Abstract
Region of provenance is defined as an area with uniform ecological conditions where stands with similar phenotypic or genetic features are found. This study assesses the effect of differing climate conditions of eight Spanish regions of provenance of Pinus nigra Arn. subsp. salzmannii on earlywood anatomical traits measured in samples from basal discs from mature trees. Results showed that variation in wood biometry between provenances was high and more pronounced than intrapopulation variation. When comparing P. nigra with other Mediterranean pines, high intertracheid wall strength values are associated with better adaptation of pines to arid conditions. However, the intraspecific variations of this parameter in P. nigra did not follow the same pattern, due to the influence of mechanical support requirements. Trees subject to greater aridity were characterised by short tracheids, apparently resulting from their poorer growth, and high frequency of rays and ray parenchyma cells, which would allow trees to store greater amounts of starch, which is the source of metabolites invested in minimising the limitations imposed by water stress. Severe winter cold spells were strongly associated with high axial resin canal frequency and large radial resin canals, creating a powerful, preformed defence system. Increased tracheid lumen involved an increase in the size of bordered pits, favouring sap flow between tracheids, in addition to an increase in the maximum diameter of cross-field pits, favouring the flow of water and metabolites between the axial and radial systems. The high influence of region of provenance on structural variation in P. nigra shows the importance of provenance in the selection of seed origin for reforestation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pinus nigra Arn. has a large area of extent in the mountains of the Mediterranean Basin, from the western half of the Iberian Peninsula to Anatolia (Turkey), including enclaves in the north of Africa (Ruiz de la Torre 2006). Populations of this species are now very fragmented, with three large areas taken into consideration: the eastern group, normally associated with the subspecies pallasiana (Lamb.) Holmboe; the central group, containing three subspecies (subsp. nigra, subsp. laricio (Poiret) Maire and subsp. dalmatita (Vis.) Franco); and the western group, with the subspecies salzmannii (Dunal) Franco. The latter group forms the basis of this study. The Spanish forests of P. nigra subsp. salzmannii occupy around 35,000 ha. They occur naturally and have the greatest resistance to summer drought of all P. nigra subspecies (Aussenac 1980). However, individual forests in Spain show major differences, both in terms of tree stem quality and growth and appearance, most likely due to the varied terrain, which has led to very distinct ecological and microecological conditions.
The region of provenance constitutes the basic unit for commercialisation of forest reproductive material (fruits, seeds, plants and plant parts) in the certification systems of the European Union and the Organisation for Economic Cooperation and Development (Alía et al. 2005). For a given species or subspecies, the region of provenance is defined as the area or group of areas subject to uniform ecological conditions where seed sources or stands with similar phenotypic or genetic features are found. Differences in the ecological conditions of regions of provenance result in specific adaptations which lead to genetic, morphological and phenological variations within a single species or subspecies. These variations need to be characterised in order to determine the characteristics of future forests in terms of adaptation, composition and growth, as well as the properties of the forest products to be obtained from them.
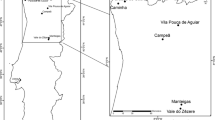
In Spain, 17 regions of provenance of P. nigra subsp. salzmannii have been defined (Fig. 1). Their area of extent covers three mountain ranges—the Pyrenees, the Iberian Mountain System and the Betic Cordillera—although many relict forests and small stands can be found in areas geographically far apart. The natural forests of P. nigra in Spain occur in distinct phytoclimates. The most frequent of these, from least to greatest aridity, are the nemoral, nemoro-Mediterranean and Mediterranean phytoclimates, although at higher altitudes they can be found in an oroboreal phytoclimate (Catalán et al. 1991; Martín et al. 1998). Forests in the Mediterranean phytoclimate must withstand severe summer drought periods, whereas in a nemoral phytoclimate, the drought period is minimal or absent. Soils where P. nigra grows in Spain are normally limestone and vary only in terms of the level of soil evolution, but the species may also be found in siliceous enclaves.
