Abstract
In managed settings, seedlings are often fertilized with the objective of enhancing establishment, growth, and survival. However, responses of seedlings to fertilization can increase their susceptibility to abiotic stresses such as drought. Seedlings acclimate to variation in soil resources by reallocating carbon among different physiological processes and compartments, such as above versus belowground growth, secondary metabolism, and support of ectomycorrhizal fungi (EMF). We examined the effects of nutrient and water availability on carbon allocation to above and belowground growth of river birch (Betula nigra), as well as partitioning among root sugars, starch, phenolics, lignin, and EMF abundance. As nutrient availability increased, total plant biomass and total leaf area increased, while percent root biomass decreased. Root sugars, total root phenolics and EMF abundance responded quadratically to nutrient availability, being lowest at intermediate fertility levels. Decreased water availability reduced total leaf area and root phenolics relative to well-watered controls. No interactions between nutrient and water availability treatments were detected, which may have been due to the moderate degree of drought stress imposed in the low water treatment. Our results indicate that nutrient and water availability significantly alter patterns of carbon allocation and partitioning in roots of Betula nigra seedlings. The potential effects of these responses on stress tolerance are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fertilization is routinely used to enhance establishment and growth of seedlings in managed settings. Although seedlings can benefit from fertilization in extremely nutrient-deficient soils where growth and photosynthesis may be severely constrained, over-fertilization in managed settings is common (Rytter et al. 2003) and can increase susceptibility to herbivores (Raupp et al. 2010), pathogens (Blodgett et al. 2005; Hagan et al. 2008; Piri 1998), and drought-related injury (Linder et al. 1987; Lloyd et al. 2006). Drought stress reduces seedling growth and establishment and can predispose seedlings to disease (Wargo 1996) and insect attack (Huberty and Denno 2004). Excessive fertilization may influence seedling physiology in ways that exacerbate drought-related injury (Kleczewski et al. 2010). Specifically, fertilization can alter patterns of seedling (1) biomass allocation; (2) concentrations/contents of carbon reserves and defense-related compounds; and (3) associations with mycorrhizal fungi (Kleczewski et al. 2010).
Tree seedlings can acclimate to soil nutrient availability by altering patterns of carbon allocation and partitioning among organs, allowing for optimal growth in a particular soil (Aerts and Chapin 2000; Kozlowski 2002; Glynn et al. 2007). Optimal allocation theory (OA) predicts that plants allocate a greater proportion of biomass to roots in nutrient-poor soils to enhance resource capture (Ibrahim et al. 1997; Ingestad and Ågren 1988; Shipley and Meziane 2002). Conversely, in soils with relatively high levels of nutrients, OA predicts that plants allocate a greater proportion of biomass towards foliage and stems as light, not soil resources, limit growth processes (Poorter and Nagel 2000). Increased carbon allocation to aboveground tissues may cause seedlings to become more susceptible to drought-related injury by increasing water demand and transpirational water loss while simultaneously reducing the capacity to acquire water (Lloyd et al. 2006).
Total non-structural carbohydrates (e.g. soluble sugars, starch) play important roles in protecting seedlings during periods of drought, as they support respiration and can facilitate fine root and mycorrhizal growth. Patterns of carbohydrate partitioning in roots can be affected by nutrient availability with fertilization generally decreasing total non-structural carbohydrate concentrations (Kobe et al. 2010; McDonald et al. 1986; Mooney et al. 1995), which can contribute to predisposing seedlings to injury from drought (Kleczewski et al. 2010). Fertilization can also reduce the content and concentration of secondary metabolites, which have roles in protection of tissues from drought-induced free radical production (Close and MacArthur 2002; Rozema et al. 1997) as well as protection of seedlings from pathogens and pests (Bennett and Wallsgrove 1994; Pearce 1996).
The growth-differentiation balance hypothesis (GDBH) attributes fertilizer effects on plant secondary metabolism to a physiological trade-off between primary and secondary metabolism that constrains constitutive secondary metabolism in rapidly growing organs and plants (Herms and Mattson 1992). The GDBH predicts that the effects of fertilization on secondary metabolism will vary in a nonlinear manner depending on an initial soil nutrient availability and fertilization rate. For example, fertilization of soils of moderate nutrient availability is predicted to increase growth and, as a consequence of the physiological trade-off, decrease constitutive secondary metabolism. Conversely, fertilization of extremely infertile soils is predicted to relax nutrient constraints on physiological processes, thereby increasing photosynthesis, growth, and constitutive secondary metabolism, resulting in positive correlations among them (Herms and Mattson 1992). These predictions of GDBH have been supported in recent studies that quantified foliar secondary metabolism (Glynn et al. 2003, 2007; Hale et al. 2005), but only a few studies have examined belowground trade-offs between growth and defense. For example, Kleczewski et al. (2010) found that fertilizer effects on root secondary metabolism can be context dependent. In their study, fertilization of Betula papyrifera L. in nutrient-deficient subsoil reduced root secondary metabolism while fertilization of moderately fertile topsoil increased contents of root phenolics and lignin relative to controls. This response was attributed to a reduction in carbon allocation to root growth and a subsequent increase in carbon partitioned to root secondary metabolism. Kleczewski et al. (2010) also documented a negative correlation between the concentration of root secondary metabolites and the abundance of ectomycorrhizal fungi (EMF).
