Abstract
Key message
Migration ability of the PWN through wood branch tissues of adult Maritime pine trees significantly differed among Iberian provenances and this variation was related to differences in anatomical and chemical defensive traits.
Abstract
The pinewood nematode or pine wilt nematode (PWN; Bursaphelenchus xylophilus) is one of the most dangerous threats to European coniferous forests, especially for the susceptible Maritime pine (Pinus pinaster), a valuable forest resource in South Western Europe. The PWN is vectored by beetles of the genus Monochamus (Coleoptera, Cerambycidae) and once inoculated in healthy branches, it quickly migrates downward to the main trunk through the resin canal system. Therefore, the anatomy of the resin canal system may modulate its migration and proliferation rates. Using material from nine Maritime pine Iberian provenances established in a common garden trial, we investigated whether these provenances differed in their (1) resin canal anatomy, (2) concentration of chemical defences (non-volatile resin and total polyphenolics) in stems and (3) ability of the PWN to migrate through the pine woody tissues in ‘in vitro’ bioassays. Whether variation in anatomical and chemical defensive traits relates to differences in PWN migration across populations was also investigated. Significant intraspecific variation in anatomical and chemical defensive traits and in nematode migration rates through pine tissues was observed. Moreover, the variation in nematode migration rate among pine provenances was related to differences in both anatomical and chemical features. Overall, this study highlights the role of plant genetics in the development of defensive traits against this harmful coniferous pest. The observed intraspecific variation should be taken into account when considering breeding as a strategy to provide areas of high risk of PWN with resistant genetic material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Range expansions of non-native pests and pathogens to new host plant species is becoming one of the characteristic environmental changes of the Anthropocene. A paradigmatic example is the pinewood nematode (PWN; Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle) which, out of its native range, causes a devastating wilt disease that kills several Pinus spp. trees within weeks or a few months (Kuroda 2008b). By blocking the water conductance in the xylem and inducing tracheid cavitation, the PWN has caused extensive damage in the pine forests of Japan, China, Korea, and Taiwan affecting several pine species including Pinus densiflora, P. thunbergii, P. massoniana and P. koraiensis (Webster and Mota 2008). The PWN is nowadays considered as one of the main threats to European coniferous forests (Vicente et al. 2012). After its introduction in the late 1990s in a P. pinaster stand in the West coastal area of the Iberian Peninsula (Mota et al. 1999), it has rapidly spread over the entire Portuguese territory (Vicente et al. 2012), and reached Spain in just a few years (Robertson et al. 2011).
The PWN is vectored by beetles of the genus Monochamus (Coleoptera, Cerambycidae), which inoculate the nematodes in branches of healthy trees during the insect´s maturation feeding (Sousa et al. 2001). It is now well accepted that, once a healthy branch is attacked by the insect, the PWN quickly migrates downward to the main stem and colonizes the whole tree through the resin canal system (Ichihara et al. 2000a; Kuroda 2008b; Son et al. 2010), particularly through the thicker resin canals of the phloem and cortex (Kawaguchi 2006). The anatomy of the resin canal system is, therefore, thought to influence the ability of the nematodes to migrate through the tree and colonize healthy tissues (Kuroda 2008b); accordingly, the number and size of resin canals have been shown to determine the migration rates of the nematodes in P. thunbergii (Kawaguchi 2006). Moreover, nematode migration has been related to the virulence of the PWN (Kuroda 2008b; Son et al. 2010). In particular, nematode migration has been found to be slower or even completely blocked in resistant conifer species (Oku et al. 1989; Nunes da Silva et al. 2013), and in resistant genetic variants (Kuroda 2004; Kuroda et al. 1991). Some examples that contradicted this were also reported, however, indicating that migration and colonization ability do not completely determine the susceptibility of pines to the PWN (Mori et al. 2008; Eo et al. 2011). The accumulation of chemical defensive compounds that repeal, immobilize or disrupt the life cycle and reproduction of nematodes (Suga et al. 1993; Hanawa et al. 2001; Zhang et al. 2013), and the ability to activate defensive responses to the infection (Ichihara et al. 2000b), may also play a key role. Reduced nematode migration and proliferation rates within the plant tissues seem to be crucial for pine resistance to the PWN (Kuroda 2008b).
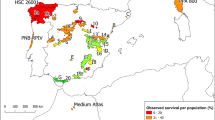
The PWN affects several pine species differently, with P. densiflora and P. pinaster being extremely susceptible and P. taeda, P. strobus and P. pinea highly resistant (Dwinell 1984; Woo et al. 2008; Dayi and Akbulut 2012). Variation of resistance within pine species to the PWN has been also reported in some previous studies (Kuroda 2004; Franco et al. 2011; Akiba et al. 2012), and prompted the launch of different breeding initiatives aimed to provide resistant genetic material to be used in areas of high risk of PWN damage (Toda and Kurinobu 2002; Nose and Shiraishi 2008; Ribeiro et al. 2012). Maritime pine (Pinus pinaster Aiton) is the most affected tree species in Portugal (Vicente et al. 2012), and the only one in Spain in which this nematode was reported (Robertson et al. 2011). Maritime pine occupies large areas in south west Europe and North Africa (more than 4 million ha). Within its natural distribution range (Fig. 1), Maritime pine has a fragmented distribution, with numerous relatively small and isolated populations (Bucci et al. 2007). Reduced gene flow among populations has favored a strong differentiation between them, which is well documented in terms of genetic (González-Martínez et al. 2002; Burban and Petit 2003) and phenotypic variation of different adaptive traits (Chambel et al. 2007; Corcuera et al. 2012; Santos del Blanco et al. 2012), other relevant traits for timber production (de la Mata and Zas 2010a; Lamy et al. 2012), and herbivore and pathogen resistance (Arrabal et al. 2005). For example, intraspecific variation in resin flux (Tadesse et al. 2001), accumulation of non-volatile resin, total polyphenols and condensed tannins in stems and needles (Sampedro et al. 2011), and resin terpene profiles (Arrabal et al. 2005; Sampedro et al. 2010) have all been reported. Variation in susceptibility to several insect herbivores (Jactel et al. 1996; Burban et al. 1999; Zas et al. 2005) and fungal pathogens (Solla et al. 2011; Vivas et al. 2012) has also been well documented. However, despite this knowledge, and the enormous threat that the PWN poses to P. pinaster, the question of whether there is intraspecific variation in resistance/susceptibility to the PWN and in other putatively related resistant traits remains unexplored.
