Abstract
Three screening methods—visual scoring (V), relative conductivity (C) and fluorometry (F)—were used to study the genetic variation in cold hardiness among six populations of maritime pine (Pinus pinaster Ait.) comprising both Atlantic and Mediterranean origins. Freezing damage assessments were carried out in three organs—needles, stems and buds—in two seasons, spring and autumn. We found high levels of genetic differentiation among populations for cold hardiness in autumn, but not in spring. Within populations, differences were always significant (p < 0.05) no matter which organ or screening method was used. Measuring F was the fastest and most easily replicated method to estimate cold hardiness and was as reliable as V and C for predicting the species performance. In autumn, there was a positive correlation between the damage measured in all three types of organs assessed, whereas in spring, correlation among organs was weak. We conclude that sampling date in spring has a crucial impact to detect genetic differences in maritime pine populations, whereas autumn sampling allows more stable comparisons. We also conclude that the fluorometry method provides a more efficient and stable comparison of cold hardiness in maritime pine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Under global warming scenarios (IPCC 2007), warmer and shorter winters will occur in many regions, increasing the risk of late spring and early autumn frosts. How these new conditions would affect productivity, quality and distribution of species is a question under debate (e.g. Lindner et al. 2010), as the response to cold is affected by the sensitivity of the species (Sutinen et al. 1992; Sakai and Larcher 1987).
Cold hardiness, i.e. the ability of plants to withstand freezing temperatures without undergoing significant damage, displays a large level of genetic variation in forest trees both among and within populations. Under common garden conditions, some populations harden in autumn more rapidly than others, depending on their origin (Díaz et al. 2009; Weng and Parker 2008), or deharden differently in spring in response to late winter and/or early spring climate conditions (Díaz et al. 2009). Differences in hardening/dehardening have been observed also between families (e.g. Darychuk et al. 2012) or clones within populations (e.g. Anekonda et al. 2000), showing the importance of cold hardiness as a selective factor in forest trees. The implications of such differences are being considered both in assisted migration programs, breeding programs or transfer guidelines for genetic materials in order to increase productivity or adaptability (e.g. Kremer et al. 2011; O’Neill et al. 2001). In most of these applications, reliable screening methods suitable for large numbers of genotypes are a bottleneck to progress in genome-wide selection programs under different climatic scenarios (Neale and Kremer 2011). The efficiency of these methods could vary depending on the used material (populations, families, clones) from different species.
Cold hardiness can be assessed by examining the freezing damages after natural frost events in field trials, but this method poses limitations related to uncontrolled conditions and lack or repeatability which in turn leads to low statistical precision for determining differences of cold hardiness among genotypes. A better solution is to subject tissue samples to different freezing temperatures under controlled conditions and to evaluate the freezing damages in those samples (Burr et al. 1990).
Several alternative screening methods for cold hardiness are available (see Burr et al. (2001) and Calkins and Swanson (1990) for a review). Among them, visual scoring (V), relative conductivity (C) and fluorometry (F) of plant tissue are the most used methods. Visual scoring is an efficient and fast method widely used in conifers (e.g. L’Hirondelle et al. 2006; Anekonda and Adams 2000; Aitken and Adams 1997). The tissue is allowed to develop symptoms of damage for several days after freezing before scoring the damages in discrete classes. The relative conductivity method measures the concentration of electrolytes leaking from the plant tissues after freezing, providing objective and quantitative results 2 days after the freezing treatment. The relative conductivity method requires small amounts of tissue and has been used for measuring cold hardiness in many conifers (Climent et al. 2009; Royo et al. 2003; Burr et al. 2001; Ryyppö et al. 1998; Colombo 1997; McKay 1994; Sutinen et al. 1992). The fluorometry method determines the efficiency of the photosystem II of the plant tissues (as the ratio of variable fluorescence to maximal fluorescence: calculated as F v/F m) and has been found to be efficient in detecting freezing damage in conifers (Corcuera et al. 2011; Peguero-Pina et al. 2008; Perks et al. 2001, 2004; Binder et al. 1996; Binder and Fielder 1996a; Lindgren and Hällgren 1993).
Pinus pinaster (maritime pine) is one of the most important forest species of the western Mediterranean Basin and the Atlantic coastal region of southern Europe, both for its economic and ecological value. The species displays large levels of intraspecific variation in morphological (Alía et al. 1995) and physiological parameters related mainly to drought tolerance (Aranda et al. 2010; Correia et al. 2008) or frost tolerance (Bouvarel 1960; Corcuera et al. 2011; Illy 1966; Le Tacon et al. 1994). Implications of the intraspecific differences in cold hardiness are of high concern for the use of reproductive material of the species in breeding or afforestation programs, as shown by the prohibition of some Iberian origins to be used in certain regions of France (European Commission 2005). Therefore, maritime pine is a good model species to test different screening methods for cold hardiness and to test the variability at different genetic levels.
In this work, we study the cold hardiness of P. pinaster at different genetic levels (families and populations), in different plant organs (needles, buds and stems), with different environmental conditions (years and seasons) and using alternative methods. The objectives of this study were (1) to detect differences in cold hardiness among maritime pine populations, (2) to compare three methods (visual scoring, relative conductivity and fluorometry) for assessing cold hardiness in maritime pine and (3) to evaluate the response of different organs to the freezing experiments.