EMF play crucial roles in acclimation of seedlings to nutrient deficiency and drought (Davies et al. 1996; Mudge et al. 1987). The extramatrical hyphae access and translocate otherwise unavailable nutrients and water to tree seedlings in exchange for host-derived carbohydrates (Nehls 2008; Tarkka et al. 2005). Fertilization (particularly N) is known to decrease EMF abundance, diversity, and growth (Newton and Pigott 1991), perhaps by reducing carbon availability to the fungus by the host (Nehls et al. 2010) or by increasing concentrations of phenolics or lignin in roots (Feughy et al. 1999; Kleczewski et al. 2010; Weiss et al. 1999).
The objective of this study was to examine within the framework of allocation theory the effects of nutrient and water availability on above and above and belowground biomass allocation and growth of river birch (Betula nigra L.), as well as carbon partitioning in roots among sugars, starch, phenolics, lignin, and EMF. River birch is an ectomycorrhizal species adapted to flood plains that grows well in high clay soils, and is sensitive to water deficit (Ranney et al. 1991; Gilman and Watson 1993; Gu et al. 2007). Specifically, we tested the following predictions of allocation theory: (1) percent root biomass and total non-structural carbohydrate content will decrease as nutrient availability increases; (2) root secondary metabolite levels and concentrations will be highest at intermediate levels of nutrient availability; (3) EMF abundance will decrease with as nutrient availability increases; and (4) as nutrient availability increases, drought stress tolerance will decrease.
Materials and methods
Plant material, soil, and growing conditions
The study took place at the Department of Horticulture and Crop Science, Howlett Greenhouses located on the Ohio State University in Columbus, OH. Seventy-two, 1-year-old dormant clonally propagated B. nigra (Schumacher Co, Sturgeon Bay, WI, USA) were planted into 13.25 L pots, placed outdoors, and watered to capacity daily until bud break (approximately 10–14 days). Large pots were used to prevent root binding and to allow adequate volume for exploration of substrate by roots and EMF hyphae.
Pots contained air-dried soils originating from an agricultural site at the Ohio Agricultural Research and Development Center (Wooster, OH; approx. Google Earth coordinates 40°47′N, 81°56′W). Soil samples were analyzed at the STAR lab at OARDC (http://oardc.osu.edu/starlab/default.asp) to estimate initial soil pH (Thomas 1996), inorganic nitrate–N [using 2 M KCl extractions (Gelderman and Beegle 1998)], available inorganic P [using a P1 extraction (Frank et al. 1998)], exchangeable K, Mg, and Ca [using ammonium acetate extraction (Helmke and Sparks 1996; Warncke and Brown 1998)], and organic matter (OM) [calculated as loss on ignition (Combs and Nathan 1998)]. Soil analysis at the start of the experiment indicated that the nutrient levels, particularly N, were relatively low (nitrate–N 19.1 μg g−1; P 35.0 μg g−1; K 163 μg g−1; Ca 3091 μg g−1; Mg 216 μg g−1), with moderate levels of organic matter (3.5%) and a pH of 7.8.
Following budbreak, plants were moved into a flat roof Cravo greenhouse equipped with retractable roof and walls (Cravo Equipment Ltd., Brantford, ON, Canada). The Cravo unit provides an environment more similar to ambient conditions than conventional greenhouses, while maintaining some control of environmental variables, particularly access to water. When closed, the retractable material allows for 80% light transmission. The retractable roof remained closed for the duration of the experiment, and walls were programed to open when the temperatures exceeded 21°C.
Experimental design and implementation
The experiment was 2 × 3 factorial, arranged in a randomized complete block design, with two levels of water availability (low and adequate) crossed with three levels of nutrient availability (no, medium, and high fertilization), with 12 replications of each treatment combination. The 72 experimental units were divided into two blocks based on location in the Cravo unit. An additional 12 river birch ramets were oven dried at 60°C for 7 days, and weighed to estimate total plant biomass when the experiment was initiated.