Maritime pine natural distribution range in the Iberian Peninsula (shaded area), provenances (dots) included in the study and location of the provenance trial (square). See Table S1 in Supplementary material for more details on the geographic and climatic characteristics of the studied provenances. The entire territory of Portugal is now considered to be affected by the PWN (dashed area). The two asterisks in the map indicate the locations in which the PWN was detected in Spain. The distribution map of P. pinaster was obtained from EUFORGEN 2009, www.euforgen.org. The CG and LE materials come from breeding programs developed in Galicia (NW Spain) upon material selected in the coastal area of this region, and in Western Australia based on original material selected within the Leiria Portuguese population, respectively (See details in the main text)
Taking advantage of a P. pinaster common garden test which includes plant material from nine Iberian provenances, in this study we explore: (1) whether there is intraspecific variation in the resin canal anatomy and the concentration of two quantitative resistance traits (non-volatile resin and total polyphenolics); (2) whether the ability of the PWN to migrate through the pine woody tissues varies across provenances, and (3) whether the variation in anatomical and chemical defensive traits could explain any variation in PWN migration rate across provenances.
Materials and methods
Plant material and experimental site
Our study was carried out using plant material belonging to a provenance trial of Maritime pine (P. pinaster) established in 2001 in the interior area of Galicia (NW Spain) by the Forestry Research Centre of Lourizán (Xunta de Galicia, Pontevedra, Spain). The trial was part of a series of provenance trials designed to search for alternative materials to be used in inland Galicia, a transitional region between the typical Atlantic and Mediterranean climates of the Iberian Peninsula, for which adapted forest reproductive materials for reforestation purposes are lacking (de la Mata and Zas 2010a). The trial, sited at Guntín (Lugo, Spain; N 42° 53.853′ W 007° 41.049′; 540 m above sea level, Fig. 1), followed a randomized complete block design and included nine Iberian provenances: seven Mediterranean provenances of Central and Eastern Spain (Bajo Tietar (BT), Sierra de Gredos (GR), Montaña de Soria-Burgos (SB), Serranía de Cuenca (SC), Sierra de Albarracín (AL), Sierra de Gata (SG) and Sierra de Segura Alcaraz (SS)) and two Atlantic origins represented by genetically improved materials (Coastal Galicia (CG) and Leiria (LE)) (Fig. 1; see also Table S1 in Online Supplementary Material). The CG provenance (NW Spain) comprises the F1 open-pollinated offspring of plus trees selected for timber production within the coastal area of Galicia (de la Mata and Zas 2010b). The LE origin was represented by a collection of families derived from crosses between plus trees selected within the Leiria provenance (Portugal) that were obtained within the frame of the Maritime pine breeding program developed in Western Australia (Butcher 2007). More details of this genetic trial can be consulted in de la Mata and Zas (2010a, b).
Sampling
During May and June 2011, when trees were 10 years old, two 2-year-old branches from ten individual trees per provenance were sampled for chemical and anatomical analyses, and for determining the ability of the PWN to migrate through wood tissues. Trees were randomly selected from the dominant trees of the trial, avoiding trees with any visual disorder (e.g., defoliation, discoloration, wounds, etc.). In each branch, the internode corresponding to the 2009 growth (ca. 1.0–2.0 cm of diameter) was sampled and immediately transported to the laboratory inside ice coolers (4 °C maintaining high humidity). The branch internode was then divided into three groups for (1) chemical, (2) histological, and (3) nematode migration assessments. Chemical analyses and histology were done at Misión Biológica de Galicia (CSIC, Spain). Nematode migration bioassays were performed at Centro de Biotecnologia e Química Fina (ESB-UCP, Porto, Portugal). To allow nematode migration assessment to be done within 36 h after branch cutting, field sampling was performed over four different dates with a 4- to 7-day interval. Sampling dates were considered in the statistical analyses, but they did not significantly affect the results.
Chemical analyses
Concentration of non-volatile resin and total polyphenolics was determined in freshly sampled branches (ca. 5 cm long pieces). Concentration of non-volatile resin was determined gravimetrically as described in Moreira et al. (2014). Non-volatile resin was extracted with hexane in an ultrasonic bath first for 15 min at 20 °C and then for 24 h at room temperature. The extract was filtered (Whatman GFF, Whatman Int. Ltd, Kent, UK) and the extraction process was repeated once again. The concentration of non-volatile resin was estimated by weighing the extracted resin to the nearest 0.0001 g after solvent evaporation, and expressed as mg of non-volatile resin g−1 dry weight (d.w.). The residual plant material was then extracted with aqueous methanol (1:1 vol:vol) in an ultrasonic bath for 15 min, followed by centrifugation and subsequent dilution of the methanolic extract. Total polyphenolic content in the extract was determined colorimetrically by the Folin-Ciocalteu method in a Biorad 650 microplate reader (Bio-Rad Laboratories Inc., Philadelphia, PA, USA) at 740 nm, using tannic acid as standard, and expressed in mg tannic acid equivalents g−1 d.w. (see more details in Moreira et al. 2014). A total of 90 samples (10 trees × 9 provenances) were analyzed with three analytical replicates.