Regions of provenance of Pinus nigra ssp. salzmannii in Spain (Catalán et al. 1991; Martín et al. 1998): 1 Western Aragonese Pre-Pyrenees, 2 Eastern Aragonese Pre-Pyrenees, 3 Catalonian Pre-Pyrenees, 4 Upper Ampurdan, 5 Lower Catalonia, 6 Upper Maestrazgo, 7 Southern Iberian Range (7A Serrania de Cuenca Range-Upper Alcarria, 7B Southern Cuenca, 7C Teruel), 8 Betic Cordillera (8A Cazorla-Alcaraz, 8B Eastern Ranges, 8C Sierra Magina Range, 8D Sierra de Baza Range, 8E Sierra de Maria Range), 9 Central Range, 10 Soria, 11 Northern Meseta sand flats
Differences in wood microstructure are thought to be the result of natural selection (Carlquist 2001), deriving from optimisation of mechanical strength, water conduction, and water and photosynthate storage functions in a variety of environments (Tyree et al. 1994). It is hypothesised that ecological differences between regions of provenance of P. nigra ssp. salzmannii have led to differences in wood microstructure. In another Mediterranean pine (Pinus halepensis Mill.), the adaptive nature of certain anatomical parameters enabling trees to compete in environments with long periods of summer drought has recently been demonstrated (Esteban et al. 2010). Although the wood anatomy of P. nigra in Spanish forests has been described previously (Castellarnau 1883; Esteban and Guindeo 1988; Esteban et al. 2002; Peraza 1964), these studies dealt only with certain populations and did not consider possible variations due to region of provenance or climate factors. The purpose of this study is to find structural features in the stem of mature P. nigra trees which may be closely related to climate factors and constitute essential elements in adaptation to stress conditions and to quantify the role of region of provenance and individual nested within each provenance in anatomical variation in the species.
Materials and methods
Regions of provenance sampled
Material used for this study was collected in eight of the 17 Spanish regions of provenance of P. nigra subsp. salzmannii (Table 1). The regions sampled fall into three distinct phytoclimates in accordance with the phytoclimate classification by Allué-Andrade (1990) for mainland Spain: Mediterranean, nemoro-Mediterranean and nemoral (Table 1). The Mediterranean climate corresponds to zones in which drought duration in a hythergraph is 3–11 months or, when drought lasts 1.25–3 months, the average temperature in the coldest month (month with the lowest average temperature) is higher than 7.5°C and annual rainfall is less than 850 mm. The nemoro-Mediterranean climate corresponds to the rest of the cases in which drought duration is 1.25–3, and when drought lasts less than 1.25 months, annual rainfall is less than 950 mm and the average minimum temperature in the coldest month is higher than 0°C. The nemoral climate corresponds to remaining cases in which drought duration is 0–1.25 (minimal or no summer drought). The best populations in terms of wood quality and growth are located in the southern Iberian Mountain System (regions 7A, 7B and 7C), in a nemoral or nemoro-Mediterranean phytoclimate (Table 1). They comprise single-species forests subject to moderate summer drought. Four regions (8A, 8B, 8D and 8E) are located in the Betic Cordillera in nemoro-Mediterranean or Mediterranean phytoclimates, where P. nigra populations withstand maximum aridity. Despite severe summer drought, regions 8A and 8B have relatively high annual rainfall, and as a result, they comprise pure stands of good quality. In regions 8D and 8E, which are very arid, trees attain moderate heights and often have twisted forms due to the action of snow and strong winds. Sampling of region 10 revealed a small inland forest with high continentality, in a nemoro-Mediterranean climate. Five adult trees (aged 80–100 years) representative of the forest were felled in each region. From each of these, a basal disc was obtained 40 cm from ground level in order to characterise the xylem anatomy.
Anatomical measurements
Samples were prepared for light microscopy observation by submerging them in water with glycerine and placing them in an oven at 80–90°C to soften them. Cross, radial and tangential sections with a thickness of 15–20 μm were prepared using a sliding microtome. The sections were dehydrated and some were stained with safranine and Sudan 4 to make the resin red (Jane 1970), and then, the three sections were mounted on the microscopic slide using synthetic resin. The anatomical descriptions follow the recommendations of IAWA Committee (2004).
Anatomical measurements were taken on the light microscopy slides using the WinCell image analysis program. The parameters measured were the following: axial tracheid length and tangential diameter (including wall), tangential diameter of axial tracheid lumen (D), axial tracheid cell wall thickness, diameter of bordered pits on the radial wall, ray height and frequency, ray parenchyma cell frequency, ray tracheid frequency, number and diameter of cross-field pits, diameter and frequency of axial and radial resin canals, and radial distance from axial resin canals to the start of the growth ring. All axial tracheid measurements were on earlywood as this is the most important tissue for sap conduction and is more vulnerable to implosion processes. Tracheid length was measured using Ladell’s indirect method (Ladell 1959). Following Hacke et al. (2001), the tangential lumen (b) and thickness (t) of the double wall of two adjacent tracheids were measured and the intertracheid wall strength (t 2/b 2) was calculated. Because t 2/b 2 varies considerably depending on the value of b, this value was calculated for b = D h, where D h = ∑D 5/∑D 4 (tracheid hydraulic diameter).
Biometry was conducted on six preparations from each tree, from three growth rings representative of the sapwood of the trees, and therefore, rings corresponding to the years 2000–2003 were chosen. On each slide, 35 random measurements were taken for each variable studied, and the mean value per tree was calculated. The number of pits per cross-field was measured on ten different rays. Ray height and frequency were measured on all rays in one mm2 in the tangential section. The number of ray parenchyma cells and ray tracheids was obtained by measuring the number of parenchyma and tracheid rows per ray in radial sections. To estimate the number of ray parenchyma cells and ray tracheids per mm2 of tangential section, the number of rays measured was equal to the ray frequency per mm2 obtained in the tangential section. All resin canals in the slides were measured using method A in IAWA Committee (2004). The radial distance from the axial resin canals to the growth ring boundary was measured from the part of the resin canal closest to the growth ring.