The adequate (W+) and low (W−) water treatments were implemented using drip irrigation. Plants in the (W+) treatment received 800 ml H2O day−1, which provided adequate soil moisture throughout the study (i.e. soils would stick together easily when squeezed in the hand). Plants in the (W−) treatment received 400 ml H2O day−1, which was designed to drought stress seedlings while minimizing seedling mortality. Relative to the (W+) treatment, soil moisture was noticeably reduced in the (W−) treatment (i.e. soil would crumble in the hand under moderate pressure).
The three levels of the fertilization treatment included non-fertilized control (ambient soil), medium (112 kg N/ha), and high (224 kg N/ha). These fertilization application rates fall within the range of standard recommendations for woody plants (ANSI 2004). In this way, we were able to achieve a gradient of nutrient availability that also mimicked fertilization rates tree seedlings might experience in a managed setting. In order to maximize initial establishment and growth, all plants received 75 mg NH4–NO3 which was topdressed onto fertilized pots on day 1. Plants in the low fertility treatment received no additional fertilizer. For the next 14 weeks, plants in the intermediate and high-fertility treatments received fertilizer containing fast- and slow-release nitrogen (30-10-7 NPK; Arbor Green PRO®-Davey Tree Co.). Plants in the intermediate- and high-fertility treatments received 216 mg N in 400 mL H2O once or twice per week, respectively. The combination of weekly additions and slow-release fertilizer was aimed to minimize nutrient leaching from pots. None of the plants exhibited symptoms of nutrient deficiency or toxicity over the course of the experiment.
Pre-dawn water potentials were measured on day 104 with a model 670 pressure bomb (PMS instruments Co.) on a leaf excised from the upper 10 cm of the stem apex. Water potential was not measured regularly to avoid excessive defoliation of the small plants. Effects of water availability were further characterized by analyzing treatment effects on total plant biomass, total leaf area, and other allocation and partitioning parameters.
Biomass allocation measurements
Immediately prior to destructive harvests, a subsample of eight fully expanded leaves was collected from each plant and photographed with a digital camera, with images used to calculate leaf areas using image analysis software (ASSESS, University of Manitoba, Manitoba, Canada). The leaves were then oven dried at 60°C for 14 days. On days 105 (block 1) and 106 (block 2), plants were destructively harvested and separated into above and belowground components. Foliage and stems were separated from one another and oven dried at 60°C for 14 days. Root systems were washed carefully with water in the field. From each plant, a subsample of approximately 50% of the root system was collected and homogenized. Approximately 25% of the subsample was placed in 50-ml centrifuge tubes containing ddH2O and stored at 4°C for assessment of EMF abundance and diversity (Kleczewski et al. 2010). The remaining 75% of the root subsample was flash frozen, lyophilized, weighed, and milled for chemical analyses. The remaining 50% of the roots were oven dried at 60°C for 14 days. For each plant, the various dry weight fractions were combined to calculate total leaf, stem, root, and whole plant biomass. The following indices of plant growth and biomass allocation were calculated according to the following formulae and units (Hunt 1978):
-
Total seedling biomass (g): root biomass + stem biomass + foliar biomass
-
Leaf mass area (g m−2): foliar biomass/foliar area
-
Total leaf area (m2): foliar biomass/leaf mass area
-
Leaf area ratio (cm2 g−1): total leaf area/total seedling biomass.
Chemical analyses
Root soluble sugars, starch, phenolics, and lignin were quantified as described in Kleczewski et al. (2010). Phytochemical concentrations may have important consequences for EMF colonization and development; however, overall partitioning of carbon to individual and total phytochemical pools in roots may be influenced by plant size (Koricheva 1999). To account for this, concentrations of root phytochemicals were also normalized to root biomass, and presented as proportion of total plant biomass [(concentration × root biomass)/total seedling biomass].
Quantification of EMF abundance and identification of EMF
To quantify EMF abundance, 200 root tips per plant were examined, and percent of total root tips colonized was recorded. Morphological characterization (morphotyping) of colonized roots conducted at 40× magnification using a dissecting microscope (Kleczewski et al. 2010) revealed only a single morphotype. Hence, no measures of EMF diversity were calculated.
To identify the morphotype, DNA of replicate mycorrhizal tips was extracted and the ITS region amplified as described in Kleczewski et al. (2010). Amplicons were sequenced at the Plant–Microbe Genomics Facility (pmgf.osu.edu). Sequences were manually edited using Chromas Lite v2.01 (Technelysium Pty Ltd.) and submitted to NCBI GenBANK for comparison against deposited fungal ITS sequences using BLAST. Matches were considered positive when sequence identity was ≥97% and E values were 0.0.