Materials and methods
Plant material and experimental site
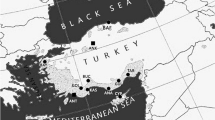
Six populations of P. pinaster covering the range of distribution (34° to 44° N and 1° to 9° W, and at 0 to 1,600 m above sea level (a.s.l)) and with contrasting climatic conditions of origin were chosen. Three of them come from the Atlantic coast (Sergude, SERG; Leiría, LEIR; and Mimizán, MIMI), while the other three come from a Mediterranean are with continental climate (Bayubas, BAYU; Cuellar, CUEL; and Tamrabta, TAMR; Fig. 1).
Location of the six P. pinaster populations sampled in the study and the experimental site (EUFORGEN 2009)
The saplings (4-year-old trees) from the six populations were located in a provenance-progeny test established in Ourense (42° 14′ N, 7° 56′ W and 460 m a.s.l., Galicia, Spain). Different artificial freezing experiments were carried out in spring and autumn over two consecutive years, 2009 and 2010, with different amount of saplings, freezing temperatures and assessed variables as detailed in Table 1. For each freezing temperature, we employed one sapling per family (9 families from each population) in the 2009 experiments and four saplings per family in the 2010 experiments (9 families from MIMI, 11 from LEIR, 12 from BAYU, 13 from TAMR, 14 from CUEL and 16 from SERG). Twigs were taken from the last lateral growth of well-exposed branches, corresponding to branches developed during the fourth growing season (collected in early spring—23 March 2009—before the growth of new shoots), the fifth growing season (collected at two times, in autumn 2009 (28 October) and in spring 2010 (23 April) in an early phenological stage of the new growth before developing new needles) and the sixth growing season (collected in autumn 2010 (5 November)). For each freezing experiment, all twigs were in a similar phenological phase. In the 2009 spring experiment, almost all twigs had elongating buds (stage 2 in a six-level scale), while in the 2010 spring experiment, almost all twigs had elongating internodes, a more advanced stage (stage 3 in a six-level scale). In both autumn experiments, all twigs had well-developed terminal buds.
Climatic conditions in the provenance-progeny test were colder in 2010 than in 2009, 1.7 °C colder on average during the 2–3 months preceding the freezing experiments. Moreover, there were much more freezing days in 2010 than in 2009 (21 versus 9 at the Gandarela weather station, 42° 17′N, 7° 97′W, 623 m, the closest station to the provenance-progeny test).
Artificial freezing experiments
Freeze-thaw treatments
For the 2009 spring experiment, the samples were randomly inserted in trays with vermiculite (Díaz et al. 2009). Two twigs were used in each freezing temperature, one for the visual scoring method and the other for both relative conductivity and fluorometry methods. In the 2009 autumn experiment and 2010 spring and autumn experiments, the samples were wrapped in a lightly moistened tissue and then in an aluminium foil (Anekonda and Adams 2000). One twig of each sapling was used for all the three screening methods.
Samples were placed in a programmable freezing chamber and exposed to the predetermined experimental temperatures: T9 (−9 ± 0.4 °C), T12 (−12 ± 0.4 °C), T15 (−15 ± 0.4 °C) and T17 (−17 ± 0.4 °C) in year 2009 and T6 (−6 ± 0.4 °C), T11 (−11 ± 0.4 °C), T14 (−14 ± 0.8 °C), T16 (−16 ± 1.0 °C) and T19 (−19 ± 0.4 °C) in year 2010. In 2009, all temperatures were tested during the same day. Plants were cooled down at the rate of 2 °C per hour, and after 30 min at each selected temperature, a group of samples was transferred into a refrigerator at 4 °C for 48 h to facilitate slow thawing. In 2010 experiments, each temperature was tested in 1 day. Twigs were cooled down at 2 °C per hour and stayed during 3 h at each selected temperature, and then the temperature was raised at a rate of 5 °C per hour until it reached 4 °C. After the freeze-thaw treatments, damage was assessed by the three methods: visual scoring, relative conductivity and fluorometry; but different measurements were performed depending on the organ (subscript N for needles, S for stems and B for buds), and season (spring or autumn) or year (2009, 2010), as detailed in Table 1.
Visual scoring
Samples remained 10 days at the greenhouse (at 20–25 °C and 90 % relative humidity maintained by a fog system) to allow visible signs of freezing damage to develop (Sakai and Larcher 1987). Visual scoring in needles (V N) was done using a scale of 0 to 6 depending on the percentage of the foliar area damaged (0 = 0 %, no damage; 1 = 1–20 %; 2 = 21–40 %; 3 = 41–60 %; 4 = 61–80 %; 5 = 81–99 %; and 6 = 100 % of foliar area damaged). Visual scoring in stems (V S) and visual scoring in buds (V B) were done after slicing them longitudinally to determine the extent of damage on a scale of 0 to 3 (0 = 0 %, no damage; 1 = 1–33 %; 2 = 34–66 %; and 3 = 67–100 %, completely damaged).