Statistical analysis
Most frequent ray height (number of cells) was obtained by fitting the distribution of the ray height frequency to a normal distribution curve. The maximum of this distribution constituted the most frequent value. The other variables were analysed using a generalised linear model (GLM) (McCullagh and Nelder 1989) approach to ANOVA with type III sum of squares, using the following model:
with Y being the response variable, μ the general mean, R the effect of the region of provenance, T(R) the effect of the tree nested within each provenance and ε the error term. Regions of provenance were compared using multiple range tests with Fisher’s least significant difference (LSD) intervals (α = 0.05).
A principal component analysis (PCA) was conducted on the anatomical variables studied so that possible groupings among provenances could be observed. This method extracts a set of uncorrelated variables as linear combinations of the original variables, reducing the dimensionality of data while preserving most of the variance. The new variables, termed components, are arranged in order of decreasing variance and those with the highest variance are termed PCs. The PCs were displayed in graph form as scatter plots of the scores, in order to observe any groupings in the data set.
Regression analyses were performed between the mean values by provenance of the anatomical variables measured and the mean values by provenance of a series of climate factors (for the 2000–2003 year period) important for plant life: altitude, duration of aridity (months in which the curve of the monthly average temperature was above the curve of monthly rainfall in an ombrothermic diagram), annual rainfall, minimum monthly summer rainfall, number of months of certainty of frost (months with mean minimum <0), number of months of probable frost (months with absolute minimums <0 and mean minimum >0), average annual temperature, lowest average monthly temperature, highest average monthly temperature, average minimum temperature in the month with the lowest average, average maximum temperature in the month with the highest average, and absolute minimum and maximum temperatures for the year interval used. A regression analysis was also carried out between individual anatomical variables.
Results
Effects of region of provenance and climate on wood biometry
The GLM results are shown in Table 2. The two sources of variation (provenance and tree) were significant in the model (P < 0.01), except in the following cases: (1) for ray tracheid frequency, the provenance factor was not significant (P > 0.05); and (2) for axial resin canal frequency, the tree factor was not significant (P > 0.08). For most of the anatomical variables (12 out of a total of 18), the provenance factor had a greater role in explaining the total variation in the model. One noteworthy aspect was the high percentage of variance explained by the provenance factor on the variable intertracheid wall strength (45.12%) and by the tree factor on radial resin canal frequency (54.66%).
The mean values of the anatomical variables by region of provenance (Table 3) showed high variability between provenances. In certain cases, an influence of geographical proximity on anatomical variation between provenances was observed. For example, in the regions in the Betic Cordillera (8A, 8B, 8D and 8E), tracheid diameter showed very similar values and tracheids were shorter than in the other provenances. Provenance 7C, subject to least aridity, showed the highest values for intertracheid wall strength and axial resin canal diameter and the lowest values for tracheid lumen diameter, bordered pit diameter, maximum cross-field pit diameter and resin canal-ring distance. Provenance 8D, subject to the driest conditions, showed the highest values for tracheid lumen diameter, ray frequency, number of cross-field pits, maximum diameter of cross-field pits and radial resin canal frequency. Region 10 showed the highest values for most frequent ray height and resin canal-ring distance.
The PCA results showed good grouping of the eight provenances in relation to phytoclimate, as seen in the scatter plot of the PC1 and PC3 scores (Fig. 2a). PC3 clearly separated the regions with a Mediterranean phytoclimate, with negative scores, from the nemoral and nemoro-Mediterranean phytoclimates, which had positive scores. The nemoral region (7C) showed the most negative scores in PC1 and was separate from the other provenances.
Principal component analysis (PCA) of the biometry of Pinus nigra ssp. salzmannii earlywood from eight Spanish provenances. Wood samples were obtained from basal discs from mature trees. a Scatter plot of PC1–PC3 scores. Each point represents a provenance (see Table 1 for description of provenances). b Projection of anatomical variables onto the component plane. Abbreviations: Td = axial tracheid diameter; Tl = axial tracheid length; Ld = axial tracheid lumen diameter; Wt = axial tracheid wall thickness; Ws = intertracheid wall strength; Pd = bordered pit diameter; Rf = ray frequency; Rh = ray height; Rfh = most frequent ray height; RPf = ray parenchyma cell frequency; RTf = ray tracheid frequency; CPf = cross-field pit frequency; CPdmax = maximum cross-field pit diameter; CPdmin = minimum cross-field pit diameter; ACd = axial resin canal diameter; ACf = axial resin canal frequency; AC-R = axial resin canal-growth ring distance; RCd = radial resin canal diameter; RCf = radial resin canal frequency
The projection of the anatomical variables onto the PC1–PC3 plane is shown in Fig. 2b. Certain variables are noteworthy for their role in separating provenances according to phytoclimate: (1) the variables intertracheid wall strength, axial resin canal diameter and axial resin canal frequency characterised the nemoral phytoclimate (in accordance with the negative PC1 gradient); (2) the variables minimum cross-field pit diameter and tracheid length characterised the nemoral and nemoro-Mediterranean phytoclimates (in accordance with the positive PC3 gradient); and (3) the variables ray frequency, number of cross-field pits and frequency of ray parenchyma cells characterised the Mediterranean phytoclimate (in accordance with the negative PC3 gradient).