Data analyses
Data were analyzed statistically using SPSS v.18 (SPSS Inc., Chicago, IL, USA). The explore function of SPSS was used examine data for normality, and percent root biomass and percent EMF colonization were arcsine transformed prior to analyses. MANOVA was used to test for overall significance of main effect factors (water and nutrient availability) and their interactions. Following significant effects of MANOVA, the effects of treatments and/or their interactions on individual variables were assessed by univariate ANOVA (Proc GLM; Type III sum of squares), with data reported as least square mean ± 1 standard error (SE). We examined relationships among means within fertility treatment (a quantitative factor) using orthogonal contrasts to test for linear or quadratic treatment effects (Chew 1976). MANOVA indicated that the fertility and water availability treatments, but not their interaction, influenced plant growth, allocation, and partitioning variables (Table 1). Therefore, results are presented as main effects averaged across all levels of the other factor.
Results
Effects of nutrient availability
Plant growth and biomass allocation
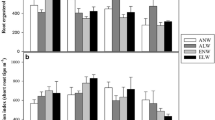
Increasing nutrient availability had a positive linear effect on total plant biomass (+44% from control to high fertilization) and total leaf area (+56% from control to high fertilization), a marginally significant (P = 0.06) positive linear effect on leaf area ratio (+9% from control to high fertilization), and a negative linear effect on percent root biomass (−6.5% from control to high fertilization) (Table 2).
Root phytochemistry
Nutrient availability had no effect on content and concentration of root starch, phenolics, and lignin (Table 3). However, root soluble sugars showed a quadratic response to nutrient availability, with both content and concentration lowest at the medium fertility level (Table 3).
EMF abundance and identity
Nutrient availability also had a quadratic effect on EMF abundance, which was 52 and 17% higher in the medium fertility treatment relative to the control and high-fertility treatment, respectively (Table 2). BLAST results indicated that the single morphotype observed on plant roots was highly similar to a Peziza ostracoderma isolate obtained from Castanea dentata (Marsh.) Borkh. (Accession EU819461.1; 1,085 bit score; E = 0.0; 97% identity). Members of this genus are known EMF associates (Smith and Read 1997).
Effects of water availability
Total leaf area was 11% lower in the (W−) relative to (W+) water treatment (Table 4). Water deficit also had a marginally significant (P = 0.06) effect on total plant biomass, which was 12% lower in the (W−) treatment, but water availability had no effect on other measures of biomass allocation, including EMF abundance (Table 4). Water availability also had no effect on root sugars, starch, or lignin (Table 5). However, total content and concentration of root phenolics were approximately 8 and 11% lower, respectively, in the (W−) relative to the (W+) water treatment (Table 5).
Discussion
This study demonstrated that above and belowground carbon allocation and partitioning of river birch are sensitive to nutrient and water availability. Responses of growth, leaf area, and root:shoot ratio were largely consistent with predictions of optimal allocation (OA) theory. However, we observed complex quadratic responses of root soluble sugars and EMF abundance to nutrient availability, and found that even moderate water deficit decreased phenolic-based root defenses.
Effects of nutrient availability on carbon allocation and partitioning
Consistent with predictions of OA theory, percent root mass of river birch increased and leaf area ratio decreased as nutrient availability decreased (Ingestad and Ågren 1988). It is often assumed that this proportional increase in root biomass results from greater production of fine roots in an attempt to maximize capture of limiting nutrients (Ostonen et al. 2007). However, another possibility is that increased belowground biomass results from increased partitioning to root phytochemicals. For example, in seven northern hardwood species, Kobe et al. (2010) found that the increased root biomass in response to lower soil nutrient availability was due largely to increased total non-structural carbohydrates and other chemical compounds. The quadratic effect of nutrient availability on soluble sugars that we observed is only partially consistent with the pattern observed by Kobe et al. (2010). For example, we found that soluble sugar content and concentration of roots decreased as nutrient availability increased from low to medium, which is consistent with the results of Kobe et al. (2010). However, as nutrient availability increased from medium to high, we observed root sugar levels to increase, which diverges from the pattern of Kobe et al. (2010). The quadratic pattern we observed also highlights the importance of testing more than two treatment levels when there is potential for nonlinear effects across resource gradients (e.g. Glynn et al. 2007).