Additional fresh branch segments (ca. 5 cm long) were used to determine the non-volatile resin separately in both the phloem-cortex and in the xylem tissues. The phloem-cortex was separated with a surgical knife that enabled its peeling away from the inner lignified wood. Non-volatile resin was determined in the two fractions following the procedure previously described. A total of 69 trees were analyzed for resin in phloem-cortex and xylem (8 or 9 trees × 9 provenances).
Histology
Branch segments of ca. 5 cm were fixed in formalin acetic acid (FAA) immediately after sampling, and then transferred to 70 % EtOH for storage until sectioning and staining (Moreira et al. 2008). Cross sections, 90 μm thick, were made using a sliding microtome. Sections were stained for 12 h with 0.1 % aqueous Safranin according to standard procedures (Ruzin 1999). Photographs were taken with a Nikon Digital Sight DS-U1, mounted on a Nikon SMZ-U binocular microscope at 20× magnification. Resin canals on digital images of two quadrants per sample (covering about 75 % of the total transectional area) were counted and diameters radially measured using the Phloemalizer v.2.12 image analysis software developed at the Pacific Forestry Centre (Victoria, BC, Canada) (Moreira et al. 2012).
The following variables in the cortex and in the xylem were obtained for each cross section: (1) resin canal density, through the number of longitudinal resin canals per unit area, (2) mean interior area of individual canals (μm2), and (3) relative conductive area (%), obtained by dividing the total transectional area occupied by the resin canals by the total area of the tissue assessed, then multiplying by 100. Digital image analysis was done at the Misión Biológica de Galicia (Pontevedra, Spain).
Nematode migration rate
The ability of virulent PWN strains to migrate through the branches of each maritime pine origin was determined by migration bioassays tests, as described in Lima et al. (2012). Pinewood nematode inocula were prepared by culturing two virulent isolates of B. xylophilus (BxHF and Bx8A, both originating from Setúbal Peninsula region, Portugal) on a culture of Botrytis cinerea growing on sterilized barley seeds. The nematodes were extracted from the grains using the Baermann funnel method, counted on a dissecting microscope, and adjusted into a solution of 10 nematodes μl−1 in deionised water. All the branches were washed with deionised water and subjected to an ultrasonic bath for 5 min to eliminate any air bubble that could prevent nematode migration, after which they were cut into 5 cm segments. Natural basal and distal ends of the branch segments were identified through visual inspection of the bark scales orientation, and segments were placed vertically on 50 ml centrifuge tubes (previously cut by the 15 ml mark). The basal section of the segments (ca. 1 cm) was immersed in 3 ml of deionised water, segments attached to the centrifuge tube with parafilm and then 200 nematodes (20 μl of solution) were inoculated on the distal surface of each segment. Once the nematode solution was absorbed, the distal surface of the segment was sealed with parafilm to avoid desiccation. The segments containing the nematodes were incubated at 25 °C in the dark for 24 h. After this time, the nematodes that migrated through the branch segments and reached the water from the basal section were counted on a dissecting microscope. The migration test was performed within the first 36 h after branch sampling in five replicated segments per tree (N = 450). Tests were performed at the Centro de Biotecnologia e Química Fina (ESB-UCP, Porto, Portugal).
Statistical analyses
Variation in chemical and anatomical traits among maritime pine provenances was analyzed with a one-way ANOVA. The analysis of the migration rate was performed with a repeated measure ANOVA in which the five samples of each tree were considered repeated measures of the same subject, accounting, therefore, for any autocorrelation among them. A compound symmetric covariance structure among repeated measures was assumed. The models also included the random effect of the sampling date (four levels) and the covariation with the stems mean diameter. Models were fitted with the PROC MIXED procedure in SAS 9.2 (SAS Institute, Cary, NC, Littell et al. 2006). When necessary, dependent variables were transformed (log(x) or √x) to achieve normality. Least square means (± standard errors) were obtained from these models for each provenance. Pearson correlation analyses were performed to explore the relationships between nematode migration rates and the chemical and anatomical traits. Correlation analyses were performed with the PROC CORR procedure in SAS 9.2.
Results
Variation in growth among pine provenances
Total height of Maritime pine trees in the common garden test significantly differed depending on the plant origin (Table 1), Atlantic (Leiria (LE) and Coastal Galicia (CG)) growing faster than Mediterranean provenances (Fig. S1). Among the Mediterranean provenances, Sierra de Gata (SG) and Montaña de Soria Burgos (SB) were the slowest in growing (Fig. S1). No significant differences among pine provenances were observed for breast height diameter (Table 1).
Variation of chemical and anatomical defensive traits
Maritime pine provenances significantly differed in the mean area of cortex resin canals, density of xylem resin canals and the concentration of defensive chemicals (Table 1). No significant differences between provenances were observed for the density and relative conductive area occupied by cortex resin canals and for the mean and relative conductive areas of the xylem resin canals (Table 1). Trees from the Bajo Tietar (BT) provenance showed the greatest concentration of non-volatile resin in the whole stem, whereas those from Sierra de Segura Alcaraz (SS) and Sierra de Gredos (GR) showed the lowest (Fig. 2a). Sierra de Segura Alcaraz trees showed the lowest concentration of total polyphenols, and those from Leiria (LE), the highest (Fig. 2b). Trees from BT, LE and GR provenances stood out for the large size of their cortex resin canals (Fig. 2c). Mean size of cortex resin canals of BT and LE trees was ca. twofold greater than the mean size of cortex resin canals in trees from Coastal Galicia (CG). Trees from LE and SG showed the lowest and the highest densities of resin canals in the xylem, respectively (Fig. 2d).
