Abstract
Background and aims
In the future, boreal forests will be growing in a warmer climate with more fluctuating soil moisture conditions. However, the knowledge about the effects of simultaneous warming and drought on boreal trees, and especially on their belowground compartments, is still scarce.
Methods
We studied the responses of four silver birch genotypes to experimental warming at normal and low watering levels after two growing seasons. Variables analysed include mycorrhizal short root growth, fungal biomass (ergosterol) in roots and extramatrical mycelium, and root morphology (root tissue density, root length density, specific root length, specific root surface area). In addition, root and shoot mass fractions were determined.
Results
Gt26 had more root mass and also more 2–5 mm diameter roots, but less short roots and ergosterol in its roots than the other genotypes. There were no treatment effects on extramatrical mycelium and mycorrhizal infection levels, but root ergosterol concentrations increased in gt12 due to warming. Drought increased root mass at the expense of shoot growth in gt12, but warming did not cause allometric growth changes in any of the genotypes.
Conclusions
Our results show some genotype-dependent responses to warming and drought but also significant within population differences in belowground allocation patterns among the silver birch genotypes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the latest IPCC report (IPCC 2013) global warming is continuing, future warming depending strongly on the greenhouse gas and black carbon emissions released during this century. At the moment, carbon dioxide emissions are on track for 3.2–5.4 °C “likely” increase in temperature above the pre-industrial levels, and large and sustained mitigation of emissions is required to keep temperature increase below 2 °C relative to 1850–1900 values (Fuss et al. 2014). A warmer climate will also result in a change in the precipitation patterns, meaning that both prolonged heat waves and heavy precipitation events are expected to occur more frequently across Europe during this century (IPCC 2013). In Finland, wintertime warming and precipitation changes are expected to be larger than those occurring during summers, but in general precipitation changes in Northern Europe will be highly variable thus increasing both the risk of flooding and drought (Jylhä et al. 2009; IPCC 2013).
Changes in environmental conditions can alter tree carbon (C) allocation patterns (Litton et al. 2007; Litton and Giardina 2008; Poorter et al. 2012). Based on C allocation theories plants will allocate relatively more mass to roots if the limiting factor of growth is below-ground, and more to shoots if limiting factor is above-ground (Brouwer 1983; Litton et al. 2007; Poorter et al. 2012). However, drought responses of plants seem to be complex, as Poorter et al. (2012) reported in their review that root mass fraction (RMF, root mass proportion of total plant mass) did not necessarily increase at the expense of stem biomass before the plants were exposed to severe drought (i.e. biomass was reduced >50 % when compared to control plants). Furthermore, Aspelmeier and Leuschner (2006) observed that drought-treated silver birches (Betula pendula Roth) had reduced shoot and root growth, but this reduction was ultimately larger in shoots than in roots and therefore resulted in allometric growth changes (i.e., increased root:shoot-ratios). Root traits associated of plant water and nutrient uptake, e.g. specific root length (SRL, root length per root dry mass) and root length density (RLD, root length per soil volume), have been observed to increase due to drought, especially at depths in soil with available water (Comas et al. 2013). However, recent meta-analysis focusing on tree root responses to drought emphasized that the drought responses have been so far mainly studied with temperate and tropical species (Brunner et al. 2015) which means that response patterns observed from these species do not necessarily apply to the boreal trees. Thus, the severity of drought and tree species itself will affect how roots will ultimately respond to the altered soil moisture conditions (Poorter et al. 2012; Brunner et al. 2015).
One of the factors limiting tree growth in boreal regions is the prevailing temperature conditions (e.g., Lahti et al. 2005; Bricedo-Elizondo et al. 2006), and therefore it has been widely assumed that boreal forest trees might at least initially benefit from climate warming. Increased productivity in boreal regions may be directly caused by warmer temperatures and longer growing seasons, but also indirectly via increased nutrient availability due to enhanced nutrient cycling (Rustad et al. 2001; Litton and Giardina 2008; Melillo et al. 2011). So far, experimental warming studies made with silver birch in field exposure system (Kasurinen et al. 2012) seem to support the assumption of increased productivity in boreal trees, as warming (c. +1 °C air temperature increase) resulted in larger trees without any clear increase or decrease in root:shoot-ratios. On the other hand, other tree species may have different responses. In a soil temperature gradient study, Vogel et al. (2008) observed that black spruce had decreased total below-ground C flux although aboveground net primary productivity increased in forests with warmer soil temperatures. In addition, Arend et al. (2011) measured growth of three oak species (Quercus robur, Quercus petraea and Quercus pubescens) after three year of warming exposure, and noticed that warming (+2–3 °C temperature increase during growing seasons) had increased shoot height growth but reduced root length growth resulting in altered allometric growth relations. Information about experimental warming effects on SRL or SRA (specific root surface area, root surface area per root mass) is limited, but Björk et al. (2007) reported that in dry tundra heath plants had increased SRL and SRA after 10 years of warming but root morphology was not altered in dry meadow plants exposed to similar warming treatment. It is likely that temperature response will depend on other co-occurring environmental factors, how close plants are to their thermal optimum for growth, intensity and duration of temperature exposure, as well as plant species or genotypes, making generalizations difficult (Dobbertin 2005; Way and Oren 2010).