Neither root starch concentration nor content was influenced by nutrient availability, which may not be surprising given the variable effects of fertilization on root starch observed in other studies (Ludovici et al. 2002; Kosola et al. 2006; Vizoso et al. 2008; Goodsman et al. 2010). Nutrient availability also had no effect on root secondary metabolism, which is more surprising given the large effect of fertility on total plant biomass, and the trade-off between growth and secondary metabolism as predicted by GDBH (Herms and Mattson 1992). Previous studies have documented increased root phenolic concentrations in response to nutrient limitation (Keski-Saari and Julkunen-Tiitto 2003; Kraus et al. 2004; Muller et al. 1989). However, in other studies, fertilization did not always affect root secondary metabolism. For example, in a 3-year study, Kosola et al. (2006) found that fertilization of hybrid poplar (Populus × canadensis cv ‘Eugeneii’) resulted in transient reductions in condensed tannin concentrations of roots.
In the present study, nutrient availability was shown to have quadratic effects on EMF abundance. This response is consistent with predictions of allocation theories (Treseder and Allen 2002; Vannette and Hunter 2011), and could explain the divergent patterns observed in other studies. Many studies have documented negative effect of fertilization on EMF abundance (Treseder 2004). Conversely, in other studies with birch, fertilization increased EMF abundance (Walker et al. 2004; Clemmensen et al. 2006; Kleczewski et al. 2010). According to Treseder and Allen (2002), a quadratic pattern of mycorrhizal growth or colonization is predicted over soil N or P gradients due to the effects of nutrient availability on plant carbon allocation and partitioning to EMF. In highly nutrient-deficient soils, plant carbon assimilation and allocation to roots is greatly constrained, which restricts mycorrhizal associations. As nutrient levels increase, nutrient limitations on carbon assimilation/allocation are relaxed, allowing for enhanced allocation to roots and mycorrhizal fungi (Treseder and Allen 2002). In fertile soils, where nutrient levels are not limiting plant growth, carbon is preferentially allocated to the production of foliage and stems, which decreases availability of carbon to support mycorrhizal growth. Our results are consistent with this hypothesis, although we did not test it directly. EMF responses to fertilization are known to be species specific, which could decrease the generality of this pattern (Avis et al. 2003; Walker et al. 2004).
Effects of water availability on carbon allocation and partitioning
Growth responses and patterns of biomass allocation and carbon partitioning were affected by water availability. In particular, total plant biomass and total leaf area increased by 12 and 13%, respectively, in the (W+) relative to the (W−) water treatment. Plants in the (W+) treatment exhibited reduced pre-dawn water potentials when measured at the end of the study. However, in comparison to previous studies with birch (e.g. Ranney et al. 1990; Kleczewski et al. 2010), the magnitude of water potential values that we observed suggest that plants in the (W−) treatment experienced mild to moderate water stress. Several physiological traits may have contributed to moderating the physiological impact of water deficit in the low water treatment. Birch species can acquire water from soils of low soil moisture content (Fort et al. 1998). Furthermore, river birch has been shown to acclimate to water deficit, thereby limiting the effects of water deficit on photosynthesis (Gu et al. 2007). The reduction in total leaf area in the (W−) treatment may have been due, in part, to premature leaf abscission, which was observed during warm summer weather. Abscission of foliage is considered a drought avoidance adaptation of river birch (http://www.dnr.state.oh.us/Home/trees/) that reduces transpirational losses (Fort et al. 1998; Gu et al. 2007).
Even in response to moderate drought stress, we observed a reduction of root phenolics in the low water treatment. Our understanding of the responses of root phytochemicals in water-stressed trees is limited, particularly the responses of secondary metabolites. The reduction in root phenolics of moderately water-stressed plants may indicate that these compounds do not play a major role in the protection B. nigra roots to drought stress injury. This is in contrast to other systems, in which increases in root phenolics or related enzymes with reduced water availability have been documented (Gray et al. 2003; Sofo et al. 2005; Weidner et al. 2009). Regardless, our results show that the root secondary metabolism of river birch can be significantly reduced under conditions of moderate water stress, which may have consequences for the susceptibility of seedling roots to pests and pathogens in natural or urban settings.
Potential impacts of fertilization on stress tolerance
Conventional wisdom holds that fertilized trees are inherently healthier than unfertilized trees (Herms and Mattson 1997). This may be true in extremely nutrient-deficient sites such as the disrupted, inverted profiles that characterize many urban soils (Craul 1994). However, a number of studies have found that fertilization can have negative effects on tree health (Herms and Mattson 1997) by decreasing their pest resistance (Herms 2002) and drought stress tolerance (Linder et al. 1987; Walters and Reich 1989; Kleiner et al. 1992; Nilsen 1995; Lloyd et al. 2006). In our study, water and nutrient availability did not interact to impact river birch growth or biomass allocation, perhaps because of the moderate nature of our drought stress treatment. However, the high fertilization did decrease percent root mass and EMF abundance, which has been associated with decreased stress tolerance.