Concentration of non-volatile resin (a) and total polyphenolics (b), and mean size of individual cortex resin canals (c), and the density of resin canals in the xylem (d) in two-year old branches of nine Maritime pine provenances. The provenances assayed were Coastal Galicia (CG), Leiria (LE), Sierra de Gata (SG), Bajo Tietar (BT), Sierra de Gredos (GR), Montaña de Soria-Burgos (SB), Sierra de Albarracín (AL), Serranía de Cuenca (SC) and Sierra Segura-Alcaraz (SS). Each provenance was represented by 10 individuals established in a common garden test located in NW Spain. Mean ± s.e. are shown
Concentration of non-volatile resin in the phloem-cortex significantly differed among pine provenances (Table 1) and showed a strong positive correlation with resin in the whole stem at the provenance level (r = 0.863, N = 9, P = 0.003). However, at the phenotypic level, concentration of non-volatile resin in the phloem-cortex was not related with that in the whole stem (r = 0.099, N = 65, P = 0.433). Concentration of non-volatile resin in the xylem did not significantly differ across provenances (Table 1).
Variation in nematode migration ability
Nematode migration through the branch segments varied significantly across Maritime pine provenances, with some origins presenting twofold more recovered nematodes than others (Fig. 3). In particular, trees from LE allowed the highest nematode migration in the bioassays (Fig. 3), in comparison with trees from genuine Mediterranean origins such as SS and Sierra de Albarracín (AL), in which nematode migration was clearly restricted (Fig. 3).
Migration ability of the pinewood nematode through branch segments of nine Maritime pine provenances measured in bioassays. Each provenance was represented by 10 individuals established in a common garden test located in NW Spain. A total of 200 nematodes were inoculated in 5-cm long branch segments and migration rate was measured after 24 h. Five replicated bioassays were performed for each individual. Provenances are ordered southwards. Mean ± s.e
Relationship between migration rate and anatomical and chemical traits
Nematode migration through the branch segments of the different Maritime pine provenances was significantly and positively correlated with the mean area of the resin canals in the cortex and the concentration of polyphenols (Table 2; Fig. 4). No other chemical or anatomical traits were significantly related to the nematode migration rates (Table 2). From the correlation analyses it could be inferred that the mean canal area, rather than the density of resin canals, was the main determinant of relative conductive area of resin canals in the cortex. On the contrary, in the xylem, the relative conductive area was influenced by the density but not by the mean area of canals (Table 2). Interestingly, a negative relationship between the relative conductive area of resin canals in the cortex and the density of resin canals in the xylem was observed (Table 2). No significant relationships were observed between the concentration of non-volatile resin and any of the measured defensive traits except for total polyphenols (Table 2).
Despite the observed significant relations at the population level, phenotypic correlations at the individual level between nematode migration rates and anatomical and chemical traits were not significant (P > 0.05).
Discussion
Our study provides three noteworthy results: first, anatomical and chemical traits putatively related to conifer resistance against biotic threats were differentially expressed depending on the origin of the maritime pine seeds; second, nematode migration throughout pine tissues significantly varied among the different Maritime pine provenances; and third, variation of nematode migration rates among pine provenances was related to the variation of anatomical and chemical traits across different pine origins.
Variation of chemical and anatomical defensive traits
Variation of non-volatile resin and total polyphenol concentrations between pine provenances agree with our previous greenhouse studies showing that these two traits are highly variable within a single Maritime pine population (Sampedro et al. 2011) and between populations (López-Goldar et al. unpublished). In the present study, variation of these two chemical defensive traits between provenances did not show a clear geographical pattern. However, it is worth mentioning that both traits were positively related at the provenance level, despite they were not related at the phenotypic level (r = −0.021, P = 0.846, N = 88), suggesting no overlap in the functionalities of chemical defences of different type, as observed in other studies (Koricheva et al. 2004). These chemicals may be present in extremely large concentration in pine tissues (in the order of dozens of mg g−1), and their accumulation is known to be costly for the plants, as it has been found to be associated with a reduction of plant growth potential (Moreira et al. 2014; Sampedro et al. 2011). In this study, although non-volatile resin was negatively correlated with tree diameter at the phenotypic level (r = −0.24, N = 85, P = 0.030), growth potential and concentration of defensive chemicals were not related across provenances (r = 0.56, N = 9, P = 0.180; r = 0.37, N = 9, P = 0.326 for non-volatile resin and total polyphenolics, respectively). In consequence, physiological constrains at the individual level seems not to have influence on the co-differentiation among population in these traits.
Maritime pine provenances differed in the density and size of resin ducts, but different patterns were observed depending on the tissue. Cortex resin canals were variable in size (mean transectional area) rather than in number across provenances, whereas xylem resin canals were more homogeneous in size but were highly variable in number. Previous studies in Maritime pine have shown that cortex resin ducts are influenced by resources availability (e.g., soil nutrients), whereas xylem resin ducts appear to be more sensitive to the biotic environment (e.g., herbivory), with proliferation of traumatic xylem resin ducts in response to herbivore damage (Moreira et al. 2008). Despite the environmental influence on resin canal traits, the variation observed in the present study was attributable to genetic differentiation processes among the studied populations, as the environmental conditions within the common garden test were fairly homogenous.
