Abstract
Aims
The role of different soil types for beech productivity and drought sensitivity is unknown. The aim of this experimental study was to compare mycorrhizal diversity between acid sandy and calcareous soils and to investigate how this diversity affects tree performance, nitrogen uptake and use efficiency (NUE).
Methods
Beech trees were germinated and grown in five different soil types (pH 3.8 to 6.7). One-and-a-half-year-old plants were exposed for 6 weeks to sufficient or low soil humidity. Tree biomass, root tip mycorrhizal colonization and community structure, root tip mortality, leaf area, photosynthesis, nitrogen concentrations, NUE and short-term 15N uptake from glutamine were determined.
Results
Soil type did not affect photosynthesis or biomass formation, with one exception in calcareous soil, where root mortality was higher than in the other soil types. Beech in acid soils showed lower mycorrhizal colonization, higher nitrogen tissue concentrations, and lower NUE than those in calcareous soils. Drought had no effect on nitrogen concentrations or NUE but caused reductions in mycorrhizal colonization. Mycorrhizal species richness correlated with nitrogen uptake and NUE. Nitrogen uptake was more sensitive to drought in calcareous soils than in acid soils.
Conclusions
Beech may be more drought-susceptible on calcareous sites because of stronger decrease of organic nitrogen uptake than on acid soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The productivity of forests is deteriorating when aridity is increasing in a changing climate (Ciais et al. 2005; Williams et al. 2013). Drought limits water uptake, suppresses photosynthesis and, thereby, leads to decreases in biomass production as well as diminished belowground carbon allocation. Consequently, energy and carbon-demanding physiological processes such as root formation and nutrient uptake can be hampered (Ruehr et al. 2009; Kreuzwieser and Gessler 2010). Reduced soil humidity further impedes soil mineralization processes and so may also decrease plant nitrogen (N) nutrition resulting in growth depression (Sardans and Penuelas 2012). A long-standing debate concerns the question whether beech (Fagus sylvatica L.), a major deciduous species in Central European forests, is particularly endangered by climate change because of restricted water supply or aggravation of N limitation (Gessler et al. 2007; Rennenberg et al. 2009). Beech trees have a number of morphological characteristics that could render the species drought-sensitive (Granier et al. 2007). However, beech is also known for its wide ecological range occurring from moist to moderately dry climatic conditions and on acid as well as on calcareous soils (Leuschner et al. 2006; Bolte et al. 2007; Ellenberg 2009). Field surveys showed that leaf biomass production and N concentrations were relatively unaffected by soil types and variation in climate (Meier and Leuschner 2008, 2014), whereas a comparison of dry-warm and cool-moist beech forests revealed significant reductions in N uptake and radial stem growth in the warmer climate (Gessler et al. 2001, 2005). These results led to divergent conclusions regarding the drought susceptibility of beech. However, potential impacts of different soil types and confounding effects of other environmental variables cannot be excluded under field conditions and therefore experimental studies are required to elucidate whether soil chemistry affects beech N nutrition and performance under drought.
Variations in soil chemistry and drought conditions are also known to affect the structures of ectomycorrhizal (EM) assemblages (Lilleskov et al. 2002; Shi et al. 2002; Kranabetter et al. 2009; Pena et al. 2010; Zhang et al. 2013; Moeller et al. 2014; Walker et al. 2014; Zavišić et al. 2016). EM fungi form symbioses with the root tips of temperate forest trees and play major roles in ecosystem nutrient cycling because of their ability to access N from different soil pools (Talbot and Treseder 2010; Hobbie and Högberg 2012; van der Heijden et al. 2015). Although relationships between soil N and EM community structures have frequently been studied (Talbot and Treseder 2010), little is known on their impact on organic N supply. It is now widely recognized that amino acids are often as abundant in soil solutions of temperate forests as inorganic N forms and can be used by plants as well as by EM fungi (Finlay et al. 1992; Keller 1996; Näsholm and Persson 2001; Persson et al. 2003; Grelet et al. 2009; Näsholm et al. 2009; Stoelken et al. 2010; Talbot and Treseder 2010). 15N labelling with mixtures of inorganic N and amino acids in a beech forest indicated that glutamine is the preferred source for N uptake by EM roots (Dannenmann et al. 2009). When other N sources are lacking, growth of EM plants benefits more strongly from amino acid-derived N than that of non-mycorrhizal plants, suggesting that EM symbionts widen the adaptability of plants to limiting resources (Turnbull et al. 1995; Plassard et al. 2000; Schmidt et al. 2006). One reason may be that composed EM assemblages as well as distinct EM taxa in their natural habitats vary in their abilities to access and utilize different N sources (Clemmensen et al. 2008; Pena et al. 2013a; Pena and Polle 2014; Valtanen et al. 2014; Kranabetter et al. 2015; Leberecht et al. 2015). Whether soils from different climatic areas and geological formations structure the root-associated EM assemblages with implications for beech performance, nitrogen use efficiency (NUE) and organic N uptake under drought is not known.