The regression analysis between climate and anatomical variables showed the following significant relations (P < 0.05): altitude was negatively related to tracheid length (r = −0.96; Fig. 3a). Minimum monthly summer rainfall was positively related to cell wall thickness (r = 0.77) and negatively related to ray frequency (r = −0.84) and ray parenchyma cell frequency (r = −0.89; Fig. 3b). Absolute minimum temperature was negatively related to axial resin canal frequency (r = −0.88; Fig. 3c). Five temperature-dependent factors showed significant correlations with radial resin canal diameter: average annual temperature (r = −0.90; Fig. 3d), lowest average monthly temperature (r = −0.88), highest average monthly temperature (r = −0.87), average minimum temperature in the month with the lowest average (r = −0.80) and number of months with certainty of frost (r = 0.87). Lastly, absolute maximum temperature was negatively related to most frequent ray height (r = −0.76).
Relations between: altitude and axial tracheid length (a); minimum summer monthly rainfall and ray parenchyma cell frequency (b); absolute minimum temperature and axial canal frequency (c); and mean annual temperature and radial resin canal diameter (d). Points correspond to the mean value for each region of provenance and lines are linear regressions. Anatomical variables were measured in earlywood from basal discs from mature P. nigra trees
Relations between anatomical variables
The regression analysis between the anatomical variables (Table 4) showed the following significant relations (P < 0.05): Bordered pit diameter was directly related to tracheid lumen diameter (r = 0.74; Fig. 4a) and negatively related to intertracheid wall strength (r = −0.79). Maximum cross-field pit diameter was directly related to tracheid lumen diameter (r = 0.85; Fig. 4b) and bordered pit diameter (r = 0.90). Axial resin canal frequency was negatively related to tracheid lumen diameter (r = −0.72) and bordered pit diameter (r = −0.81).
Relations between axial tracheid lumen diameter and: bordered pit diameter (a) and maximum cross-field pit diameter (b). Points correspond to the mean value for each region of provenance and lines are linear regressions. Anatomical variables were measured in earlywood from basal discs from mature P. nigra trees
Discussion
This study characterises the earlywood anatomy of P. nigra ssp. Salzmannii from eight Spanish regions of provenance using samples obtained from basal discs from mature trees. For most of the anatomical variables studied, variation due to provenance was greater than intrapopulation variation (due to individual trees), which shows that the microstructure of P. nigra ssp. salzmannii is highly influenced by region of provenance. The influence of provenance was also seen in the fact that regions in close geographical proximity (e.g. 7A, 7B and 7C) had very different biometries (Table 3).
Variation in intertracheid wall strength due to provenance was particularly high (Table 2). It has been suggested that high intertracheid wall strength values are associated with arid environments, given the need to withstand greater implosion loads in the conduit walls because of the negative pressure caused in situations of severe drought (Hacke et al. 2001; Sperry et al. 2006). However, the highest value for intertracheid wall strength in the present study was seen in the region subject to least aridity (nemoral region 7C), and this parameter also showed high variation between provenances within a single phytoclimate or geographical area (Table 3). Therefore, the variation observed in intertracheid wall strength is difficult to interpret. The wall of the conduit must withstand both implosion loads caused during water conduction in situations of water stress as well as gravity and wind loads. The combination of both types of load will condition and define the strength of the conduit (Sperry et al. 2006). In this respect, the positive correlation found between cell wall thickness and minimum monthly summer rainfall suggests that the former is possibly more closely related to mechanical support requirements than to water stress resistance, as trees that are less restricted by summer drought grow better (Table 1) and have to withstand greater gravity and bending loads. A similar tendency was recently observed in P. halepensis (Esteban et al. 2010).