Intraspecific variation in anatomical defensive structures has been reported for other coniferous species (e.g., Martín et al. 2010; Moreira et al. 2012; Esteban et al. 2012), but no information regarding across provenances genetic variation of resin canal anatomy is available for Maritime pine. Maritime pine populations are known to greatly vary in several life-history traits such as growth (de la Mata and Zas 2010a), reproduction (Santos del Blanco et al. 2012), fire tolerance (Fernandes and Rigolot 2007), drought tolerance (Gaspar et al. 2013) and cold tolerance (Prada et al. 2014). Based on the relationships between the phenotypic expression of these traits and the environmental conditions in the place of origin, it has been inferred that the differentiation among provenances could be related to adaptive processes. The between provenances variation observed in defensive traits in the present study might have also originated from adaptive processes, as geographically distant Maritime pine populations might have been subjected to different selection pressures by biotic threats. However, inferring the adaptive value of the observed differences would be difficult as the biotic environment in which the different origins have evolved is largely unknown. Additionally, many other factors, including demographic processes (Bucci et al. 2007), environmental effects driving population differentiation in defensive traits (Martín et al. 2010; Estaban et al. 2012) or trade-offs among different fitness related traits (Moreira et al. 2014) could also be relevant.
Variation in nematode migration ability
Maritime pine provenances differed in terms of PWN migration ability in stem tissues. Using a simple in vitro bioassay (Lima et al. 2012), similar to that used in other related studies (Son et al. 2010), we found that migration rates of the PWN through branches of some provenances were more than twofold higher than in other provenances. Nematode migration is a key component of the capability of nematodes to colonize healthy pines, and decreased migration rates can be assumed to be related, at least in part, to pine resistance against nematodes (Kuroda 2008b; Son et al. 2010). The observed variation among maritime pine provenances is a first step in providing some insight about the variation in susceptibility to the PWN along the vast and heterogeneous area occupied by this pine species in the Iberian Peninsula. It should be noted that the migration tests were done on excised branch segments. Although we were especially careful to avoid desiccation and physical deterioration of the samples, we cannot assert that the migration ability of the PWN in excised tissues would be identical from that in living branches. Further research should test this uncertainty.
Interestingly, the Leiria (LE) material showed the highest nematode migration rate. This provenance naturally distributes in an area especially damaged by the PWN (Rodrigues 2008), close to where the PWN was first detected in Europe (Mota et al. 1999). The potentially high susceptibility of LE, inferred by the high nematode migration observed, is consistent with the rapid expansion and devastating effects of the PWN across Portugal. The LE material analyzed here was derived from a breeding program developed in Western Australia upon the material selected within the Leiria Portuguese provenance. The breeding program was designed to improve stem straightness and tree vigor (Butcher 2007), as was the case for the breeding program developed in Spain with the CG material (e.g., Zas and Merlo 2008). The material studied here from LE and CG can be considered, thus, representative of the material used for reforestation purposes in Atlantic areas of the Iberian Peninsula.
Although the LE material was also the fastest grower in the provenance trial (de la Mata and Zas 2010a), growth potential and nematode migration rates were not significantly related (r = 0.56, N = 9, P = 0.118). Other fast-growing origins appeared to restrict nematode migration to a higher extent. Of particular note in this respect was the other Atlantic selected material (CG), which also stands out as being fast growing (de la Mata and Zas 2010a). While this origin is assumed to be genetically close to the Leiria provenance (Bucci et al. 2007), CG was much more resistant than LE in terms of nematode migration rate.
Overall, differences among populations in nematode susceptibility did not follow any clear climatic or geographical patterns. Mediterranean origins, for instance, showed a large variation in nematode susceptibility, and included populations that appeared to be highly susceptible. Simulation studies predict that the PWN could easily spread under a warming climate if both vectoring insects and susceptible trees exist (i.e., Fernández and Solla 2008). Besides, temperature is regarded as the most important factor for the progression of the disease (Evans and Futai 2008), because high summer temperatures and large seasonal variation in water availability increase the risk of wilt expression (Evans et al. 2008). As summer temperatures and summer drought are strong in these Mediterranean origins, the apparent high susceptibility to the PWN found in the present study can be seen as a warning signal of the risk of expansion across the Mediterranean Basin.
Relationship between migration rate and anatomical and chemical traits
Results, indicating a positive relation between the mean area of axial resin canals in the cortex and nematode migration, agree with previous findings (Kawaguchi 2006), and suggest that cortex resin canals may be the most important paths of nematode dispersal in two-year-old branches. Nematode migration rate was previously related to the size of the resin canals of the cortex (Kawaguchi 2006), and cortex canals are known to be one of the most important routes for nematode dispersal inside the tree (Ichihara et al. 2000a; Kuroda 2008b; Son et al. 2010). However, cortex tissues are ephemeral in pine species, dying in a few years and leading to periderm formation. Resin canals in the cortex would thus become dysfunctional in older branches, and PWN would likely move to the vertical resin canals of the xylem through the horizontal radial canals. Consequently, in older branches, both vertical and radial xylem resin canals could be also relevant for PWN migration. Nematodes are able to migrate through the xylem canal system and xylem tracheids (Kuroda 2008a; Son et al. 2010). Our results suggest, however, a secondary relevance of those migration paths, at least in two-year-old branches, and a more relevant role of resin canals in the cortex.