To close this knowledge gap, we conducted a pot study, in which European beech seedlings were raised in five different soil types that represented acid and calcareous conditions. Beech was chosen because of its relevance as a keystone species in temperate forests, its wide ecological range and its ability to form diverse EM assemblages. We hypothesized that the beech seedlings in different soil types (i) develop different EM communities under common climatic conditions, (ii) exhibit differences in 15N uptake from glutamine and (iii) that N uptake and NUE are more resilient to drought in soils from dry habitats than in soils from moist conditions. To test these hypotheses, biomass, growth parameters, photosynthesis, and root vitality and mycorrhizal colonization were analyzed in relation to NUE and uptake of 15N from glutamine.
Materials and methods
Beech cultivation and drought treatment
Beech (Fagus sylvatica L.) nuts (certificate no.: D-03,001 2 0031 09, FSB Oerrel, Oerrel, Germany) were germinated in humid cloths at 4 °C in darkness. After 6 weeks the seed coat of germinating nuts was removed. The seedlings were incubated in a sterilization solution [5 ml of Proplant (Stähler, Stade, Germany), 5 ml Tween 80 (Roth, Karlsruhe, Germany), 500 mg tetracycline] overnight and subsequently washed with tap water. Three germinating beech nuts were planted together in 2-L pots. The soils were collected immediately before use in March 2011 in five beech forests with closed canopy that differed in climatic and edaphic conditions (Table 1). The soils from Unterluess (UL) and Calvörde (CL, CS) are highly acidic nutrient poor sandy soils originating from Pleistocene fluvio-glacial sands from the penultimate Saalian ice age with up to 30 % sand fraction, classified as moderate to intense podzolic Umbrisols (Müller-Haubold et al. 2013). The mineral soils are covered by a thick organic layer (9-4 cm). The soils in the Tuttlingen forests are characterized as Rendzic Leptosols derived from limestone (Weißjura beta and gamma series) and are shallow with a thin organic layer (Gessler et al. 2005). Top soils of the first 0.2 m were used after sieving (20 × 20 mm mesh width).
The seedlings were grown until May 2011 in a greenhouse and then placed randomized outside in a nursery (Forest Botanical Garden, University of Göttingen, longitude 9°57’E, latitude 51°33’N, 171 m above sea level). The two weakest seedlings were removed, keeping only one seedling per pot. The plants were watered as necessary and kept outside from May 2011 until the end of the experiment in August 2012 (mean annual air temperature 9.9° in 2011 and 9.4 °C in 2012).
In June 2012, the soil humidity was regularly determined by a sensor (ML2X, HH2, Delta-T Devices, Cambridge, UK) in each individual pot, and kept in the range from 0.2 to 0.3 m3 m−3. Watering was stopped for half of the plants in each soil treatment on 25th June until the soil humidity had dropped to about 0.1 m3 m−3. Thereafter, the drought-exposed plants were watered individually to maintain the reduced soil humidity of about 0.08 to 0.1 m3 m−3 until the harvest (10th August) (n = 5 per soil type and water level).