Another possible interpretation of the higher intertracheid wall strength values in nemoral region 7C than in the more arid areas could be the result of parameters associated with xylem capacitance. A recent study on Douglas-fir (Pseudotsuga menziesii) and ponderosa pine (Pinus ponderosa) showed that the vulnerability of sapwood to embolism is greater in populations of both species adapted to more arid environments (Barnard et al. 2011). However, capacitance was also greater in these populations, suggesting that under conditions of high transpiration, xylem capacitance can play an important role in avoiding levels of tension that would cause excessive embolism. These factors may also be involved in the adaptation of P. nigra to the most arid conditions, which could compensate the need for structural modifications to increase resistance to implosion. The fact that the trees subject to greater summer water stress are characterised by a higher frequency of rays and ray parenchyma cells (Figs. 2, 3b) may indicate a greater water storage capacity in the sapwood, which could buffer excessive tensions on the water column under situations of high transpiration.
On comparing P. nigra with other Mediterranean pine species (P. halepensis and P. sylvestris), the intertracheid wall strength values of each species appear to follow a gradient corresponding to the adaptation of the species to drought, as the average values for Spanish populations of P. halepensis, P. nigra and P. sylvestris are (mean ± SE) 0.101 ± 0.009, 0.040 ± 0.001 and 0.037 ± 0.001, respectively (authors, recent measurements). In terms of microthermal and hydrophilic features, P. nigra is between P. sylvestris, adapted to oroboreal phytoclimates, and P. halepensis, adapted to Mediterranean phytoclimates with severe summer drought. From this, it can be concluded that high intertracheid wall strength values are associated with adaptation of pines in the Mediterranean to arid conditions, although the intraspecific variations in this parameter do not follow this pattern. The pit microstructure may also have a role in defining wall strength requirements in arid environments, given that during a water stress period the greatest implosion forces occur in the shared wall between a functional and an embolised conduit, and vulnerability to cavitation is mainly determined by pit microstructure (Tyree and Zimmermann 2002). In fact, a direct relation has been described between intertracheid wall strength and cavitation resistance (Hacke et al. 2001).
The population most exposed to prolonged drought (8D, with 3.9 months of aridity) had the largest average axial tracheid lumen diameter, whereas the population subject to the shortest interval of drought (7C, with 1.1 month of aridity) had the smallest lumen diameter. The Hagen Poiseuille law states that hydraulic conduction is proportional to the fourth power of the conduit diameter (Tyree and Zimmermann 2002). Therefore, large lumen diameters require lower investment in tissue to reach a certain hydraulic conductivity and have greater capacity to tolerate higher transpiration rates (Sperry 2003). In line with these findings, populations of P. sylvestris (Eilmann et al. 2009; Martín et al. 2010) and Pinus ponderosa Dougl. (Maherali and DeLucia 2000) have larger tracheid lumen diameters in arid conditions than that in more mesic conditions. Increased conductivity associated with larger tracheid lumen in provenance 8D than in 7C could be interpreted as a way to improve total water conductivity of the tree without extra carbon costs. Disregarding the provenances with the longest and shortest periods of drought (8D and 7C), no similar tendencies were observed on comparing the other provenances. For example, in provenance 8E, with similar climate features to 8D except for a shorter summer drought period, tracheid lumen diameter was the same as or smaller than that of other regions with a more favourable climate (e.g. 7A, 7B and 10). There may be a drought threshold, beyond which it becomes necessary to increase lumen diameter to maintain conduction efficiency, which means that differences become apparent only in the case of contrasting climates. Moreover, given that lumen diameter affects intertracheid wall strength, it is not only water factors but also structural factors that will affect lumen diameter (Sperry et al. 2006), which makes the search for relations between structure and function more complicated.
Another factor that can determine lumen diameter size is freezing-induced xylem cavitation. This occurs when the sap in the xylem freezes and bubbles are formed in the conduit lumen, as air is soluble in liquid water but not in ice (Zimmermann 1983). The larger the conduit diameter is, the more air it will contain in dissolution and the larger the diameter of the bubbles will be, which means the air bubbles will expand more easily during thawing. In conifers, it has been noted that freezing-induced cavitation causes considerable loss of hydraulic conductivity beyond a mean tracheid lumen diameter of 30 μm (Pittermann and Sperry 2003). Although the area of extent P. nigra in Spain is subject to considerable frosts (Table 1), no relation was observed between tracheid lumen diameter and the climate variables most associated with frost. Thus, it is unlikely that frost causes significant loss of hydraulic conductivity in the studied populations. However, given that propensity to freezing-induced cavitation also increases as a result of preceding drought events (Wilson Willson and Jackson 2006), it is possible that in the more arid areas subject to heavy frost, tracheid lumen is partially limited by this factor.
An increase in lumen diameter does not necessarily involve an increase in total conductivity, as water flows not only through the lumen but also through intertracheal pits (Sperry et al. 2006), which are estimated to be responsible for at least 50% of total xylem resistivity (Choat et al. 2008). The correlation found between tracheid lumen size and bordered pit diameter concurs with the findings of Hacke et al. (2004) on comparing 17 species of gymnosperms and suggests that an increase in conductivity associated with larger lumens is associated with an increase in pit conductivity, as larger pits lead to greater conductivity (Rosner et al. 2007). The negative correlation found between bordered pit diameter and intertracheid wall strength may be a result of this, given that when wall thickness is constant, the strength of the conduit decreases the larger the lumen is.