Relative conductivity
Eight pieces of 1 cm length from eight randomly chosen needles, two stem pieces of 3 cm and the half of the bud were transferred to test tubes, separately for each organ, with 20 ml of distilled water for needles and stem and with 10 ml for buds. The tubes were capped and were left for 18 h at room temperature. Following other studies (Bower and Aitken 2006; Hannerz et al. 1999), the tubes were placed on a shaker for 1 h at room temperature to speed up and stabilize electrolyte diffusion from damaged tissues, and then the initial conductivity (C 1) was measured with an electrical conductivity meter (HI 2300, Hanna Instruments, S.L.). The tubes were then oven-heated to 85 °C for 90 min to kill any possible surviving cells and ensure complete electrolyte leakage (Guardia et al. 2013; Repo et al. 2008). Samples were left during 18 h at room temperature; they were shaken for 1 h, and the electrical conductivity was measured again to yield the maximum conductivity (C 2). The percent relative conductivity (C) was calculated for each organ (C N, C S and C B for needles, stems and buds, respectively) as C = 100 × C 1/C 2 (Luoranen et al. 2004).
Fluorometry
Measurements were made in needles (F N) and in twig phloem (F S). First, twigs were left to adapt to darkness for at least 30 min. Then, measurements on needles were done at the top, middle and bottom of a group of needles per each tree. Afterwards, a 1-cm basal piece of the twig stem was detached, and the next 2-cm stem section of the stem was collected. The section was cut longitudinally, the xylem was removed and the remaining tissue was divided into two to fit the leaf clip holder. The minimum (F 0) and maximum (F m) chlorophyll fluorescence were measured to calculate the variable chlorophyll fluorescence parameter, F v, as F v = F m − F 0 (Genty et al. 1989). The dark-acclimated, maximum potential photosystem II (PSII) efficiency was calculated as F v/F m (Peguero-Pina et al. 2008). High values of F v/F m reveal undamaged tissue, while low values are indicative of freezing damage. To compare outcomes between methods, this variable was expressed as a percentage of the maximum damage as follows:
where F is the percentage of damage estimated by the measurement of the fluorescence, F v/F m is the maximum potential PSII efficiency of the sample and F vc/F mc is the maximum potential PSII efficiency of the control. In the first experiment, in spring 2009, fluorometry measurements were done after 1, 3 and 7 days at greenhouse conditions (20 °C and 90 % relative humidity maintained by a fog system). Readings of fluorometry on the 3 days were highly correlated (data not shown), confirming that damages were irreversible (L’Hirondelle et al. 2006); therefore, we decided to use the results on day 3 for this first experiment and to measure fluorometry only after 3 days in the next experiments. Fluorescence measurements were made by means of a pulse amplitude-modulated fluorometer (MINI PAM, Walz, Effeltrich, Germany) equipped with a 2030-B leaf clip holder, featuring an integrated micro-quantum sensor and a thermocouple.
Statistical analysis
Freezing damage was evaluated separately for each organ, method, temperature, season and year.
The population mean (μ) and coefficient of variation (CV) were estimated for 54 individuals (2009 experiments) and for 300 individuals (2010 experiments).
Different mixed models were used for traits assessed each year due to their different datasets, with population and family as random effects and field block as fixed effect. For 2009 dataset, damages were analysed by the following mixed model:
where X ij is the damage value of the j th tree (j = 1 to 54), μ is the overall mean, P i is the effect of population (i = 1 to 6) and ε ij is the experimental error.
For 2010 dataset, we used the following mixed model:
where X ijk is the damage value of the l th tree (l = 1 to 4) from the j th family within the i th population and k th block, μ is the overall mean, P i is the effect of the i th population (i = 1 to 6), F(P) j(i) is the effect of the j th family within the i th population (j = 1–9, 1–11, 1–12, 1–13, 1–14 or 1–16), B k is the effect of the field block (k = 1 to 4) and ε ijk is the experimental error.
Variance components were estimated by the restricted maximum likelihood (REML) method, assuming a normal distribution of the random effects. Several covariance structures were tested to model the residuals, and a first-order autoregressive (AR(1)) structure was selected by the Bayesian information criterion (BIC) (Littell et al. 2006). The significance of variance components was tested using log-likelihood ratio tests. We included population as a random effect to make inference at species level and to obtain an unbiased estimate of heritability and genetic population differentiation (Lamy et al. 2011; Wilson 2008).
The BIC was chosen to compare the fit statistics between methods in spring 2009 and between methods in autumn 2009, as models were the same within each season and year (Littell et al. 2006).
Pooled narrow-sense heritability over populations (h 2) was estimated after removing the population effect, since natural selection seems to occur within populations (Lamy et al. 2011):
where σ 2 A is the within-population additive variance, σ 2 F(P) is the family within population variance and σ 2 ε is the residual variance. In our study, σ 2 A was estimated by σ 2 A = 4σ 2 F(P) assuming that trees from the same family were half-sibs (open-pollinated seeds). The standard error of the heritabilities was estimated as follows (Visscher 1998):
where s is the number of offspring per family (4), and f is the number of families (75).
The estimate of genetic differentiation among populations, Q st, was calculated as described by Wright (1951):
The best linear unbiased predictors (BLUP) were obtained for population random effects. The Spearman correlations were carried out on individual and population mean bases to explore the relationships between methods, organs and seasons. All statistical analyses were done with the SAS System (SAS 9.1, SAS Institute Inc. 2004).