Plant performance
In the week before harvest, four plants per drought and soil treatment were used for measurements of gas exchange. A mean value for an individual plant was obtained by eight measurements on that plant and plant means were used for further data analyses. All measurements were conducted between 9:00 h und 14:00 h with a photosynthesis analyzer (LCpro+, ADC, Hoddesdon, UK) at a photosynthetic light intensity of 435 μmol photons m−2 s−1, a mean air temperature of 22.6 ± 0.5 °C and an ambient CO2 concentration of 385 ± 11 μmol CO2 mol.
Plant height and stem diameter at soil level were measured, and the total number of leaves per plant was counted the day before harvest.
Labeling with 15N glutamine
The labeling with L-glutamine (C5H10 15N2O3, 98 %, LOT # PR-19,070, Cambridge Isotope Laboratories, Andover, USA) was achieved by soil injection. Each pot received 7.8 mg glutamine dissolved in 30 ml deionized water. The glutamine solution was injected at 15 positions at soil depths of 60, 30, and 0 mm. The plants were harvested 5 h after soil injection. Five plants per soil and drought treatment were used. Non-labelled plants were treated with water in same way.
Harvest
Plants were carefully removed from the pots. Leaves and above-ground woody tissues were separated and weighed. Five leaves per plant (sample leaves) were weighed separately, photographed with a camera KP-C551 (Hitachi, Tokyo, Japan) and used for leaf area determination (ImageJ 1.47v, National Institute of Health, Bethesda, USA). Whole plant leaf area was calculated as leaf area of sample leaves x whole plant leaf mass / mass of sample leaves.
Roots were washed, separated into coarse roots (> 2 mm diameter) and fine roots (< 2 mm diameter) and weighed. Fine roots were kept in humid tissue papers at 4 °C in darkness until analysis of mycorrhizal colonization.
Aliquots of all fresh tissues were dried for 7 days at 60 °C and used to determine the dry biomass.
The soil from each pot was mixed carefully, and 15 g were removed and stored for soil analyses at −20 °C.
Soil analyses
Five g of fresh soil (stored frozen) was suspended in 12.5 ml deionized water, shaken for 4 h at 230 rpm (Shaker 3018, GFL, Burgwedel, Germany) and then used to determine the pH (pH 538, WTW, Weilheim, Germany).
For elemental analyses soil aliquots were extracted in 65 % HNO3 for 12 h at 170 °C (Heinrichs et al. 1986). After filtering the extracts were analyzed by inductively coupled plasma atomic emission spectroscopy (Spectro Analytic Instruments, Kleve, Germany).
Root tip and mycorrhizal analyses
Fine roots were cut into about 20 mm long pieces and spread under a compound microscope (205 FA, Leica, Wetzlar, Germany). Adhering soil particles were removed. Randomly selected root segments were used for counting root tips. In each sample, a total number of 700 root tips was counted and classified as vital mycorrhizal, vital non-mycorrhizal or dead according to their morphological appearances (Winkler et al. 2010). Vital mycorrhizal root tips were assigned to morphotypes after morphological characters such as color, mantle structures, ramnification, presence/absence of hyphae or rhizomorphs (Agerer 2006). Morphotypes were documented with a digital camera (DFC420 C, Leica, Wetzlar, Germany). Aliquots of different morphotypes were collected and used for ITS sequencing to determine fungal identities as described elsewhere (Lang et al. 2011). The sequences were deposited in Genbank under the accessions numbers KU564080 - KU564088 and KX355262 (Table S1).
Carbon and nitrogen analysis in plant tissues and soil
Dry aliquots of all plant tissues were ground to a fine powder in a ball mill (Retsch, Düsseldorf, Germany). Aliquots of about 1 mg were weighed into tin cartouches (5 × 9 mm, IVA Analysetechnik) and subjected to N and C analyses in a CNS analyzer (EA 1108, Carlo Erba Strumentazione, Rodano, Italy). Dry, milled soil samples of 1.2 mg were used for N and C analyses in soil. Whole plant N content was determined as Nleaves*massleaves + N(stem+branches)*mass(stem+branches) + Ncoarse roots * masscoarse roots + Nfine roots*massfine roots with N = N concentration (mg g−1 dry mass). Whole plant C content was determined correspondingly. Mean plant N concentration (mg g−1) was determined as whole plant N content (mg) / whole plant biomass (g). Nitrogen use efficiency (NUE) was determined as the annual amount of biomass produced per amount of N taken up. NUE = (biomass at harvest) / (whole plant Ncontent at harvest) / 1.5 years according to Finzi et al. (2007). Because the plants were raised from the same seed lot, the amounts of N and C at the beginning (t = 0) were the same and tiny for all treatments and therefore neglected. We used 1.5 years as the time period because the plants were harvested after about 1.5 full growth periods.