Both drought and warming effects on mycorrhizas can be either direct or indirect. Indirect effects include drought- or temperature-induced changes in host C allocation to symbionts and/or changes in net nitrogen mineralization rates in soil (Rustad et al. 2001; Litton et al. 2007; Melillo et al. 2011). Mycorrhizal abundance in plant roots have shown variable responses to drought, responses ranging from increase to decrease (Mohan et al. 2014). The information about extramatrical mycelium (EMM) growth responses to drought is scarce, but Majdi et al. (2008) have reported that in a dry year EMM production was decreased in a well-drained Scots pine (Pinus sylvestris) forest when compared to EMM production in a wet year. Also Pritchard et al. (2008) have reported mycorrhizal growth to be influenced by drought as rhizomorph length growth was reduced in a dry year in a temperate forest system. Ostonen et al. (2011) have showed that there was a higher mycorrhizal root tip abundance but a smaller proportion of long-distance exploration type forming mycorrhizas (i.e. less EMM) along natural climate gradient from subarctic to temperate ecosystems in Norway spruce (Picea abies (L.) Karst) stands. In contrast to above climate gradient study, experimental warming in Arctic tussock tundra showed that warming significantly increase ergosterol concentrations (i.e., fungal biomass) in soil (Clemmensen et al. 2006), and in Arctic heath tundra, a long-term experimental warming favoured the growth of ectomycorrhizal species with relatively high biomass (Deslippe et al. 2011). Thus, to date responses of mycorrhizal roots and EMM to drought and warming have been variable.
Silver birch has a large genotypic variation especially within but also among populations, and as a pioneer species it is considered to be effective in its growth habitat exploitation (Eriksson et al. 2003; Rusanen et al. 2003; Hynynen et al. 2010; Ostonen et al. 2013; Possen et al. 2014). In the previous experiments with silver birch, a genotype-dependent root growth response to experimental warming was observed without a clear change in root:shoot-ratios (Kasurinen et al. 2012), whereas Possen et al. (2011) reported that drought reduced root growth in all studied silver birch genotypes without clear change in their root:shoot-ratios. In this work, we studied simultaneous warming and drought effects on four silver birch genotypes and their belowground compartments in a greenhouse experiment. Besides root mass fraction (RMF), shoot mass fraction (SMF, proportion of stem, branch and leaf mass of total plant mass) was determined in order to see if there are any allometric growth changes. Other measured variables included the specific root length (SRL), specific root surface area (SRA) and root length density (RLD). In addition, mycorrhizal infection levels were studied by microscopy and fungal biomass in roots as well as extramatrical mycelium biomass were measured with ergosterol analysis. Based on above literature, we expected that silver birches may change their root morphology (e.g. increase SRL) and either have increase, decrease or no change in their root growth under drought conditions, whereas warming might enhance the root growth but root morphology changes might be more variable. In addition, we studied if warming and watering treatments modify each other’s effects on below-ground responses, and if there are any genotype-dependent responses to warming and watering treatments.
Materials and methods
Birch material and experimental setup
For this greenhouse experiment, 10 silver birch genotypes (2, 4, 8, 12, 14, 18, 19, 23, 25, 26) were grown in two adjacent, identical, 45 × 8 m greenhouses at the Suonenjoki Research Nursery (the Natural Resources Institute Finland, Suonenjoki Unit, Finland, 62°38’N, 27°03’E). The genotypes used were micropropagated from single-stemmed, well-spaced trees selected randomly from a mixed, naturally regenerated silver and downy birch (B. pubescens Ehrh.) stand in Punkaharju, Finland (61°48’N, 29°21’E).
During the growing season in 2010, no treatments were applied and the plantlets only received the standard nursery protocol until the end of October 2010, when they were stored in sealed plastic bags at −5 °C for winter (see Possen et al. 2015). In early May 2011 the plantlets were transferred to the two greenhouses, and watering and warming treatments began in early June 2011 ca. 14 months after the rooting phase of the plantlets. After the 2011 growing season and first harvest, the remaining plantlets were placed in sealed plastic bags and overwintered again in −5 °C in preparation for the 2012 studies. At the beginning of the experiment (2011), the average height of the all plantlets was 0.44 m (Possen et al. 2015). To prevent nutrient limitations, all plantlets were fertilized once a year with standard forest fertilizers (Kekkilä Forest Superex NPK: 22–5-16 in 2011 and Osmocote Exact Standard 5-6 M; NPK: 15–4-8 in 2012), and to prevent stress due to infection with aphids, all plantlets were treated with a liquid suspension of 0.3 % Pirimor (Syngenta Nordic, Denmark) twice during summer 2011 (June 6th and July 25th) and 2012 (May 31st and July 9th). In mid-August 2011 an application of 0.2 % Candit (BASF Chemical Co., Germany) was applied against mildew. During the first exposure season 2011, plantlets were grown in 7.5 L pots exterior painted white and filled with peat (Novarbo Metsätaimiturve B1F, Eura, Finland), but just before the initiation of the exposure season 2012, remaining plantlets were planted to new 20 L pots (exterior painted white) filled with nursery peat (Puutarhaturve, Kekkilä Oy, Vantaa, Finland).