Tracheid lumen diameter was also correlated with maximum cross-field pit diameter. Cross-field pits are very important in conifers, as they are the point of contact for the axial water transport system and the biochemically active ray parenchyma cells. This facilitates water transport from the axial tracheids to the rays and allows the metabolic products synthesised in the parenchyma cells to be deposited in the axial tracheid system. For example, in Pinus radiata (Hillis 1968) and Pinus canariensis C. Smith (Esteban et al. 2005), resin impregnation of axial tracheids is carried out from the ray parenchyma cells through the cross-field pits. The results suggest that the larger the tracheid is, the larger its communication system with the ray system has to be in order to maintain the same flow of water or metabolites between the radial and axial systems.
Tracheid length affects aspects associated with hydraulics. Longer tracheids allow the water flow to cross fewer end-wall crossings per unit of length, which means that total hydraulic resistance is lower (Sperry et al. 2006). The longest tracheids were found in the most favourable regions, where increased tracheid length is a hydraulic advantage, as the taller the tree grows, the greater the distance water has to travel from the ground to the leaves. Therefore, it seems conceivable that the best provenances in terms of growth would have the longest tracheids, as seen in P. sylvestris (Hannrup et al. 2000). It is difficult to separate the effect of altitude on tracheid length (Fig. 3a) from the effect of climate, as the driest regions are also located at the highest altitudes. However, the same tendency of shorter tracheids at higher altitudes has been observed in other pine species, such as Pinus ponderosa Laws. (Echols 1973), Pinus taiwanensis Hayata and Pinus patula Schlecht et Cham (Zobel and van Buijtenen 1989). Although other studies noted a positive influence of air temperature on tracheid length (e.g. Watt et al. 2008 and references therein), this relation was not observed in the present study. It may be that the range of temperatures between the regions of provenance studied is not broad enough for this difference to be noted.
One noteworthy aspect is seen in the correlations found between average radial resin canal diameter and five climate parameters related to temperature, which show that in colder environments P. nigra forms larger radial resin canals. Similarly, axial resin canal frequency was highly negatively correlated with absolute minimum temperature. The functions of resin canals are not clear, although it is widely accepted that their main function is to deter herbivores (Franceschi et al. 2005) and protect trees from certain fungal infections (Woodward 1992), particularly in the case of traumatic resin canals formed as a result of mechanical or fungal wounds. Nonetheless, whether normal resin canals (non-traumatic) also provide this type of protection is not so clear. The continuity existing between the lumen of radial and axial resin canals results in a three-dimensional protective structure which facilitates resin flow to the outer regions of the bark (Nagy et al. 2000). Moreover, in loblolly pine, a correlation has been found between resin flow and axial resin canal frequency (Blanche et al. 1992). This suggests that in P. nigra the combination of large radial resin canals and high axial resin canal frequency constitutes a preformed defence system that is structurally more powerful than small radial resin canals and low axial resin canal frequency. It is possible that the stress caused to the plant by severe winter cold spells is a factor of predisposition to disease, in the same way that high temperatures and summer droughts affect P. sylvestris by stimulating resin canal formation (Rigling et al. 2003). Resin canal formation is associated with growth hormones (Wimmer and Grabner 1997). Ethylene in particular appears to play a key role in linking exogenous factors and resin canal formation. The two principal factors inducing ethylene production are extreme temperatures and drought (Abeles et al. 1992), which could explain the relations observed.
The PCA results indicate that the Mediterranean phytoclimate is associated with high ray frequency and high ray parenchyma cell frequency, suggesting that the more intense summer drought is, the more ray parenchyma cells are produced by the tree. This is supported by the high negative correlations found between minimum monthly summer rainfall and ray frequency and ray parenchyma cell frequency (Fig. 3b). A high amount of ray parenchyma cells implies a high number of metabolically active cells capable of synthesising a large amount of products and accumulating reserve metabolites. High levels of carbohydrates, mainly starch, have been related to the drought tolerance of Picea rubens Sarg. (Amundson et al. 1993) and Quercus ilex L. (Bussotti et al. 2002). During prolonged drought, stomatal conductance, photosynthesis and cell water content decrease. Energy reserves are then invested in minimising the limitations imposed by stress through accumulation of organic osmolytes, whose principal source is starch (Amundson et al. 1993). Therefore, it seems conceivable that populations most adapted to drought would have greater abundance of ray parenchyma cells in the stem capable of accumulating starch. It is possible that the abundance of rays and ray parenchyma is determined by the minimum energy cost of maintaining the plant during the water stress period. Moreover, the effect of provenance on ray tracheid abundance was not significant (Table 2), showing quite uniform average values between all provenances (Table 3). This suggests that in P. nigra axial tracheid abundance is a factor which allows little environmental variation.