15N and 13C was measured in milled fine roots at the service unit KOSI (Kompetenzzentrum für Stabile Isotope, University Göttingen, Germany) on a Delta Plus mass spectrometer (Finnigan MAT, Bremen, Germany; Interface: Conflo III, Finnigan MAT, Bremen, Germany; elemental analyzer: NA2500, CE Instruments, Rodano, Milano, Italy). Stable isotope ratios of 13C to 12C are expressed with the delta notation (δ13C ‰) relative to the Vienna Pee Dee Belemnite standard. Stable isotope ratios of 15N to 14N were calculated as APE (15N atom% excess) with 15N APE = atom %sample – atom %natural abundance with
Fine root 15N uptake rate (μg g−1 × h−1) was determined as (1000 × 15N APEfine roots*10*Nfine roots)/5 h. 15N recovery (%) in roots was determined as (1000 × 15N APEfine roots x Nfine roots x dry mass of fine whole plant fine roots)/7.8.
Statistical analyses
Data are means of n = 5 biological replicates (± SE) or of n = 4 (± SE) for the gas exchange measurements. Statistical analyses were conducted with the program Statgraphics Centurion XVII (Statpoint Technologies, Inc. Warrenton, Virginia, USA). Data were tested for normal distribution by the Shapiro-Wilk’s test and variance homogeneity by the Levine test. When these requirements were not fulfilled, data were log-transformed to achieve normal distribution. Two-way-ANOVA was conducted for the main factors soil type and drought treatment. P values ≤0.05 were considered to indicate significant differences. LSD tests were conducted post hoc to determine the means that differed at P < 0.05. Multiple variable regression analyses were based on Pearson product moment correlations and regression coefficients (R); P values <0.05 were considered to indicate significant relationships. Multiple regression analyses were conducted by stepwise addition of variables. To compare EM communities, analyses of similarities (ANOSIM) with the Bay-Curtis indices as similarity measure were conducted with 9999 permutations using the free software package PAST 3.08 (http://folk.uio.no/ohammer/past/, Hammer et al. 2001).
Results
Performance and drought response of beech seedlings in different soil types
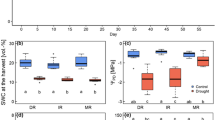
There were no significant differences in biomass among the beech seedlings raised in acid (CS, CL, UL) and calcareous soils (TN), except plants in TS soil (Fig. 1a). They exhibited significantly lower biomass than those in the other soils tested here. Reduced water supply for 6 weeks resulted in a decline of the relative soil humidity from a mean of 0.267 ± 0.003 across all control treatments to a mean of 0.066 ± 0.005 m3 m−3 during the last week of the drought treatments (Fig. 2), but had no significant effect on plant biomass (Fig. 1a). In addition to biomass, we also determined plant height, stem diameter, and leaf area. All performance parameters were strongly correlated among each other (Pearson product moment correlations for all combinations, P < 0.001) showing no drought effects, but significantly lower values for beeches in TS than in the other soil types.