Temperature and watering treatments
The exposure experiment consisted of three replicates (blocks), each consisting of six treatments (combination of two temperature and three watering treatments) in separate plots. Each plot contained four plantlets for each of the 10 genotypes in 2011 and two plantlets per genotype in 2012, and was surrounded by a row of shelter plants. Three infrared heaters per plot (CIR110, Frico AB, Göteborg, Sweden, 1000 W, wavelengths >800 nm) were placed in six plots resulting in c. + 1 °C degree air temperature elevation, whereas the other six plots were kept at ambient temperature. The ambient temperature plots were fitted with wooden dummy heaters of the same size to mimic possible shading effects. Warming was started after the transfer of the plantlets to the greenhouses (June 6th and April 18th in 2011 and 2012, respectively) and ended when the plantlets were either leafless for overwintering (24 October 2011) or harvested (18 September 2012). The height of the heaters and dummies was adjusted throughout the experiment to ensure a distance of 1.0 m between the heaters and the top of the plantlets. Temperatures in warming and ambient temperature plots established in both greenhouses were monitored every 15 min at a height of 1.2 m from the heaters (i.e. 20 cm under the top of the plantlets throughout the whole experiment for each plot) using thermocouples and temperature sensors (Hobo H08–032-08, Onset, Bourne, MA, USA). Air temperature increase in the warming plots was on average + 0.88 °C in 2011 and +0.68 °C in 2012, and thus close to +1 °C target in both years. In soil, the temperature increase was on average + 0.7 °C in 2011 and +0.3 °C in 2012. However, according to the leaf temperature measurements (Possen et al. 2015), the difference in leaf surface temperatures between the ambient temperature and warming plots was on average + 1.9 °C, this result being comparable to an earlier field study (Riikonen et al. 2009) where similar warming system was employed.
In order to study the soil water availability effects on birches, three watering treatments were established: low watering (volumetric water content, VWC, 20–30 %), normal watering (VWC 40–50 %) and excess watering (VWC >60 %). VWC in the low treatment was close to the wilting point of the peat used, while VWC in the excess treatment represents the water-holding-capacity of the peat. After the plants were transferred to the greenhouses from the winter storage, VWC was kept close to normal for all plantlets until the start of the watering treatments, when VWC was gradually increased or decreased to the target VWC within a period of one week. After the watering treatments were ended, VWC was allowed to return to normal for all plantlets within a period of one week. In 2011, the watering treatments lasted for five weeks (from July 11th till August 12th) whereas in 2012 the watering treatment lasted for seven weeks (from June 17th till August 8th). VWC was different between moisture treatments only during the application of watering treatments (Possen et al. 2015).
Meshbag and root analyses
For the purposes of this root study, the in-growth meshbags for the quantification of extramatrical mycelium (Wallander et al. 2001) were installed to genotypes 12, 14, 18 and 26 (referred as gt12, gt14, gt18 and gt26 from hereon) in April 2012 at the same time as plantlets were planted to larger pots. At this stage the average height of these four genotypes ranged already from ca 1.5 to 1.6 m, and at the end of the experiment from ca. 2.5 to 2.8 m. Meshbags were thus planted only to the above randomly selected genotypes kept at low and normal VWC in ambient temperature and warming plots. Meshbags were prepared from nylon mesh with mesh size of 50 μm (LK-Suodatin Ltd., Ylöjärvi, Finland). The bags were 50 × 100 mm and each contained 25 g of acid washed quartz sand. Meshbags were installed into the pots horizontally (one mesh-bag per pot) at a depth of 5 cm resulting in three replicates per treatment for each genotype. Hereafter following abbreviations for the treatments are used: ambient temperature + normal VWC = ANW, ambient temperature + low VWC = ALW, elevated temperature + normal VWC = ENW and elevated temperature + low VWC = ELW.
Root samples and meshbags were harvested in September 2012 during the final harvest of the entire experiment. Meshbags were stored in plastic bags in a freezer (−20 °C) until the ergosterol analysis. Root samples were harvested by removing the whole root system from the pot and cutting it into two parts, from top to bottom. Then one half of the root system was cut again into parts depth wise and from this quarter, a subsample was taken (subsample volume of c. 500 cm3 and collected from 0 to 20 cm depth) for the root scanning analysis. Root samples were then individually washed over a sieve (mesh size 1 mm), and all adhering debris was removed with tweezers while soaking the sample in tap water on a glass tray. Washed root sample was stored back in a freezer (−20 °C) until root scanning.
Roots were scanned using a WinRhizo Pro 2009c software and an Epson Perfection V700/V750 scanner, adapted with a Regent positioning system (Regent Instruments, Quebec, Canada) at the Department of Environmental Sciences, University of Helsinki in spring 2013. Prior to scanning, the roots were placed on a 20 × 25 cm clear plastic tray with 400 ml of water. The tray was then placed on the scanner bed and scanned to determine the root length and surface area for each root fraction (fractions 0–1, 1–2, 2–3, 3–4, 4–5,5–6, 6–7,7–8, 8–9,9–10 mm, i.e. ten root size classes were separated according to their diameter) and the whole root sample. After scanning, excess water was removed from the root samples and the samples were weighed for fresh weight and stored in a freezer (−20 °C) until microscopy and root ergosterol analysis at the Department of Environmental and Biological Sciences, University of Eastern Finland, Kuopio campus.