The present study shows how varying microclimate conditions in the regions of provenance of P. nigra have brought about significant structural differences in its wood. This demonstrates the importance of provenance when selecting seed origin for reforestation in Mediterranean areas, as observed in the case of reforestation of Pinus pinaster Ait. after forest fires (Gil et al. 2009). Inadequate seed selection can lead to very poorly adapted plantations or trees of very low quality, even more so considering that global warming would accentuate summer drought in Mediterranean environments.
References
Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in plant biology. Academic Press, San Diego
Alía R, Alba N, Agúndez D, Iglesias S, (coord.) (2005) Manual para la comercialización y producción de semillas y plantas forestales. Materiales de base y de reproducción. Serie forestal. DGB, Madrid
Allué-Andrade JL (1990) Atlas fitoclimático de España. Taxonomías. Instituto Nacional de Investigaciones Agrarias. Ministerio de Agricultura Pesca y Alimentación, Madrid
Amundson RG, Kohut RJ, Laurence JA, Fellows S, Colavito LJ (1993) Moderate water-stress alters carbohydrate content and cold tolerance of red spruce foliage. Environ Exp Bot 33:383–390
Aussenac G (1980) Comportement hydrique de rameaux excisés de quelques espèces de sapins et de pins noirs en phase de dessiccation. Ann For Sci 37:201–215
Barnard DM, Meinzer FC, Lachenbruch B, Mcculloh KA, Johnson DM, Woodruff DR (2011) Climate-related trends in sapwood biophysical properties in two conifers: avoidance of hydraulic dysfunction through coordinated adjustments in xylem efficiency, safety and capacitance. Plant Cell Environ 34:643–654
Blanche CA, Lorio PL, Sommers RA, Hodges JD, Nebeker TE (1992) Seasonal cambial growth and development of loblolly-pine: xylem formation, inner bark chemistry, resin ducts, and resin flow. For Ecol Manage 49:151–165
Bussotti F, Bettini D, Grossoni P, Mansuino S, Nibbi R, Soda C, Tani C (2002) Structural and functional traits of Quercus ilex in response to water availability. Environ Exp Bot 47:11–23
Carlquist SJ (2001) Comparative wood anatomy: systematic, ecological, and evolutionary aspects of dicotyledon wood, 2nd edn. Springer, Berlin
Castellarnau JM (1883) Estudio micrográfico del sistema leñoso de las coníferas y en general del género Pinus. Anales de la Sociedad Española de Historia Natural XII:136–219
Catalán G, Gil P, Galera RM, Martín S, Agúndez D, Alía R (1991) Las regiones de procedencia de Pinus sylvestris L. y Pinus nigra Arn. subsp. salzmannii (Dunal) Franco en España. Instituto Nacional para la Conservación de la Naturaleza, Madrid
Choat B, Cobb AR, Jansen S (2008) Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytol 177:608–625. doi:10.1111/j.1469-8137.2007.02317.x
Echols RM (1973) Effects of elevation and seed source on tracheid length in young ponderosa pine. For Sci 19:46–49
Eilmann B, Zweifel R, Buchmann N, Fonti P, Rigling A (2009) Drought-induced adaptation of the xylem in Scots pine and pubescent oak. Tree Physiol 29:1011–1020. doi:10.1093/treephys/tpp035
Esteban LG, Guindeo A (1988) Anatomía e identificación de las maderas de coníferas españolas. Aitim, Madrid
Esteban LG, de Palacios P, Guindeo A, García L, Lázaro I, González L, Rodríguez Y, García Fernández F, Bobadilla I, Camacho A (2002) Anatomía e identificación de maderas de coníferas a nivel de especie/anatomy and identification of conifers wood as a species. Fundación Conde del Valle de Salazar—Ediciones Mundi Prensa, Madrid
Esteban LG, Gasson P, Climent JM, de Palacios P, Guindeo A (2005) The wood of Pinus canariensis and its resinous heartwood. IAWA J 26:69–77
Esteban LG, Martín JA, de Palacios P, García-Fernández F, López R (2010) Adaptive anatomy of Pinus halepensis trees from different Mediterranean environments in Spain. Trees Struct Funct 24:19–30. doi:10.1007/s00468-009-0375-3
Franceschi VR, Krokene P, Christiansen E, Krekling T (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167:353–375. doi:10.1111/j.1469-8137.2005.01436.x
Gil L, López R, García-Mateos A, González-Doncel I (2009) Seed provenance and fire-related reproductive traits of Pinus pinaster in central Spain. Int J Wildland Fire 18:1003–1009. doi:10.1071/WF08101
Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA (2001) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126:457–461
