf traits with leaf age and canopy position in 28 Australian shrubs and trees. Funct. Plant Biol. 33 407–419
Wright IJ, Reich PB, Westoby M, et al. 2004 The worldwide leaf economics spectrum. Nature 428 821–827
Wright IJ, Reich PB, Cornelissen JH, et al. 2005 Modulation of leaf economic traits and trait relationships by climate. Glob. Ecol. Biogeogr. 14 411–421
Acknowledgements
Financial support from the Department of Science and Technology (DST), New Delhi, India (SERBNo:DST/IS-STAC/CO2-SR-181/13 G), [DST/SPLICE/CCP/NMSHE/TF2015/JNU/2014[G]] and DST PURSE is acknowledged. This research was carried out as part of the Ph.D. program of RKJ, which was financially supported by University Grants Commission (UGC), India. We express our gratitude to the editor and the two anonymous reviewers for their invaluable suggestions and comments, which greatly contributed to enhancing the quality of this manuscript.
Author information
Authors and Affiliations
Contributions
SCG and RKJ conceived the idea; RKJ, AM, and RG, designed and conducted the field experiment and analyzed the data; RKJ, RG, and AM contributed reagents/materials/analysis tools; RKJ wrote the manuscript, and SCG, AM, and RG edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Corresponding editor: Agepati Raghavendra
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joshi, R.K., Mishra, A., Gupta, R. et al. Leaf and tree age-related changes in leaf ecophysiological traits, nutrient, and adaptive strategies of Alnus nepalensis in the central Himalaya. J Biosci 49, 24 (2024). https://doi.org/10.1007/s12038-023-00385-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-023-00385-9