For the mesh bag ergosterol (extramatrical mycelium growth) and root ergosterol analysis, samples were freeze-dried. Before freeze-drying, the root samples were weighed for their fresh weight. Root ergosterol extraction and high-pressure liquid chromatography analysis followed that of Nylund and Wallander (1992) with modifications described in Kasurinen et al. (2001). For the mesh bag ergosterol analysis, the protocol was similar to the root ergosterol method described in Kasurinen et al. (2001), except a larger sample size of 15 g of quartz sand was used. In the microscopy analysis, the number of short root tips was counted and their mycorrhizal status was determined from a 0.5 m-long fine root (diameter less than 1 mm) sample as previously done e.g. in Kasurinen et al. (2012). On the basis of microscopy data, total root ramification index (short roots per m of fine root), mycorrhizal root tips per m of fine root, and total mycorrhizal infection level (% mycorrhizal of all short roots) were calculated. After scanning and microscopy analyses, root samples were dried at +60 °C to a constant weight before the measurement of root dry weight. The dry weight of roots from samples taken for ergosterol analysis was determined on the basis of fwt/dwt ratio measured from the scanned + microscopy roots, and added to their weight in order to obtain the dry weight data for the whole scanned root sample.
Statistical analysis
Before the statistical analyses, several testable variables were calculated on the basis of scanning data. As over 90 % of all scanned roots belonged to 0–1 mm fraction, the number of root fractions was reduced by combining coarser root classes together before the statistical analyses. Thus, the new root size classes used in the statistical test were 0–1, 1–2, 2–5 and >5–10 mm fractions. Root length density (RLD; root length divided by sample soil volume, cm cm−3 soil) were calculated for all fractions combined and each four fractions separately. On the basis of total root length, total root surface area and total root dry mass, specific root length (SRL; root length per root mass, m g DW−1) and specific root surface area (SRA; root surface area per root mass, m2 kg DW−1) were obtained for the whole scanned root system. In addition, root tissue density (RTD, root mass per soil volume; mg DW cm−3 soil) for the whole scanned root sample was calculated. Other testable variables were soil and root ergosterol concentrations, total mycorrhizal infection level (proportion of mycorrhizal short roots of all short roots) and total ramification index (tips m−1 of fine root). Possen et al. (2015) present leaf morphology, physiology and shoot:root-ratio responses for all 10 genotypes, but here we calculated root (RMF, root mass divided by the total tree mass, g g−1) and shoot mass fractions (SMF, shoot mass divided by total tree mass, g g−1) for these four genotypes only in order to see how genotype and treatments affect the total belowground and aboveground mass.
Treatment differences were tested with the Linear Mixed Models ANOVA (LMM ANOVA) with design where the fixed factors were birch genotype, temperature and watering levels, and random factors were plot and block. Differences were considered statistically significant at P ≤ 0.05 and statistically marginally significant at P ≤ 0.1. Statistically significant or statistically marginally significant main effects (i.e. genotype, temperature or watering main effects) were compared with pairwise comparison adjusted with Bonferroni correction. If there were statistically significant or statistically marginally significant two- or three-way interactions, main effects within other factors were further tested with a Simple Main effects (SME) test adjusted with Bonferroni correction.
Results
Genotype effects on roots and mycorrhiza
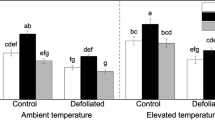
Genotype effects were observed in root ergosterol and total root ramification index (p < 0.05 genotype main effect for both variables, Table 1, Fig 1a-b), whereas extramatrical mycelium production and total mycorrhizal infection % levels were not affected by genotype (Table 1). Gt26 had the smallest root ergosterol concentration of the four genotypes, this difference being clearest when gt26 was compared to gt12 (Fig 1a, gt26 vs gt12, pairwise comparison, p = 0.014). Gt26 also had the smallest total ramification index (short root tips m−1, Table 1, Fig 1b), but now this difference became statistically significant when compared to gt14 (gt26 vs gt14 pairwise comparison, p = 0.004) and marginally statistically significant when compared to gt18 (gt26 vs gt18 comparison, p = 0.088). Root tissue density (RTD) was highest in gt26 and lowest in gt12 and gt18 (Tables 2 and 3), gt26 differing most from gt12 and gt18 (gt26 vs gt12 and gt26 vs gt18, P < 0.1 for both pairwise comparisons). In general, gt26 had the highest RLD in each root fraction (Tables 2 and 3), but especially in root fraction 3 (2–5 mm roots) this genotype difference became marginally statistically significant when gt26 was compared to gt18 (gt26 vs gt18, p = 0.075). However, the total RLD did not differ between the four genotypes (Tables 2 and 3).