Results
Cold hardiness variation among and within populations
In spring, we did not find significant differences in cold hardiness among populations for any of the methods and plant organs, neither for any sampling year (Tables 2 and 3, Fig. 2). By contrast, we found highly significant differences among populations in autumn, especially in 2010 when we also increased the assessed number of trees and families per population. Populations from central Iberian Peninsula (BAYU and CUEL) showed lower levels of damage than those from coastal Iberian populations (LEIR and SERG) (see Fig. 2 for needles assessed by fluorometry).
Additive genetic variation within populations was significant in both the spring and autumn 2010 experiments, especially for needles. The estimated pooled narrow-sense heritability (h 2) for cold hardiness was high in the two seasons (h 2 > 0.6). We obtained estimates higher than 1 in 6 out of the 19 estimates (3 by visual scoring in needles, 2 by visual scoring in stems and 1 by the fluorometry method in needles) (Table 3). The genetic differentiation (Q st) among populations was very low in spring (0.02–0.05) for all screening methods and varied between 0.03 and 0.66 in autumn, depending on the organ, method and experimental temperature (Table 3).
Comparison of screening methods
We compared the three methods assessing needles in year 2009. Damage curves showed that, in general, the methods performed similarly, especially fluorometry and relative conductivity. In spring, visual scoring gave the highest freezing damage levels, whereas in autumn, the comparison was not that clear; hence, the ranking depended on the studied temperature (Fig. 3a, b). In general, fluorometry was more stable across temperatures, since the intrapopulation variability in needles, determined by the CV, had the lowest values (for needles, between 87 and 117 in spring 2009 and between 79 and 107 in autumn 2009; Table 2). Regarding the BIC, in spring 2009, relative conductivity had the lowest values, whereas in autumn 2009, the lowest values were with fluorometry (Table 2).
Mean freezing damage (%, n = 54 in 2009, and n = 300 in 2010) in needles at different temperatures and methods. Black square, visual scoring method; black triangle, relative conductivity method; multiplication symbol, fluorometry method. Error bars represent standard errors. Different letters show statistically significant (p < 0.05) differences. Letters were depicted only when methods were statistically different
In year 2010, we compared visual scoring and fluorometry in needles, obtaining more freezing damages with visual scoring in spring and with fluorometry in autumn (Fig. 3c, d). In spring 2010, the lowest BIC values were with fluorometry, while in autumn, it depended on the considered temperature, being fluorometry the method giving the lowest values in most cases (data not shown).
Correlations between methods assessing needles for the temperatures which caused levels of freezing damage among 30–70 % are shown in Table 4. These correlations were always significant for individual data, and considering population means, they were highly significant in year 2010 between visual scoring and fluorometry.
We compared methods using the other two organs—stems and buds—and the best correlation was achieved with stems in autumn 2010, at T19, between visual scoring and fluorometry (r = 0.83 (p < 0.05) for population means; data not shown).
Comparison of freezing damages in different plant organs
Freezing damages differed among organs and years in the spring experiments. Needles were the most damaged organ in 2009 (V and C, Table 2); by contrast, in 2010, when the collection was done 1 month later and the twigs were exposed to the selected temperatures for a longer period of time, buds were the most damaged organ, and needles and stems had similar damage levels (Table 3).
In autumn 2009, buds showed slightly higher levels of damage than needles and the stems were the least damaged organ (Table 2). In autumn 2010, buds and needles showed similar damage levels, and stems were again the least damaged organ (Table 3).
Correlation inferences between organs for the temperatures which caused levels of freezing damage among 30–70 % are shown in Table 5. Individual correlations between plant organs were significant and moderate, both in spring and autumn of years 2009 and 2010. At the population level, we found highly significant correlations between needles and stems in autumn of years 2009 and 2010 with relative conductivity and visual scoring methods. However, in spring of both years, correlations between organs for population means were weak.
Discussion
Genetic variation of cold hardiness
We observed a high variability in additive variance for cold hardiness among populations in maritime pine, in accordance with the previous knowledge on the adaptive genetic structure of this species (Aranda et al. 2010; González-Martínez et al. 2002). Moreover, genetic differentiation for cold hardiness among populations was much higher in autumn than in spring, in consonance with results of various studies in other pine species (Bower and Aitken 2006; Jonsson et al. 1986; Nilsson 2001). The nonsignificant population differentiation in cold hardiness observed in spring could suggest no differences in timing of dormancy release (Morgenstern 1996), but considering the contrasting origins of the assessed populations, we believe that it is hard to work out the precise moment when spring assessment can be more discriminant between populations, since deacclimation occurs rapidly (Bower and Aitken 2006; Kalberer et al. 2006).
The populations evaluated in this study can be grouped according to their sensitivity to freezing damage in autumn. The least cold-hardy populations were the Iberian Atlantic coastal populations (SERG and LEIR), and the most cold-hardy, the Iberian continental populations (BAYU and CUEL). Population ranking was highly consistent when freezing experiments were applied in different years, plant organs and methods, and results are also concordant with previous works (Corcuera et al. 2011). These differences likely reflect adaptation to their different source environments, through directional selection driven by a faster decrease in minimum daily temperatures during autumn in the high-elevation continental areas (O’Neill et al. 2001). Likewise, these differences mimic those encountered among pine species related to their thermal niche (Climent et al. 2009).