References
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–65
ANSI (2004) American National Standard for tree care operations—tree, shrub, and other woody plant maintenance—standard practices (Fertilization). ANSI A300 (Part 2)—2004 Fertilization revision of ANSI A 300 (Part 2)—1998
Avis PG, McLaughlin DJ, Dentinger BC, Reich PB (2003) Long-term increase in nitrogen supply alters above and below ground ectomycorrhizal communities and increases the dominance of Russula spp. in a temperate oak savanna. New Phytol 160:239–253
Bennett RN, Wallsgrove RM (1994) Secondary metabolites in plant defense mechanisms. New Phytol 127:617–633
Blodgett JT, Herms DA, Bonello P (2005) Effects of fertilization on red pine defense chemistry and resistance to Sphaeropsis sapinea. For Ecol Manag 208:373–382
Chew V (1976) Comparing treatment means: a compendium. Hortscience 11:348–357
Clemmensen KE, Michelson A, Jonasson S, Shaver GR (2006) Increased ectomycorrhizal fungal abundance after long-term fertilization and warming of two artic tundra ecosystems. New Phytol 171:391–404
Close DC, MacArthur C (2002) Rethinking the role of many plant phenolics: protection from photodamage not herbivores? Oikos 99:166–172
Combs SM, Nathan MV (1998) Soil organic matter. Recommended chemical soil test procedures for the North Central region, NCR Publication no. 221. Missouri Agricultural Experiment Station, Columbia, pp 53–58
Craul PJ (1994) Urban soils: an overview and their future. In: Watson GW, Neely D (eds) The landscape belowground. International Society of Arboriculture, Champaign
Davies FT, Svenson SE, Cole JC, Phavaphutanon L, Duray SA, Olaide-Portugal V, Meyer CE, Bo SH (1996) Non-nutritional stress acclimation of mycorrhizal woody plants exposed to drought. Tree Physiol 16:985–993
Feughy L, Strullu DG, Poupard P, Simoneau P (1999) Induced defence responses limit Hartig net formation in ectomycorrhizal birch roots. New Phytol 144:541–547
Fort C, Muller F, Label P, Granier A, Dreyer E (1998) Stomatal conductance, growth and root signaling in Betula pendula seedlings subjected to partial soil drying. Tree Physiol 18:769–776
Frank K, Beegle D, Denning J (1998) Phosphorus. Recommended chemical soil test procedures for the North Central region, NCR Publication No. 221. Missouri Agricultural Experiment Station, Columbia, pp 21–23
Gelderman RH, Beegle D (1998) Nitrate–nitrogen. Recommended chemical soil test procedures for the North Central region, NCR Publication No. 221. Missouri Agricultural Experiment Station, Columbia, pp 17–20
Gilman EF, Watson DG (1993) Betula nigra: river birch. Fact sheet ST-94. University of Florida, Institute of Food and Agricultural Sciences, pp 1–3
Glynn C, Herms DA, Egawa M, Hansen RC, Mattson WJ (2003) Effects of nutrient availability on dry matter allocation, and constitutive and induced insect resistance of poplar. Oikos 101:385–397
Glynn C, Herms DA, Orians CM, Hansen RC, Larsson S (2007) Testing the growth-differentiation balance hypothesis: dynamic responses of willows to nutrient availability. New Phytol 176:623–634
Goodsman DW, Lieffers VJ, Landhaeusser SM, Erbilgin N (2010) Fertilization of lodgepole pine trees increased diameter growth but reduced root carbohydrate concentrations. For Ecol Manag 260:1914–1920
Gray DE, Pallardy SG, Garrett HE, Rottinghaus GE (2003) Acute drought stress and plant age effects on alkamide and phenolic acid content in purple coneflower roots. Planta Med 690:50–55
Gu MM, Rom CR, Robbins JA, Oosterhuis DM (2007) Effect of water deficit on gas exchange, osmotic solutes, leaf abscission and growth of four birch genotypes (Betula L.) under a controlled environment. Hortscience 42:1383–1391
Hagan AK, Akridge JR, Bowen KL (2008) Nitrogen and flowering dogwood. I. Impact of nitrogen fertilization rate on the occurrence of spot anthracnose, powdery mildew, and Cercospora leaf spot and their effect on tree growth. J Environ Hort 26:197–203
Hale BK, Herms DA, Hansen RC, Clausen TP, Arnold D (2005) Effects of drought stress and nutrient availability on dry matter allocation, phenolic glycosides, and rapid induced resistance of poplar to two lymantriid defoliators. J Chem Ecol 31:2601–2620
Helmke PA, Sparks DL (1996) Lithium, sodium, potassium, rubidium, and cesium. Methods of soil analysis, Part 3: Chemical methods. Soil Science Society of America, Madison, pp 568–569