a Root ergosterol concentrations and b total root ramification index (values are means ± standard errors of means) in temperature and watering treatments for each genotype separately. Abbreviations: ANW = ambient temperature + normal watering, ALW = ambient temperature + low watering, ENW = elevated temperature + normal watering and ELW = elevated temperature + low watering (n = 2–3 per treatment for each genotype)
Treatment effects on fungal biomass and root morphology
Root ergosterol concentration data also showed a statistically significant genotype x temperature interaction (Table 1, Fig 1a). In short, gt12 had 52 % higher fungal biomass in roots when ENW and ELW treatments were compared to ANW and ALW treatments (ANW + ALW vs ENW + ELW, p = 0.034 for SME test in gt12), whereas in other studied genotypes warming in fact decreased root ergosterol concentrations by 19–24 % (Fig. 1a, ANW + ALW vs ENW + ELW, but p > 0.1 for this SME test in gt14, gt18 and gt26). Extramatrical mycelium production and total mycorrhizal infection levels did not show statistically significant treatment differences (Table 1). Of root morphology variables, SRL showed some temperature and watering treatment interactions (Fig. 2a, T x W interaction P < 0.05). At ambient temperature low soil moisture reduced SRL clearly (SRL reduced by 21 %, ALW vs ANW, SME test p = 0.015), whereas in warming exposure soil moisture effect was not seen. In addition, SRL was 26 % higher in ELW than in ALW (ALW vs ELW, SME test p = 0.022), warming thus removing the drought effect on SRL. SRA also showed a marginally statistically significant T x W interaction (Fig 2b) P < 0.1 for temperature x watering interaction). At ambient temperature low soil moisture reduced SRA by 18 % (ALW vs ANW, SME test p = 0.036), but in warming this drought-caused reduction was not seen. Warming in fact increased SRA in low soil moisture by 15 %, but now this difference between ALW and ELW did not become statistically significant (ALW vs ELW, SME test p > 0.1) (Fig. 2b).
a SRL (specific root length) and b SRA (specific root surface area) means ± standard errors of means per treatment (genotypes aggregated together). Abbreviations: ANW = ambient temperature + normal watering, ALW = ambient temperature + low watering, ENW = elevated temperature + normal watering and ELW = elevated temperature + low watering (n = 11–12 per treatment)
Root and shoot mass fractions
Root mass fraction (RMF) showed a statistically significant genotype x watering interaction (P = 0.004 for RMF, Fig 3). In short, gt12 had increased RMF under drought conditions regardless of temperature treatment (ALW and ELW both had 36 % higher RMF when compared to ANW and ENW, P = 0.004 for SME test in gt12), whereas in other genotypes drought did not affect RMF significantly (ALW and ELW vs ANW and ENW, P > 0.1 for SME tests in gt14, gt18 and gt26). RMF was smallest in gt12 (gt12 average 0.35 g g−1) and largest in gt26 (gt26 average 0.45 g g−1) indicating that gt26 invested more on root mass production than other studied genotypes. The shoot mass fractions (SMF) ranged from 0.43 g g−1 to 0.77 g g−1 among the four genotypes when treatments were aggregated together, and in gt12 drought thus reduced SMF (SMF was 0.60 g g−1 in ALW + ELW vs 0.71 g g−1 in ANW + ENW).
Root mass fractions (values are means ± standard errors of means) in temperature and watering treatments for each genotype separately. Abbreviations: ANW = ambient temperature + normal watering, ALW = ambient temperature + low watering, ENW = elevated temperature + normal watering and ELW = elevated temperature + low watering (n = 2–3 per treatment for each genotype)
Discussion
In this study, warming and drought effects on four micropropagated silver birch genotypes were investigated in a greenhouse experiment after two growing seasons (2011–2012). We observed an increase in root mass fraction and a decrease in shoot mass fraction due to drought in one genotype, but these trends were not seen in the three other genotypes. Furthermore, root SRL and SRA were decreased due to drought, though warming totally or partly cancelled these drought effects. Root fungal biomass increased in one genotype in warming exposure, but otherwise fungal compartments (mycorrhizal infection levels and EMM growth) were not affected by the treatments.
Of all four genotypes studied here, gt26 had the lowest root ergosterol concentrations and the smallest number of short root per fine root length. According to the genotype comparison made by Possen et al. (2015) for year 2012 data, this genotype was also separated from gt12, gt14 and gt18, as it had lower leaf mass fraction (LMF) but higher net assimilation rates (NAR) than the above genotypes. Lower leaf mass fractions and higher NARs were related to good growth in terms of total dry mass of plants (Possen et al. 2015). Our result indicates that gt26 with good overall growth invested less resources to the growth of short roots and fungal compartments in the fine roots in general. This result is consistent with a study by Kasurinen et al. (2005) who observed that birch genotype with lower growth and physiological activity produced slightly more extramatrical mycelium and also had higher Peziza badia-sporocarp production than genotype with higher growth and physiological activity. However, in this earlier study the two silver birch genotypes originated from different latitudes, the more southern one having larger sporocarp production (Kasurinen et al. 2005), whereas in the current study all the genotypes used were micropropagated from one local stand in Punkaharju, Finland (61°48’N, 29°21’E) and were thus considered to have the same latitudinal origin.