The significant variation observed among families within populations suggests that there is a sufficient additive genetic variation for selection by cold hardiness within populations (Salmela et al. 2011). The family component of variation was similar for spring and autumn sampling, suggesting that cold hardiness in maritime pine is under strong genetic control.
The family effect was nonsignificant in 12 out of the 31 combinations of temperature and screening method, and therefore, it was not possible to estimate an additive genetic variance. For the rest of the 19 cases, we got very high values of heritability (higher than 0.6) both in spring and autumn, obtained by considering all the 75 families as belonging to the same population by removing the population effect; for 6 out of the 19 estimates, the value was higher than 1. These results are affected by the level of damage; as to make good statistical inferences, we should take into account only the intermediate levels of damage (30–70 %) (Anekonda and Adams 2000). Considering this range, only one of the cases presented heritability greater than 1 (visual scoring method in needles, at a temperature of −19 °C and collected in autumn 2010), where the standard error of the estimation is high. The subjective estimation in the visual scoring method could have affected this overestimation (indicated by the discrepancy with the more reliable fluorometry method). We can discard an overestimation based on a higher correlation paternity in maritime pine that would result in a combination of half-sib and full-sib seedlings as deduced by sampling in the natural populations of the species (Gaspar et al. 2011), similar to the sampling performed when establishing provenance-progeny tests. Also, we can discard an environmental effect based on individuals of the same family sharing the same plot (e.g. Dutkowski et al. 2002; Lamy et al. 2013), as we have corrected in our model this environmental correlation of the individuals. We cannot discard the effect of the reduced number of individuals per family, which resulted in high standard errors in the estimation, but the high values of the genetic correlation indicate the reliability of our results in accordance with other studies (Aitken and Adams 1997; Persson et al. 2010). Also, the heritability values could be affected by some population effect (Corcuera et al. 2010) that, in our case, can be linked to a greater additive variance in some of the populations (e.g. TAMR, CUEL; see Supplementary Fig. S1). To what extent this greater variability is caused by some evolutionary effects—linked to different demographic and adaptive processes in these populations (Grivet et al. 2011)—or to a confounding effect with the size of the plant or to differences in the families environmental harshening would need a deeper study.
Comparison of screening methods
When working on needles, the three methods compared (visual scoring, relative conductivity and fluorometry) were well correlated between them (L’Hirondelle et al. 2006).
In the literature, visual scores have been found to be strongly correlated (r 2 > 0.90) with damage assessed quantitatively using relative conductivity (Shortt et al. 1996) and fluorometry methods (Binder et al. 1996). In our study, although in some cases visual scores could overestimate the level of damage compared to relative conductivity and fluorometry, all methods were strongly correlated in most cases, especially when the three analyses were done on the same twig (autumn 2009). Also, the sample preparation system used in spring 2009 could have led to odd results, such as the unexpected percentage of damage recorded, lower at temperature T15 than at temperature T12, with fluorometry and relative conductivity.
In general, visual scoring was less precise than relative conductivity and fluorometry methods; it is subjected to further error depending on the skill and experience of the scorer, and it depends on the timing of the examination, since if it is too early, it could not discriminate lethal from nonlethal damage (Deans et al. 1995). If it is too late, as it seems to have occurred in some cases in the present work, it could lead to overestimation of the damage level. To our opinion, these drawbacks overpass the possible advantages of this method, namely its simplicity (Anekonda and Adams 2000).
Relative conductivity improved consistency and yielded statistically better results than visual scoring in the present work, both for detecting differences among populations and for heritability estimates. However, the relative conductivity method was more labour intensive than the other two studied methods (Jensen and Deans 2004).
Accordingly to our results, fluorometry is the most recommendable method, since it was the fastest among the three tested methods (L’Hirondelle et al. 2006; Perks et al. 2004), and it was more objective than the visual scoring and more reliable than both the visual and the conductivity methods for assessing cold hardiness, in consonance with other published works (Binder and Fielder 1996b; Weng and Parker (2008). In our study, we performed the fluorometry 3 days after the freeze-thaw treatment in order to best accommodate the different tests, but this method can provide results within a few hours after the freezing treatment, enabling high-throughput screening of many genotypes in multilocation field trials. In such a way, high-throughput phenotyping platforms (HTPP) could be particularly useful for obtaining detailed measurements of plant characteristics that collectively provide reliable estimates of phenotypic traits.
Effect of plant organ sampling
Needles are the most assessed organ in freezing damage studies in conifers, but the importance of assessing different organs and tissues has been recognized by several authors (Berrang and Steiner 1986; Burr et al. 1990; Sakai and Weiser 1973). In the present study, stems were the most cold-hardy organ in both seasons, fitting in with the results of other authors (Corcuera et al. 2011). The ranking in cold hardiness in spring for buds and needles seems to depend mainly on bud phenology. In year 2009, twigs were collected at the beginning of the spring (81 growing degree days at the 22nd of March) with the buds in a moderately swollen stage but still not burst, and in that occasion, buds were less damaged than needles. In spring 2010, twigs were collected 1 month later (186 growing degree days at the 22nd of April), with buds showing a more advanced phenological stage, and buds were more damaged than needles. In autumn, some authors found that buds of other conifer seedlings were considerably less cold-hardy than needles (Burr et al. 1990; O’Neill et al. 2001), but in our study, cold hardiness between needles and buds was similar, with buds showing slightly less hardiness than needles.