We found a positive relationship between nematode migration and polyphenol concentration of branch segments, contradicting previous research reporting that PWN resistant species or varieties accumulate more phenolic compounds than susceptible ones in response to PWN infection (Kuroda et al. 2011; Nunes da Silva et al. 2013). Some particular phenolic compounds found in resistant pine species, i.e., stilbenoids, have been reported to show nematicidal activity to the PWN in in vitro assays (Suga et al. 1993), although their role as a factor of resistance in vivo has been questioned (Zhang et al. 2013). On the other hand, phenolic compounds are also known to accumulate in response to the PWN infections when compared to control trees (Futai 2003). Most studies reporting positive relations between phenolic concentrations and PWN resistance failed to differentiate whether this link was due to variation in constitutive (as observed here) or induced levels of polyphenols. Further research will be needed to clarify the role of phenolic compounds in the intraspecific variation in PWN resistance in Maritime pine.
Relationships between nematode migration rates and anatomical and chemical traits were only significant at the provenance level, but not at the individual (phenotypic) level. This result calls for caution when interpreting our results and suggest that other unstudied phenotypic traits may be also relevant for nematode migration at the individual level. Despite this lack of phenotypic relationships, genetic differentiation among provenances in cortex resin canals and total polyphenolics parallels that in nematode migration rate. It should be noted, however, that the significant relationships observed at the population level appear to be highly influenced by the particular population of Leira, which has outstanding values for all these traits. Further research including more Maritime pine origins and other anatomical and chemical traits is needed to clarify the complex equation of nematode susceptibility.
Overall, this study highlights the relevance of tree genetics, anatomy and chemical defensive traits as resistance factors against the PWN. These traits could be extremely valuable for future breeding initiatives aimed at obtaining resistant genetic material.
References
Akiba M, Ishihara M, Sahashi N, Nakamura K, Ohira M, Toda T (2012) Virulence of Bursaphelenchus xylophilus isolated from naturally infested pine forests to five resistant families of Pinus thunbergii. Plant Dis 96:249–252
Arrabal C, Cortijo M, Fernández de Simón B, García Vallejo MC, Cadahía E (2005) Differentiation among five Spanish Pinus pinaster provenances based on its oleoresin terpenic composition. Biochem Syst Ecol 33:1007–1016
Bucci G, Gonzalez Martinez SC, Le Provost G et al (2007) Range-wide phylogeography and gene zones in Pinus pinaster Ait. revealed by chloroplast microsatellite markers. Mol Ecol 16:2137–2153
Burban C, Petit RJ (2003) Phylogeography of maritime pine inferred with organelle markers having contrasted inheritance. Mol Ecol 12:1487–1495
Burban C, Petit RJ, Carcreff E, Jactel H (1999) Rangewide variation of the maritime pine bast scale Matsucoccus feytaudi Duc. (Homoptera: matsucoccidae) in relation to the genetic structure of its host. Mol Ecol 8:1593–1602
Butcher TB (2007) Achievements in forest tree genetic improvement in Australia and New Zealand. Maritime pine and Brutian pine tree improvement programs in Western Australia. Aus For 70:141–151
Chambel MR, Climent J, Alía R (2007) Divergence among species and populations of Mediterranean pines in biomass allocation of seedlings grown under two watering regimes. Ann For Sci 64:87–97
Corcuera L, Gil-Pelegrín E, Notivol E (2012) Differences in hydraulic architecture between mesic and xeric Pinus pinaster populations at the seedling stage. Tree Physiol 32:1442–1457
Dayi M, Akbulut S (2012) Pathogenicity testing of four Bursaphelenchus species on conifer seedlings under greenhouse conditions. For Pathol 42:213–219
de la Mata R, Zas R (2010a) Performance of maritime pine Spanish Mediterranean provenances in a transitional region between Atlantic and Mediterranean climates in NW Spain. Silvae Genet 59:8–17
de la Mata R, Zas R (2010b) Transferring Atlantic maritime pine improved material to a region with marked Mediterranean influence in inland NW Spain: a likelihood-based approach on spatially adjusted field data. Eu J For Res 129:645–658
Dwinell LD (1984) Relative susceptibilities of 5 southeastern pine species to the pinewood nematode, Bursaphelenchus xylophilus. Phytopathology 74:870
Eo J, Takemoto S, Otobe K (2011) Is there a relationship between the intrinsic rate of propagation and in vitro migration and virulence of the pinewood nematode, Bursaphelenchus xylophilus? Eu J Plant Pathol 130:231–237
Esteban LG, Martín JA, de Palacios P, García-Fernández F (2012) Influence of region of provenance and climate factors on wood anatomical traits of Pinus nigra Arn. subsp. salzmannii. Eu J For Res 131:633–645
Evans H, Futai K (2008) Ecology and modeling. In: Vieira P, Mota M (eds) Pine wilt disease: a worldwide threat to forest ecosystems. Springer, Germany, pp 255–257
Evans S, Evans H, Ikegami M (2008) Modelling PWN-induced wilt expression: A mechanistic approach. In: Vieira P, Mota M (eds) Pine wilt disease: a worldwide threat to forest ecosystems. Springer, Germany, pp 259–278