Average mycorrhizal infection levels ranged from 63 % to 85 %, the lower end of the range being clearly lower than those reported for birch stands (Ostonen et al. 2013) and earlier field exposure studies (e.g., Kasurinen et al. 2005, 2012). The reason for lower infection levels is likely that these birches were grown in greenhouse conditions throughout the experiment and they were also overwintered in a cold storage, which reduced the magnitude of natural background infection though air exchange between greenhouses and outdoor air occurred during the exposure seasons. In addition, experimental plantlets were exchanged to new pots and growth media before the season 2012, and this probably affected the infection levels. According to Persson (2002) fine roots (diameter < 1–2 mm) can account for more than 95 % of the total root length in arboreal plants. Our finding is consistent with this observation as in total ca. 95–96 % of the total root length consisted of fine root fractions (i.e., of 0–1 and 1–2 mm fractions). Here the total root ramification indexes for most of the studied genotypes were c. 500–1000 tips m−1 for gt12, gt14 and gt18, except for gt26 in which root ramification index was only 300–600 tips m−1. Our result is also in accordance with the finding of Ostonen et al. (2013) who reported that young silver birches usually have a high number of short roots in their fine root systems, as a pioneer tree species it needs to exploit soil fast after establishing.
Treatment effects were seen in root fungal biomass, SRL and SRA
Warming effects on root ergosterol concentrations were genotype-dependent, the responses ranging from significant increase (gt12) to negligible decrease (gt14, gt18 and gt26) among the studied genotypes. In a previous warming experiment, Kasurinen et al. (2012) showed that root fungal biomass increased in silver birch roots due to warming regardless of silver birch genotype. Above studies thus indicate that fungal biomass in silver birch tree roots can increase due to warming, but responses are complex being partly dependent on tree genotype and also on the fungal symbionts occurring in the roots. There were no drought effects on total mycorrhizal infection levels, root fungal biomass or EMM growth in general. Thus, it seems that the mycorrhizal types present in the roots and soil were not very sensitive to drought stress or then the drought stress was not severe enough to produce response in mycorrhizal growth, or both. However, though the drought treatments in the current study lasted for only seven weeks in 2012, they nonetheless resulted in increased water use efficiency via closure of stomata (Possen et al. 2015). This shows that trees were employing a strategy where they minimized the water loss via stomatal conductance rather than investing to fungal compartments in order to improve their water acquisition from soil. In addition, Kleczewski et al. (2010) also reported that ectomycorrhizal abundance in young, pot-grown paper birch (Betula papyrifera) roots was not affected by drought treatment though whole seedling and root growth were clearly reduced.
Recently Ostonen et al. (2013) reported that high SRL of ectomycorrhizal roots (i.e., thinner and longer roots) is an acclimation strategy of silver birch in stress, unfavourable growing conditions and at young age (birches under 10-years old). In general, the SRL values reported here were similar to those reported by Ostonen et al. (2007) for birch fine roots (diameter < 2 mm) but much lower than those for finer ectomycorrhizal roots indicating that SRL was dominated by root fractions 0–1 and 1–2 mm. In contrast to our expectation, SRL did not increase due to drought treatment in this study but was in fact decreased in it, and warming was able to cancel the negative effects of drought on SRL. Meta-analyses of tree root responses to drought (Ostonen et al. 2007; Brunner et al. 2015), however, indicated that SRL responses in different tree species have been highly variable, from no change to increase or decrease. Furthermore, Olmo et al. (2014) showed that the responses of SRL to drought can vary even between root fractions and soil layers, as in their study the SRL of roots of finer root fractions (i.e. those under 0.5 mm diameter) in deeper soil layers increased, but there was no so clear change in the SRL of coarser roots (0.5–2 mm and >2 mm). Here we however measured only total SRL for the whole root sample which might have masked some of the treatment effects on root length growth. In addition, changes in SRA have been suggested to be related to the changes in the environment (Lõhmus et al. 1989) and to be connected with the physiological activity of fine roots (Ostonen et al. 1999). Again, in contrast to our hypothesis, SRA decreased due to drought, but only in ambient temperature. Both reduced SRL and SRA suggest that less root length and surface area were produced per unit of root mass and roots were less effective in soil exploration, but as Ostonen et al. (2007) points out, in pot studies reductions in SRL to various soil manipulations have been commonly observed. Like our study, pot studies are usually shorter term experiments using artificial growth media and young seedlings or plantlets which limits their applicability to field or stand conditions. Hence, it is clear that warming and drought effects on older trees and their root morphology need to be studied in more natural growth conditions.
Allometric growth change due to drought was seen in one genotype
In silver birch, genotype-dependent responses to changing growth conditions are common (e.g. Kasurinen et al. 2012). RMF and SMF results are in agreement with our expectation that silver birch can have some genotype-dependent responses to drought. Drought-induced increase in root growth in gt12 also follows the carbon allocation theories (Brouwer 1983, Litton and Giardina 2008; Poorter et al. 2012) which predict root growth to increase if some belowground factor becomes limiting. Since shoot mass in gt12 was reduced due to drought, it indicates that increased root growth occurred at the expense of shoot growth. There are also other studies where drought have resulted in allometric growth changes. For instance, Arend et al. (2011) reported significant shoot growth reductions, but no change in root length growth in three oak species (Quercus sp), the shoot growth reductions nonetheless resulting in increased root length:shoot height-ratios.
Conclusion
Our results indicate that belowground compartments in boreal birch stands are not necessarily quickly changed due to moderate drought periods and warming, as significant genotypic variation in belowground compartments exist even within local silver birch populations. However, warming and drought interactions are complex and need to be studied in longer-term studies with soil-growing trees.