Hacke UG, Sperry JS, Pittermann J (2004) Analysis of circular bordered pit function—II. Gymnosperm tracheids with torus-margo pit membranes. Am J Bot 91:386–400
Hannrup B, Ekberg I, Persson A (2000) Genetic correlations among wood, growth capacity and stem traits in Pinus sylvestris. Scand J For Res 15:161–170
Hillis WE (1968) Chemical aspects of heartwood formation. Wood Sci Technol 2:241–259
IAWA Committee (2004) IAWA list of microscopic features for softwood identification. IAWA J 25:1–70
Jane FW (1970) The structure of wood, 2nd edn. Adam & Charles Black, London
Ladell JL (1959) A new method of measuring tracheid length. Forestry 32:124–125
Maherali H, DeLucia EH (2000) Xylem conductivity and vulnerability to cavitation of ponderosa pine growing in contrasting climates. Tree Physiol 20:859–867
Martín S, Díaz-Fernández PM, de Miguel J (1998) Regiones de procedencia de las especies forestales españolas. Géneros Abies, Fagus, Pinus y Quercus. Organismo Autónomo de Parques Nacionales, Madrid
Martín JA, Esteban LG, de Palacios P, Fernández FG (2010) Variation in wood anatomical traits of Pinus sylvestris L. between Spanish regions of provenance. Trees Struct Funct 24:1017–1028. doi:10.1007/s00468-010-0471-4
McCullagh P, Nelder JA (1989) Generalized linear models, 2nd edn. Chapman & Hall, London
Nagy NE, Franceschi VR, Solheim H, Krekling T, Christiansen E (2000) Wound-induced traumatic resin duct development in stems of Norway spruce (Pinaceae): anatomy and cytochemical traits. Am J Bot 87:302–313
Peraza C (1964) Estudio de las maderas de coníferas españolas y de la zona norte de Marruecos. Instituto Forestal de Investigaciones y Experiencias, Madrid
Pittermann J, Sperry JS (2003) Tracheid diameter is the key trait determining the extent of freezing-induced embolism in conifers. Tree Physiol 23:907–914
Rigling A, Brühlhart H, Bräker OU, Forster T, Schweingruber FH (2003) Effects of irrigation on diameter growth and vertical resin duct production in Pinus sylvestris L. on dry sites in the central Alps, Switzerland. For Ecol Manage 175:285–296
Rosner S, Klein A, Müller U, Karlsson B (2007) Hydraulic and mechanical properties of young Norway spruce clones related to growth and wood structure. Tree Physiol 27:1165–1178
Ruiz de la Torre J (2006) Flora mayor. Organismo Autónomo Parques Nacionales. Dirección General para la Biodiversidad, Madrid
Sperry JS (2003) Evolution of water transport and xylem structure. Int J Plant Sci 164:S115–S127
Sperry JS, Hacke UG, Pittermann J (2006) Size and function in conifer tracheids and angiosperm vessels. Am J Bot 93:1490–1500
Tyree MT, Zimmermann MH (2002) Xylem structure and the ascent of sap. Springer, Berlin
Tyree MT, Davis SD, Cochard H (1994) Biophysical perspectives of xylem evolution: is there a tradeoff hydraulic efficiency for vulnerability to dysfunction? IAWA J 15:335–360
Watt MS, D’Ath R, Leckie AC, Clinton PW, Coker G, Davis MR, Simcock R, Parfitt RL, Dando J, Mason EG (2008) Modelling the influence of stand structural, edaphic and climatic influences on juvenile Pinus radiata fibre length. For Ecol Manag 254:166–177
Willson CJ, Jackson RB (2006) Xylem cavitation caused by drought and freezing stress in four co-occurring Juniperus species. Physiol Plantarum 127:374–382
Wimmer R, Grabner M (1997) Effects of climate on vertical resin duct density and radial growth of Norway spruce [Picea abies (L.) Karst]. Trees Struct Funct 11:271–276
Woodward S (1992) Responses of gymnosperm bark tissues to fungal infections. In: Blanchette A, Biggs R (eds) Defense mechanisms of woody plants against fungi. Springer, Berlin, pp 62–75
Zimmermann MH (1983) Xylem structure and the ascent of sap. Springer, Berlin
Zobel BJ, van Buijtenen JP (1989) Wood variation: its causes and control. Springer, Berlin
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Seifert.
Luis G. Esteban and Juan A. Martín contributed equally to this work.
Rights and permissions
About this article
Cite this article
Esteban, L.G., Martín, J.A., de Palacios, P. et al. Influence of region of provenance and climate factors on wood anatomical traits of Pinus nigra Arn. subsp. salzmannii . Eur J Forest Res 131, 633–645 (2012). https://doi.org/10.1007/s10342-011-0537-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-011-0537-x