Fernandes PM, Rigolot E (2007) The fire ecology and management of maritime pine (Pinus pinaster Ait.). For Ecol Manage 241:1–13
Fernández JM, Solla A (2008) Mapas de riesgo de aparición y desarrollo de la enfermedad del marchitamiento de los pinos (Bursaphelenchus xylophilus) en Extremadura. For Syst 15:141–151
Franco AR, Santos C, Roriz M, Rodrigues R, Lima MRM, Vasconcelos MW (2011) Study of symptoms and gene expression in four Pinus species after pinewood nematode infection. Plant Gen Res 9:272–275
Futai K (2003) Abnormal metabolites in pine wood nematode-inoculated Japanese black pine. Jap J Nematol 33:45–56
Gaspar MJ, Velasco T, Feito I, Alía R, Majada J (2013) Genetic variation of drought tolerance in Pinus pinaster at three hierarchical levels: a comparison of induced osmotic stress and field testing. PLoS One 8(11):e79094
González-Martínez SC, Alía R, Gil L (2002) Population genetic structure in a Mediterranean pine (Pinus pinaster Ait.): a comparison of allozyme markers and quantitative traits. Heredity 89:199–206
Hanawa F, Yamada T, Nakashima T (2001) Phytoalexins from Pinus strobus bark infected with pinewood nematode Bursaphelencus xylophilus. Phytochemistry 57:223–228
Ichihara Y, Fukuda K, Suzuki K (2000a) Early symptom development and histological changes associated with migration of Bursaphelenchus xylophilus in seedling tissues of Pinus thunbergii. Plant Dis 84:675–680
Ichihara Y, Fukuda K, Suzuki K (2000b) The effect of periderm formation in the cortex of Pinus thunbergii on early invasion by the pinewood nematode. For Pathol 30:141–148
Jactel H, Kleinhentz M, Marpeau-Bezard A, Marion-Poll F, Menassieu P, Burban C (1996) Terpene variation in maritime pine constitutive oleoresin related to host tree selection by Dyorictria sylvestrella Ratz. (Lepidoptera, Pyralidae). J Chem Ecol 22:1037–1050
Kawaguchi E (2006) Relationship between the anatomical characteristics of cortical resin canals and migration of Bursaphelenchus xylophilus in stem cuttings of Pinus thunbergii seedlings (in Japanes with English summary). J Jap For Soc 88:240–244
Koricheva J, Nykänen H, Gianoli E (2004) Meta-analysis of trade-offs among plant antiherbivore defenses: are plants jacks-of-all-trades, masters of all? Am Nat 163:E64–E75
Kuroda K (2004) Inhibiting factors of symptom development in several Japanese red pine (Pinus densiflora) families selected as resistant to pine wilt. J For Res 9:217–224
Kuroda K (2008a) Defense systems of Pinus densiflora cultivars selected as resistant to pine wilt disease. In: Vieira P, Mota M (eds) Pine wilt disease: a worldwide threat to forest ecosystems. Springer, Germany, pp 313–320
Kuroda K (2008b) Physiological incidences related to symptom development and wilting mechanism. In: Zhao B, Futai K, Sutherland JR, Takeuchi Y (eds) Pine wilt disease. Springer, Japan, pp 204–222
Kuroda K, Yamada T, Ito S (1991) Bursaphelenchus xylophilus induced pine wilt: factors associated with resistance. Eu J For Pathol 21:430–438
Kuroda H, Goto S, Kazumi E, Kuroda K (2011) The expressed genes of Japanese red pine (Pinus densiflora) involved in the pine wilt disease severity. BMC Proceedings 5(Suppl. 7):92
Lamy J-B, Lagane F, Plomion C, Cochard H, Delzon S (2012) Micro-evolutionary patterns of juvenile wood density in a pine species. Plant Ecol 213:1781–1792
Lima M, Ramos M, Sampedro L, Moreira X, Zas R, Vasconcelos M (2012) Teste de susceptibilidade ao nematodo da madeira do pinheiro–optimizaçao para utilizaçao en larga escala. Cuad Soc Esp Cien For 36:21–26
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS System for mixed models, 2nd edn. SAS Institute, Cary
Martín JA, Esteban LG, de Palacios P, García-Fernández F (2010) Variation in wood anatomical traits of Pinus sylvestris L. between Spanish regions of provenance. Trees 24:1017–1028
Moreira X, Sampedro L, Solla A, Zas R (2008) Alterations of the resin canal system of Pinus pinaster seedlings after fertilization of a healthy and of a Hylobius abietis attacked stand. Trees 22:771–777
Moreira X, Alfaro RI, King J (2012) Constitutive defenses and damage in Sitka spruce progeny obtained from crosses between white pine weevil resistant and susceptible parents. Forestry 85:87–97
Moreira X, Mooney KA, Rasmann S, Petry WK, Zas R, Sampedro L (2014) Trade-offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecol Lett 17:537–546
Mori Y, Miyahara F, Tsutsumi Y, Kondo E (2008) Relationship between resistance to pine wilt disease and the migration of proliferation of pine wood nematodes. Eu J Plant Pathol 122:529–538
Mota MM, Braasch H, Bravo MA, Penas AC, Burgermeister W, Metge K, Sousa E (1999) First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1:727–734
Nose M, Shiraishi S (2008) Breeding for resistance to pine wilt disease. In: Zhao B, Futai K, Sutherland JR, Takeuchi Y (eds) pine wilt disease. Springer, Japan, pp 334–350
Nunes da Silva M, Lima MRM, Vasconcelos MW (2013) Susceptibility evaluation of Picea abies and Cupressus lusitanica to the pine wood nematode (Bursaphelenchus xylophilus). Plant Pathol 62:1398–1406
Oku H, Shiraishi T, Chikamatsu K (1989) Active defense as a mechanism of resistance in pine against pine wilt disease. Ann Phytopathol Soc Jap 55:603–608
Prada E, Alía R, Climent J, Díaz R (2014) Seasonal cold hardiness in Maritime pine assessed by different methods. Tree Genet Genom 10:698–701