References
Arend M, Kuster T, Günthardt-Goerg MS, Dobbertin M (2011) Provenance-specific growth responses to drought and air warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol 31:287–297
Aspelmeier S, Leuschner C (2006) Genotypic variation in drought response of silver birch (Betula pendula Roth): leaf and root morphology and carbon partitioning. Trees – Struct Funct 20:42–52
Björk RG, Majdi H, Klemedtsson L, Lewis-Jonsson L, Molau U (2007) Long-term warming effects on root morphology, root mass distribution, and microbial activity in two dry tundra plant communities in Northern Sweden. New Phytol 176:862–873
Bricedo-Elizondo E, Garcia-Gonzalo J, Peltola H, Matala H, Kellomäki S (2006) Sensitivity of growth of scots pine, Norway spruce and silver birch to climate change and forest management in boreal conditions. For Ecol Manag 232:152–167
Brouwer R (1983) Functional equilibrium: sense or nonsense? Neth J Agric Sci 31:335–348
Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C (2015) How tree roots respond to drought. Front Plant Sci 6(547):1–16
Clemmensen KE, Michelsen A, Jonasson S, Shaver GR (2006) Increased ectomycorrhizal fungal abundance after long-term fertilization and warming of two arctic tundra ecosystems. New Phytol 171:391–404
Comas LH, Becker SR, Von Mark VC, Byrne PF, Dierig DA (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4(442):1–16
Deslippe JR, Hartmann M, Mohn WW, Simard SW (2011) Long-term experimental manipulation of climate alters the ectomycorrhizal community of Betula nana in Arctic tundra. Glob Chang Biol 17:1625–1636
Dobbertin M (2005) Tree growth as indicator of tree vitality and of tree reaction to environmental stress: a review. Eur J Forest Sci 124:319–333
Eriksson G, Black-Samuelsson S, Jensen M, Myking T, Rusanen M, Skrøppa T, et al. (2003) Genetic variability in two tree species, Acer platanoides L. And Betula pendula Roth, with contrasting life-history traits. Scand J For Res 18:320–331
Fuss S, Canadell JG, Peters GP, Tavoni M, Andrew RW, Ciais P, Jackson RB, Jones CD, Kraxner F, Nakicenovic N, Le Quéré C, Raupach MR, Sharifi A, Smith P, Yamagata Y (2014) Betting on negative emissions. Nat Clim Chang 4:850–853
Hynynen J, Niemistö P, Viherä-Aarnio A, Brunner A, Hein S, Velling P (2010) Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in Northern Europe. Forestry 83:103–119
IPCC (2013) Summary for Policymakers. In: Stocker ,TF, Qin D, Plattner ,G-K, Tignor M, SK A, Boschung J, Nauels A, Xia Y, Bex V, PM M (eds) In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK, p. 2013
Jylhä K, Ruosteenoja K, Räisanen J, Venäläinen A, Tuomenvirta H, Ruokolainen L, Saku S, Seitola T (2009) The changing climate in Finland, estimates for adaptation studies. ACCLIM project report 2009. Finnish Metereological Institute Reports.
Kasurinen A, Holopainen T, Anttonen S (2001) Mycorrhizal colonization of highbush blueberry and its native relatives in Central Finland. Agr Food Sci Finland 19:113–119
Kasurinen A, Keinänen MM, Kaipainen S, Nilsson L-O, Vapaavuori E, Kontro MH, Holopainen T (2005) Below-ground responses of silver birch trees exposed to elevated CO2 and O3 levels during three growing seasons. Glob Chang Biol 10:1654–1665
Kasurinen A, Biasi C, Holopainen T, Rousi M, Mäenpää M, Oksanen E (2012) Interactive effects of elevated ozone and temperature on carbon allocation of silver birch (Betula pendula) genotypes in an open-air field exposure. Tree Physiol 32:737–751
Kleczewski NM, Herms DA, Bonello P (2010) Effects of soil type, fertilization and drought on carbon allocation to root growth and partitioning between secondary metabolism and ectomycorrhizae of Betula papyrifera. Tree Physiol 30:807–817
Lahti M, Aphalo PJ, Finér L, Ryyppö A, Lehto T, Mannerkoski H (2005) Effects of soil temperature on shoot and root growth and nutrient uptake of 5-year-old Norway spruce seedlings. Tree Physiol 25:115–122
Litton CM, Giardina CP (2008) Below-ground carbon flux and partitioning: global patterns and response to temperature. Funct Ecol 22:941–954
Litton CM, Raich JW, Ryan MG (2007) Carbon allocation in forest ecosystems. Glob Chang Biol 13:2089–2109
Lõhmus K, Oja T, Lasn R (1989) Specific root area: a soil characteristic. Plant Soil 119:245–249
Majdi H, Truus L, Johansson U, Nylund J-E, Wallander H (2008) Effects of slash retention and wood ash addition on fine roots biomass and production and fungal mycelium in a Norway spruce stand in SW Sweden. For Ecol Manag 255:2109–2117
Melillo JM, Butler S, Johnson J, Mohan J, Steudler P, Lux H, Burrows E, Bowles F, Smith R, Scott L (2011) Soil warming, carbon-nitrogen interactions and forest carbon budgets. PNAS 108:9508–9512