Herms DA (2002) Effects of fertilization on insect resistance of woody ornamental plants: reassessing an entrenched paradigm. Environ Entomol 31:923–933
Herms DA, Mattson WJ (1992) The dilemma of plants—to grow or defend. Q Rev Biol 67:283–335
Herms DA, Mattson WJ (1997) Trees, stress, and pests. In: Lloyd JE (ed) Plant health care for woody ornamentals. International Society of Arboriculture, Champaign, pp 13–25
Huberty AF, Denno RF (2004) Plant water stress and its consequences for herbivorous insects: a new synthesis. Ecology 85:1383–1398
Hunt R (1978) Plant growth analysis. E. Arnold, London
Ibrahim ML, Proe F, Cameron AD (1997) Main effects of nitrogen supply, and drought stress upon whole plant carbon allocation in poplar. Can J For Res 27:1413–1419
Ingestad T, Ågren DI (1988) Nutrient-uptake and allocation at steady-state nutrition. Physiol Plantar 72:450–459
Keski-Saari S, Julkunen-Tiitto R (2003) Resource allocation in different parts of juvenile mountain birch plants: effect of nitrogen supply on seedling phenolics and growth. Physiol Plantar 118:114–126
Kleczewski NM, Herms DA, Bonello P (2010) Effects of soil type, fertilization and drought on carbon allocation to root growth and partitioning between secondary metabolism and ectomycorrhizae of Betula papyrifera. Tree Physiol 30:807–813
Kleiner KW, Abrams MD, Schultz JC (1992) The impact of water and nutrient deficiencies on the growth, gas exchange and water relations of red oak and chestnut oak. Tree Physiol 11:271–287
Kobe RK, Iyer M, Walters MB (2010) Optimal partitioning theory revisited: non-structural carbohydrates dominate root mass responses to nitrogen. Ecology 91:166–179
Koricheva J (1999) Interpreting phenotypic variation in plant allelochemistry: problems with the use of concentrations. Oecologia 119:467–473
Kosola KR, Parry D, Workmaster BAA (2006) Responses of condensed tannins in poplar roots to fertilization and gypsy moth defoliation. Tree Physiol 26:1607–1611
Kozlowski TT (2002) Acclimation and adaptive responses of woody plants to environmental stresses. Bot Rev 68:270–334
Kraus TEC, Zasoski RJ, Dahlgren RA (2004) Fertility and pH effects on polyphenol and condensed tannin concentrations in foliage and roots. Plant Soil 262:95–109
Linder S, Benson ML, Myers BJ, Raison RJ (1987) Canopy dynamics and growth of Pinus radiata. I. Effects of irrigation and fertilization during drought. Can J For Res 17:1157–1165
Lloyd JE, Herms DA, Rose M, Wagoner JV (2006) Fertilization rate and irrigation scheduling in the nursery influence growth, insect performance, and stress tolerance of “Sutyzam” crabapple in the landscape. Hortscience 41:442–445
Ludovici KH, Allen HL, Albaugh TJ, Dougherty PM (2002) The influence of nutrient and water availability on carbohydrate storage in loblolly pine. For Ecol Manag 159:261–270
McDonald AJS, Ericsson A, Lohammar T (1986) Dependance of starch storage on nutrient availability and photon flux density in small birch Betula pendula Roth. Plant Cell Environ 9:433–438
Mooney HA, Fichtner K, Schulze ED (1995) Growth, photosynthesis, and storage of carbohydrates and nitrogen in Phaseolus lunatus in relation to resource availability. Oecologia 104:17–23
Mudge KM, Diebolt KS, Whitlow TH (1987) Ectomycorrhizal effect on host plant response to drought stress. J Environ Hortic 5:183–187
Muller RN, Kalisz PJ, Luken JO (1989) Fine root production of astringent phenolics. Oecologia 79:563–565
Nehls U (2008) Mastering ectomycorrhizal symbiosis: the impact of carbohydrates. J Exp Bot 59:1097–1108
Nehls U, Gohringer F, Wittulsky S, Dietz S (2010) Fungal carbohydrate support in the ectomycorrhizal symbiosis: a review. Plant Biol 12:292–301
Newton AC, Pigott CD (1991) Mineral nutrition and mycorrhizal infection of seedling oak and birch 2. The effect of fertilizers on growth, nutrient uptake, and ectomycorrhizal infection. New Phytol 117:45–52
Nilsen P (1995) Effect of nitrogen on drought strain and nutrient uptake in Norway spruce Picea abies (L.) Karst.) trees. Plant Soil 172:73–85
Ostonen I, Puttsepp U, Biel C, Alberton O, Bakker MR, Lohmus K, Majdi H, Metcalfe D, Olsthoorn AFM, Pronk A, Vanguelova E, Weih M, Brunner I (2007) Specific root length as an indicator of environmental change. Plant Biosyst 141:426–442