Ribeiro B, Espada M, Vu T, Nobrega F, Mota M, Carrasquinho I (2012) Pine wilt disease: detection of the pinewood nematode (Bursaphelenchus xylophilus) as a tool for a pine breeding programme. For Pathol 42:521–525
Robertson L, Cobacho Arcos S, Escuer M, Santiago Merino R, Esparrago G, Abellera A, Navas A (2011) Incidence of the pinewood nematode Bursaphelenchus xylophilus Steiner and Buhrer, 1934 (Nickle, 1970) in Spain. Nematology 13:755–757
Rodrigues JM (2008) National eradication programme for the pinewood nematode. In: Mota M, Vieira P (eds) Pine wilt disease: a worldwide threat to forest ecosystems. Springer, Germany, pp 5–14
Ruzin SE (1999) Plant microtechnique and microscopy. Oxford University Press, New York
Sampedro L, Moreira X, Llusia J, Peñuelas J, Zas R (2010) Genetics, phosphorus availability, and herbivore-derived induction as sources of phenotypic variation of leaf volatile terpenes in a pine species. J Exp Bot 61:4437–4447
Sampedro L, Moreira X, Zas R (2011) Costs of constitutive and jasmonate-induced pine tree chemical defences emerge only under low nutrient availability. J Ecol 99:818–827
Santos del Blanco L, Climent J, González-Martínez SC, Pannell JR (2012) Genetic differentiation for size at first reproduction through male versus female functions in the widespread Mediterranean tree Pinus pinaster. Ann Bot 110:1449–1460
Solla A, Aguín O, Cubera E, Sampedro L, Mansilla P, Zas R (2011) Survival time analysis of Pinus pinaster inoculated with Armillaria ostoyae: genetic variation and relevance of seed and root traits. Eur J Plant Pathol 130:477–488
Son JA, Komatsu M, Mtsushita N, Hogetsu T (2010) Migration of pine wood nematodes in the tissues of Pinus thunbergii. J For Res 15:186–193
Sousa E, Bravo MA, Pires J, Naves P, Penas AC, Bonifacio L, Mota MM (2001) Bursaphelenchus xylophilus (Nematoda; Aphelenchoididae) associated with Monochamus galloprovincialis (Coleoptera; Cerambycidae) in Portugal. Nematology 3:89–91
Suga T, Ohta S, Munesada K, Ide N, Kurokawa M, Shimizu M, Ohta E (1993) Endogenous pine wood nematicidal substances in pines, Pinus massoniana P. strobus and P. palustris. Phytochemistry 33:1395–1401
Tadesse W, Auñón FJ, Pardos JA, Gil L, Alía R (2001) Evaluación precoz de la producción de miera en Pinus pinaster Ait. Inv Agr Sist Rec For 10:141–150
Toda T, Kurinobu S (2002) Realized genetic gains observed in progeny tolerance of selected Red pine (Pinus densiflora) and Black pine (P. thunbergii) to pine wilt disease. Silvae Genet 51:42–44
Vicente C, Espada M, Vieira P, Mota M (2012) Pine Wilt Disease: a threat to European forestry. Eu J Plant Pathol 133:89–99
Vivas M, Zas R, Solla A (2012) Screening of Maritime pine (Pinus pinaster) for resistance to Fusarium circinatum, the causal agent of Pitch Canker disease. Forestry 85:185–192
Webster J, Mota M (2008) Pine wilt disease: global issues, trade and economic Impact. In: Mota M, Vieira P (eds) Pine wilt disease: a worldwide threat to forest ecosystems. Springer, pp 1-5
Woo K-S, Lee D-H, Koo Y-B, Yeo J-K (2008) Inoculation of seven pine species or hybrid seedlings with Korean isolates of pinewood nematode under greenhouse conditions. Ann For Sci 65:811
Zas R, Merlo E (2008) El programa de mejora de Pinus pinaster en Galicia. Boletin CIDEU 5–6:5–24
Zas R, Sampedro L, Prada E, Fernández-López J (2005) Genetic variation of Pinus pinaster Ait. seedlings in susceptibility to Hylobius abietis L. Ann For Sci 62:681–688
Zhang F, Kajiwara J, Mori Y, Ohira M, Tsutsumi Y, Kondo E (2013) Metabolites from resistant and susceptible Pinus thunbergii after inoculation with pine wood nematode. Am J Plant Sci 4:512–518
Author contribution statement
LS, RZ and MV conceived the study. LS and XM conducted the sampling and field assessments. XM performed the chemical analyses and the histological analyses with the assistance of LS and AS. MR and ML performed the PWN migration bioassays with the assistance of MNS. RZ performed the statistical analyses, and primarily wrote the manuscript. All authors contributed to the writing and revisions.
Acknowledgments
This study was financed by the Spanish National Research Grants AGL2012-40151 (FENOPIN), co-financed by EU-FEDER, and by the bilateral action Spain-Portugal PRI-AIBPT-2011-1152 (NEMARES). The Portuguese Fundo Florestal Permanente, Instituto de Financiamento da Agricultura e Pescas I.P., Autoridade Florestal Nacional is also acknowledged for funding. We thank Manuel Mota (Universidade de Évora, Portugal) for providing B. xylophilus isolates. The genetic trials in which the samples were collected are part of the experimental set up of the Maritime pine breeding program developed by the Centro de Investigación Forestal de Lourizán, Xunta de Galicia. We thank David Brown for language edition, and Alvin Yanchuk for valuable comments and discussion.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Penuelas.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zas, R., Moreira, X., Ramos, M. et al. Intraspecific variation of anatomical and chemical defensive traits in Maritime pine (Pinus pinaster) as factors in susceptibility to the pinewood nematode (Bursaphelenchus xylophilus). Trees 29, 663–673 (2015). https://doi.org/10.1007/s00468-014-1143-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-014-1143-6