In our analysis of maritime pine, freezing damage in needles and stems was strongly correlated in autumn; besides, correlations among needles and buds were significant in autumn 2010. Correlations between organs in spring were weaker, contrarily to findings by Aitken and Adams (1996, 1997) in Douglas fir. We hypothesize that the weaker correlation between organs in spring is caused by a different response of each organ to the cumulative effects of chilling and heat sum during the dehardening process. On the other hand, in autumn, all organs were developing during the previous months, and therefore, they should be at a similar hardening status, justifying the stronger correlation between them at this season.
The moderately strong correlations of freezing damage levels between organs in autumn indicate that selecting for cold hardiness based on a single organ can be acceptable for minimizing phenotyping costs (O’Neill et al. 2001). Following O’Neill et al. (2001), the choice of the best organ to score should consider (a) ease of measurement, (b) heritability, (c) correlations with other organs and (d) correlations with the economic impact of frost damage in the field. Although occasional needle freezing damage is not expected to significantly impact survival, growth rate or stem form, practical simplicity, heritability and correlations with other organs make needles highly recommendable for evaluating cold hardiness in autumn in maritime pine. Further investigation is needed in spring cold hardiness evaluations, especially in buds.
Conclusions
We have shown the importance of the sampling date and year in the estimation of genetic variation of cold hardiness in maritime pine, as well as the effect of the screening method used and the analysed organ.
These experiments have demonstrated an important genetic variation among P. pinaster populations in autumn. Within-population genetic variation was always significant in both seasons. Thus, there is much potential for improving spring and autumn cold hardiness in maritime pine through selection and breeding.
Based on our results, we recommend the use of the fluorometry method for cold hardiness assessment in P. pinaster. It is the fastest method of the three tested, more objective than visual scoring and as reliable as the visual scoring and relative conductivity methods for predicting field performance. These characteristics allow fluorometry to be used in high-throughput phenotyping systems, improving the precision of selection and being a useful tool in modelling for predicting genotypic performance in different climate scenarios.
We suggest using needles to evaluate cold hardiness in maritime pine in autumn, since needles are the easiest organ for testing, and needle freezing damage levels in autumn were well correlated to those in stem and buds. More investigation is needed in spring cold hardiness evaluations, especially in buds.
References
Aitken SN, Adams WT (1996) Genetics of fall and winter cold hardiness of coastal Douglas-fir in Oregon. Can J For Res 26:1828–1837. doi:10.1139/x26-208
Aitken SN, Adams WT (1997) Spring cold hardiness under strong genetic control in Oregon populations of Pseudotsuga menziesii var. menziesii. Can J For Res 27:1773–1780
Alía R, Gil L, Pardos JA (1995) Performance of 43 Pinus pinaster Ait. provenances on 5 locations in Central Spain. Silvae Genet 44:75–80
Anekonda TS, Adams WT (2000) Cold hardiness testing for Douglas-Fir tree improvement programs: guidelines for a simple, robust, and inexpensive screening method. West J Appl For 15:129–136
Anekonda TS, Adams WT, Aitken SN et al (2000) Genetics of cold hardiness in a cloned full-sib family of coastal Douglas-fir 1. Can J For Res 30:837–840
Aranda I, Alía R, Ortega U et al (2010) Intra-specific variability in biomass partitioning and carbon isotopic discrimination under moderate drought stress in seedlings from four Pinus pinaster populations. Tree Genet Genomes 6:169–178. doi:10.1007/s11295-009-0238-5
Berrang PC, Steiner KC (1986) Seasonal changes in the cold tolerance of pitch pine. Can J For Res 16:408–410
Binder WD, Fielder P (1996a) Chlorophyll fluorescence as an indicator of frost hardiness in white spruce seedlings from different latitudes. New For 11:233–253
Binder WD, Fielder P (1996b) Seasonal changes in chlorophyll fluorescence of white spruce seedlings from different latitudes in relation to gas exchange and winter storability. New For 11:207–232
Binder WD, Fielder P, Mohammed GH, L’Hirondelle SJ (1996) Applications of chlorophyll fluorescence for stock quality assessment with different types of fluorometers. New For 13:63–89
Bouvarel P (1960) Note sur la resistance au froid de quelques provenances de pin maritime. Rev For Fr 12:495–508
Bower AD, Aitken SN (2006) Geographic and seasonal variation in cold hardiness of whitebark pine. Can J For Res 36:1842–1850. doi:10.1139/x06-067
Burr KE, Tinus RW, Wallner SJ, King RM (1990) Comparison of three cold hardiness tests for conifer seedlings. Tree Physiol 6:351–369