Pearce RB (1996) Antimicrobial defenses in the wood of living trees. New Phytol 132:203–233
Piri T (1998) Effects of vitality fertilization on the growth of Heterobasidion annosum in Norway spruce roots. Eur J For Pathol 28:391–397
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27:1191
Ranney TG, Whitlow TH, Bassuk NL (1990) Response of 5 temperate deciduous tree species to water stress. Tree Physiol 6:439–448
Ranney TG, Bir RE, Skroch WA (1991) Comparative drought resistance among 6 species of Birch (Betula): Influence of mild water-stress on water relations and leaf gas exchange. Tree Physiol 8:351–360
Raupp MJ, Shrewsbury PM, Herms DA (2010) Ecology of herbivorous arthropods in urban landscapes. Annu Rev Entomol 55:19–38
Rozema J, vande Staaij J, Bjorn LO, Caldwell M (1997) UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol Evol 12:22–28
Rytter L, Ericsson T, Rytter R (2003) Effects of demand-driven fertilization on nutrient use, root:plant ratio, and field performance of Betula pendula and Picea abies. Scand J For Res 18:401–412
Shipley B, Meziane D (2002) The balanced-growth hypothesis and the allometry of leaf and root biomass. Funct Ecol 16:326–331
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic Press, San Diego
Sofo A, Dichio B, Xiloyannis C, Masia A (2005) Antioxidant defences in olive trees during drought stress: changes in activity of some antioxidant enzymes. Funct Plant Biol 32:45–53
Tarkka M, Nehls U, Hampp R (2005) Physiology of ectomycorrhizae. Prog Bot 66:247–276
Thomas GW (1996) Soil pH and soil acidity. Methods of soil analysis, Part 3: Chemical methods. Soil Science Society of America, Madison, pp 475–490
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorous, and athmospheric CO2 in field studies. New Phytol 164:347–355
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515
Vannette RL, Hunter MD (2011) Plant defense theory re-examined: nonlinear expectations based on the costs and benefits of resource mutualisms. J Ecol 99:66–76
Vizoso S, Gerant D, Guehl JM, Joffre R, Chalot M, Gross P, Maillard P (2008) Do elevation of CO2 concentration and nitrogen fertilization alter storage and remobilization of carbon and nitrogen in pedunculate oak saplings? Tree Physiol 28:1729–1739
Walker RF, McLaughlin SB, West DC (2004) Establishment of sweet birch on surface mine spoil as influenced by mycorrhizal inoculation and fertility. Restor Ecol 12:8–19
Walters MB, Reich PB (1989) Response of Ulmus americana seedlings to varying nitrogen and water status. 1 Photosynthesis and growth. Tree Physiol 5:159–172
Wargo PM (1996) Consequences of environmental stress on oak: predisposition to pathogens. Ann Sci For 53:359–368
Warncke D, Brown JR (1998) Potassium and other basic cations. Recommended chemical soil test procedures for the North Central region, NCR Publication No. 221. Missouri Agricultural Experiment Station, Columbia, pp 31–33
Weidner S, Karolak M, Karamac M, Kosinska A, Amarowicz R (2009) Phenolic compounds and properties of antioxidants in grapevine roots (Vinis vinifera L.) under drought stress followed by recovery. Acta Soc Bot Pol 78:97–103
Weiss M, Schmidt J, Neumann D, Wray V, Chriss R, Strack D (1999) Phenylpropanoids in mycorrhizas of the Pinaceae. Planta 208:491–502
Acknowledgments
We thank Victoria Caceres and two anonymous reviewers for insightful comments on earlier drafts of this work, and David Snodgrass, Duan Wang, Justin Whitehill and Jim Vent for their assistance with many technical aspects of this work. This research was supported by USDA Forest Service National Urban and Community Forestry Advisory Grant No. 03-DG-11244225-428 to D.A.H. and P.B., a Tree Research and Education Endowment Fund John Z. Duling Grant to P.B. and D.A.H., an Ohio Agricultural Research and Development Center SEEDS Graduate Student Grant to N.M.K., and by State and Federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Koike.
Rights and permissions
About this article
Cite this article
Kleczewski, N.M., Herms, D.A. & Bonello, P. Nutrient and water availability alter belowground patterns of biomass allocation, carbon partitioning, and ectomycorrhizal abundance in Betula nigra . Trees 26, 525–533 (2012). https://doi.org/10.1007/s00468-011-0613-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-011-0613-3