Mohan E, Cowden CC, Baas P, Dawadi A, Frankson PT, Helmick K, Hughes E, Khan S, Lang A, Machmuller M, Taylor M, Witt A (2014) Mycorrhizal fungi mediation of terrestrial ecosystem response to global change: mini-review. Fungal Ecol 10:3–19
Nylund J-E, Wallander H (1992) Ergosterol analysis as a means of quantifying mycorrhizal biomass. Method Microbiol 24:77–78
Olmo M, Lopez-Iglesias B, Villar R (2014) Drought changes the structure and elemental composition of very fine roots in seedlings of ten woody tree species. Implications for drier climate. Plant Soil 384:113–129
Ostonen I, Lõhmus K, Lasn R (1999) The role of soil conditions in fine root ecomorphology in Norway spruce (Picea abies (L.) karst.). Plant Soil 208:283–292
Ostonen I, Püttsepp Ü, Biel C, Alberton O, Bakker MR, Lõhmus K, Majdi H, Metcalfe D, Olsthoorn AFM, Pronk A, Vanguelova E, Weih M, Brunner I (2007) Specific root length as an indicator of environmental change. Plant Biol 141(3):426–442
Ostonen I, Helmisaari H, Borken W, Tedersoo L, Kukumägi M, Bahram M, Lindroos A-J, Nöjd P, Uri V, Merilä P, Asi E, Lõhmus K (2011) Fine root foraging strategies in Norway spruce forests across a european climate gradient. Glob Chang Biol 17:3620–3632
Ostonen I, Rosenvald K, Helmisaari H-S, Godbold D, Parts K, Uri V, Lõhmus K (2013) Morphological plasticity of ectomycorrhizal short roots in Betula sp and Picea abies forests across climate and forest succession gradients: its role in changing environments. Front Plant Sci 4(335):1–10
Persson HA (2002) Root system of arboreal plants. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant Roots: The Hidden Half, 3rd edn. Marcel Dekker Inc., New York, USA, p 287–313
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50
Possen BJHM, Oksanen E, Rousi M, Ruhanen H, Ahonen V, Tervahauta A, Heinonen J, Heiskanen J, Kärenlampi S, Vapaavuori E (2011) Adaptability of birch (Betula pendula Roth) and aspen (Populus tremula L.) genotypes to different soil moisture conditions. For Ecol Manag 262:1387–1399
Possen BJHM, Rousi M, Silfver T, Anttonen MJ, Ruotsalainen S, Oksanen E, Vapaavuori E (2014) Within-stand variation in silver birch (Betula pendula Roth) phenology. Trees – Struct Funct 28:1801–1812
Possen BHJM, Heinonen J, Anttonen MJ, Rousi M, Kontunen-Soppela S, Oksanen E, Vapaavuori E (2015) Trait syndromes underlying stand-level differences in growth and acclimation in 10 silver birch (Betula pendula Roth) genotypes. For Ecol Manag 343:123–135
Pritchard SG, Strand AE, McCormack ML, Davis MA, Oren R (2008) Mycorrhizal and rhizomorph dynamics in a loblolly pine forest during 5 years of free-air-CO2-enrichment. Glob Chang Biol 14:1–13
Riikonen J, Mäenpää M, Alavillamo M, Silfver T, Oksanen E (2009) Interactive effect of elevated temperature and O3 on antioxidant capacity and gas exchange in Betula pendula saplings. Planta 230:419–427
Rusanen M, Vakkari P, Blom A (2003) Genetic structure of Acer platanoides and Betula pendula in Northern Europe. Can J For Res 33:1110–1115
Rustad L, Campbell J, Marion G, Norby R, Mitchell M, Hartley A, Cornelissen J, Gurevitch JA (2001) Meta-analysis of the response of soil respiration, net nitrogen mineralization and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Vogel JG, Bond-Lamberty B, Schuur EAG, Gower ST, Mack MC, O′Connell KEB, Valentine DW, Ruess RW (2008) Carbon allocation in boreal black spruce forests across regions varying in soil temperature and precipitation. Glob Chang Biol 14: 1503–1516
Wallander H, Nilsson L-O, Hagerberg D, Bååth E (2001) Estimation of the biomass and seasonal growth of external mycelium of ectomycorrhizal fungi in the field. New Phytol 151:753–760
Way D, Oren R (2010) Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30:669–688
Acknowledgments
We thank the staff of the Natural Resources Institute Finland, Suonenjoki Research Unit, for their help with the establishment and maintenance of the greenhouse experiment. We also thank laboratory technician Jaana Rissanen for her assistance with the root sampling and root ergosterol analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was financially supported by University of Eastern Finland (spearhead project “Changing climate and biological interactions related to forests”) and Academy of Finland (projects 133084 and 266532).
Additional information
Responsible Editor: Duncan D. Cameron.
Rights and permissions
About this article
Cite this article
Kasurinen, A., Koikkalainen, K., Anttonen, M.J. et al. Root morphology, mycorrhizal roots and extramatrical mycelium growth in silver birch (Betula pendula Roth) genotypes exposed to experimental warming and soil moisture manipulations. Plant Soil 407, 341–353 (2016). https://doi.org/10.1007/s11104-016-2891-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2891-4