Burr KE, Hawkings CDB, L’Hirondelle S et al (2001) Methods for measuring cold hardiness of conifers. In: Bigras FJ, Colombo SJ (eds) Conifer cold hardiness. Kluwer Academic, Dordrecht, pp 369–401
Calkins JB, Swanson BT (1990) The distinction between living and dead plant tissue—viability tests in cold hardiness research. Cryobiol 27:194–211
Climent J, Costa e Silva F, Chambel MR et al (2009) Freezing injury in primary and secondary needles of Mediterranean pine species of contrasting ecological niches. Ann For Sci 66:407–407. doi:10.1051/forest/2009016
Colombo SJ (1997) Frost hardening spruce container stock for overwintering in Ontario. New For 13:449–467
Corcuera L, Gil-Pelegrin E, Notivol E (2010) Phenotypic plasticity in Pinus pinaster δ13C: environment modulates genetic variation. Ann For Sci, p67
Corcuera L, Gil-Pelegrin E, Notivol E (2011) Intraspecific variation in Pinus pinaster PSII photochemical efficiency in response to winter stress and freezing temperatures. PloS One 6(12):e28772. doi:10.1371/journal.pone.0028772
Correia I, Almeida MH, Aguiar A et al (2008) Variations in growth, survival and carbon isotope composition (delta(13)C) among Pinus pinaster populations of different geographic origins. Tree Physiol 28:1545–1552
Darychuk N, Hawkins BJ, Stoehr M (2012) Trade-offs between growth and cold and drought hardiness in submaritime Douglas-fir. Can J For Res 42:1530–1541. doi:10.1139/x2012-092
Deans JD, Billington HL, Harvey FJ (1995) Assessment of frost damage to leafless stem tissues of Quercus petraea: a reappraisal of the method of relative conductivity. Forestry 68:25–34
Díaz R, Johnsen O, Fernández-López J (2009) Variation in spring and autumn freezing resistance among and within Spanish wild populations of Castanea sativa. Ann For Sci 66:708–720
Dutkowski GW, Silva JC, Gilmour AR, Lopez GA (2002) Spatial analysis methods for forest genetic trials. Can J For Res 32:2201–2214. doi:10.1139/x02-111
EUFORGEN (2009) Distribution map of maritime pine (Pinus pinaster). www.euforgen.org. Accessed 1 Oct 2013
European Commission (2005) 2005/853/EC: Commission decision of 30 November 2005 authorising France to prohibit the marketing to the end user, with a view to seeding or planting in certain regions of France, of reproductive material of Pinus pinaster Ait. of Iberian Peninsula origin. 14–16
Gaspar MJ, Alves A, Louzada JL, Morais J, Santos A, Fernandes C, Almeida MH et al (2011) Genetic variation of chemical and mechanical traits of maritime pine (Pinus pinaster Aiton). Correlations with wood density components. Ann For Sci 68(2):255–265. doi:10.1007/s13595-011-0034-x
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Grivet D, Sebastiani F, Alía R et al (2011) Molecular footprints of local adaptation in two Mediterranean conifers. Mol Biol Evol 28:101–116. doi:10.1093/molbev/msq190
Guardia M, Díaz R, Savé R, Aleta N (2013) Autumn Frost resistance on several walnut species: methods comparison and impact of leaf fall. For Sci 1–7
Hannerz M, Aitken SN, King JN, Budge S (1999) Effects of genetic selection for growth on frost hardiness in western hemlock. Can J For Res 29:509–516. doi:10.1139/x99-019
Illy G (1966) Recherches sur l’amélioration génétique du Pin maritime. Ann Sci For 23:765–948. doi:10.1051/forest/19660401
Institute Inc SAS (2004) SAS® 9.1 SQL Procedure user’s guide. SAS Institute Inc, Cary
IPCC (2007) Summary for policymakers. In: Climate change 2007: The physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. (Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.Bk Av
Jensen JS, Deans JD (2004) Late autumn frost resistance of twelve north European provenances of Quercus species. Scand J For Res 19:390–399
Jonsson A, Eriksson G, Franzen A (1986) Within-population variation in frost damage in Pinus contorta Dougl. seedlings after simulated autumn or late-winter conditions. Silvae Genet 35:96–102
Kalberer SR, Wisniewski M, Arora R (2006) Deacclimation and reacclimation of cold-hardy plants: current understanding and emerging concepts. Plant Sci 171:3–16. doi:10.1016/j.plantsci.2006.02.013
Kremer A, Vincenti B, Alia R et al (2011) Forest ecosystem genomics and adaptation: EVOLTREE conference report. Tree Genet Genom 7:869–875
L’Hirondelle SJ, Simpson DG, Binder WD (2006) Overwinter storability of conifer planting stock: operational testing of fall frost hardiness. New For 32:307–321
Lamy J-B, Bouffier L, Burlett R, Plomion C, Cochard H, Delzon S (2011) Uniform selection as a primary force reducing population genetic differentiation of cavitation resistance across a species range. PloS One 6(8):e23476. doi:10.1371/journal.pone.0023476
Lamy J-B, Delzon S, Bouche PS et al (2013) Limited genetic variability and phenotypic plasticity detected for cavitation resistance in a Mediterranean pine. New Phytol. doi:10.1111/nph.12556
Le Tacon F, Bonneau M, Gelpe J et al (1994) Le dépérissement du pin maritime dans les landes de gascogne à la suite des introductions de graines d’origine ibérique et des grands froids des années 1962–1963 et 1985. Rev For Fr 46:474–484
Lindgren K, Hällgren JE (1993) Cold acclimation of Pinus contorta and Pinus sylvestris assessed by chlorophyll fluorescence. Tree Physiol 13:97–106
Lindner M, Maroschek M, Netherer S et al (2010) Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For Ecol Manag 259:698–709. doi:10.1016/j.foreco.2009.09.023
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for Mixed models, Secondth edn. SAS Institute Inc, Cary, p 840
Luoranen J, Repo T, Lappi J (2004) Assessment of the frost hardiness of shoots of silver birch (Betula pendula) seedlings with and without controlled exposure to freezing. Can J For Res 34:1108–1118
McKay HM (1994) Frost hardiness and cold-storage tolerance of the root system of Picea sitchensis, Pseudotsuga menziesii, Larix kaempferi and Pinus sylvestris bare-root seedlings. Scand J For Res 9:203–213
Morgenstern E (1996) Geographic variation in forest trees: genetic basis and application of knowledge in silviculture. UBC, Vancouver, 209 p
Neale DB, Kremer A (2011) Forest tree genomics: growing resources and applications. Nat Rev Genet 12(2):111–122. doi:10.1038/nrg2931
Nilsson JE (2001) Seasonal changes in phenological traits and cold hardiness of F1-populations from plus-trees of Pinus sylvestris and Pinus contorta of various geographical origins. Scand J For Res 16:7–20. doi:10.1080/028275801300004361
O’Neill GA, Adams WT, Aitken SN (2001) Quantitative genetics of spring and fall cold hardiness in seedlings from two Oregon populations of coastal Douglas-fir. For Ecol Manag 149:305–318. doi:10.1016/S0378-1127(00)00564-8
Peguero-Pina JJ, Morales F, Gil-Pelegrín E (2008) Frost damage in Pinus sylvestris L. stems assessed by chlorophyll fluorescence in cortical bark chlorenchyma. Ann For Sci 65:813, p1–p6
Perks MP, Monaghan S, O’Reilly C et al (2001) Chlorophyll fluorescence characteristics, performance and survival of freshly lifted and cold stored Douglas fir seedlings. Ann For Sci 58:225–236
Perks MP, Osborne BA, Mitchell DT (2004) Rapid predictions of cold tolerance in Douglas-fir seedlings using chlorophyll fluorescence after freezing. New For 28:49–62
Persson T, Andersson B, Ericsson T (2010) Relationship between autumn cold hardiness and field performance in northern Pinus sylvestris. Silva Fenn 44(2):255–266
Repo T, Mononen K, Alvila L, Pakkanen TT, Hänninen H (2008) Cold acclimation of pedunculate oak (Quercus robur L.) at its northernmost distribution range. Environ Exp Bot 63:59–70
Royo A, Fernandez M, Gil L, Pardos JA (2003) Assessing the hardiness of Aleppo pine, maritime pine, and holm oak seedlings by electrolyte leakage and water potential methods. Tree Planters’ Notes 50:38–43
Ryyppö A, Repo T, Vapaavuori E (1998) Development of freezing tolerance in roots and shoots of Scots pine seedlings at nonfreezing temperatures. Can J For Res 28:557–565
Sakai A, Larcher R (1987) Frost survival of plants—responses and adaptation to freezing stress. Springer, Berlin, 321 pp
Sakai A, Weiser CJ (1973) Freezing resistance of trees in North America with reference to tree regions. Ecol 54:118–126
Salmela MJ, Cavers S, Cottrell JE et al (2011) Seasonal patterns of photochemical capacity and spring phenology reveal genetic differentiation among native Scots pine (Pinus sylvestris L.) populations in Scotland. For Ecol Manag 262:1020–1029. doi:10.1016/j.foreco.2011.05.037
Shortt RL, Hawkings BJ, Woods JH (1996) Inbreeding effects on the spring frost hardiness of coastal Douglas-fir. Can J For Res 26:1049–1054
Sutinen ML, Palta JP, Reich PB (1992) Seasonal differences in freezing stress resistance of needles of Pinus nigra and Pinus resinosa: evaluation of the electrolyte leakage method. Tree Physiol 11:241–254
Visscher PM (1998) On the sampling variance of intraclass correlations and genetic correlations. Genetics 149:1605–1614
Weng YH, Parker WH (2008) Adaptive variation in fall cold hardiness of aspen from northwestern Ontario. Tree Physiol 28:143–150
Wilson AJ (2008) Why h2 does not always equal V A/V P? J Evol Biol 21(3):647–650. doi:10.1111/j.1420-9101.2008.01500.x
Wright S (1951) The genetical structure of populations. Ann Eugenics 15(4):323–354
Acknowledgments
This research was developed as part of the project 09MDS019502PR from the Galician government. The field trial was installed as part of project TREESNIPS (QLK3-CT2002-01973) and currently is part of the Spanish Network of Genetic Trials (GENFORED). We thank all persons and institutions linked to the establishment of the field trials and to the maintenance of the network. We thank the C.I.F. Lourizán staff, and also Antonio Soliño and the students Rebeca Rodas, Marta Paraños and Carmen Muiña for field and laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. González-Martínez
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(DOCX 95 kb)
Rights and permissions
About this article
Cite this article
Prada, E., Alía, R., Climent, J. et al. Seasonal cold hardiness in maritime pine assessed by different methods. Tree Genetics & Genomes 10, 689–701 (2014). https://doi.org/10.1007/s11295-014-0714-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-014-0714-4