Biomass (g plant−1) (a), net photosynthesis (μmol CO2 m−2 s−1) (b) and carbon discrimination δ13C (‰) in fine roots (c) of 1.5-year-old beech (Fagus sylvatica) trees raised in different soil types and exposed for 6 weeks to diminished water supply. Abbreviations indicate soil types (CL = Calvörde, clay, Calvörde, sand, UL = Unterlüß, clay, TN = Tuttlingen, calcareous, north exposition, TS = Tuttlingen, calcareous, south exposition) as described in detail in Table 1. Bars indicate means (±SE) of well irrigated controls (black) and drought-treated plants (white). Different letters indicate significant differences with p ≤ 0.05
Time course of the decrease in soil humidity in five different soil types after reduced water supply (white symbols) and with sufficient irrigation (black symbols). Abbreviations indicate soil types (CL = Calvörde, clay, Calvörde, sand, UL = Unterlüß, clay, TN = Tuttlingen, calcareous, north exposition, TS = Tuttlingen, calcareous, south exposition) and treatments (C = well irrigated controls, D = drought treatment) as described in detail in Table 1. Data indicate means of n = 5 (± SE) pots per treatment
Net photosynthesis of control plants showed no significant variations among well-watered plants in different soil types (Fig. 1b). Drought treatment caused significant reductions in photosynthesis, with the exception of the smaller seedlings in TS soil and the UL seedlings (Fig. 1b). Photosynthesis was unrelated to biomass (Pearson product moment, P = 0.778). Other gas exchange parameters (transpiration, stomatal conductance) were significantly correlated with photosynthesis (Pearson product moment correlations for all combinations, P < 0.001).
To characterize the impact of drought, we determined δ13C in fine roots (Fig. 1c). It was notable that the control roots showed differences in 13C discrimination among the soil types, with the strongest discrimination in roots in TN and the lowest in CL and UL soils (Fig. 1c). Drought resulted in significant decreases in 13C discrimination in CL, TS, and TN roots, reaching the lowest values in CL roots (Fig. 1c). On the contrary, the variation of δ13C in response to drought in CS and UL roots was not significant (Fig. 1c).
Mycorrhizal assemblages of beech in different soils in response to drought
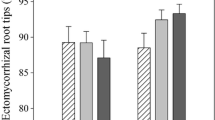
An unexpected result was that the root tips of beech plants in the acid soil (CL, CS, UL) were significantly less colonized by mycorrhizas than those in the calcareous soils (TN, TS, Fig. 3a). Drought resulted in significant reductions in mycorrhizal colonization of beech plants in calcareous soils, which were stronger in TS than in TN soil (Fig. 3a).
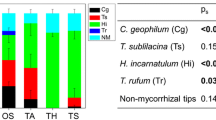
Ectomycorrhizal colonization of root tips (% of vital root tips) (a) ectomycorrhizal species composition (% of mycorrhizal root tips of the controls) (b) and root tip mortality (% of all counted root tips) (c) of 1.5-year-old beech (Fagus sylvatica) trees raised in different soil types and exposed for 6 weeks to diminished water supply. Abbreviations indicate soil types (CL = Calvörde, clay, Calvörde, sand, UL = Unterlüß, clay, TN = Tuttlingen, calcareous, north exposition, TS = Tuttlingen, calcareous, south exposition) as described in detail in Table 1. Ectomycorrhizal species names: Sein: Sebacina incrustans, unHe: uncultured Hebeloma, Hytu: Hydnotrya tulasnei, Cege: Cenococcum geophilum, Tusp1: uncultured Tuber, Hesp1: uncultured Heliotales, Gete: Geopora tenuis, Toca: Tomentella castanea syn. T. sublilacina, Tosp1: uncultured Tomentella, Hesp2: Uncultured Helotiales. Further details are shown in supplement Table S1. Bars indicate means of n = 5 plants per treatment (± SE) of well irrigated controls (black) and drought-treated plants (white). Different letters indicate significant differences with p ≤ 0.05
Beech seedlings in different soil types also developed different EM fungal communities at the root tips (Fig. 3b). Roots of well-watered controls were colonized by 5 to 10 EM species and those of drought-stressed beech plants by 3 to 7 EM taxa per soil origin (Fig 3b). The plants in acid soils exhibited lower EM species richness than those in calcareous soils resulting in significant differences between the acid and the calcareous EM assemblages under control conditions (Fig. 3b, Table 2). While no significant differences were found among EM assemblages of the well-watered controls in acid soils (CS, CL, UL), the communities on roots in calcareous soil from TS were significantly different from those in TN soil (Table 2). Because of EM species loss in response to drought, the significant differences among the communities disappeared among the beech seedlings in different soil types in response to drought (Table 2).
Cenococcum geophilum was the only fungal species that was found in all treatments, but its abundance was generally lower on roots grown in calcareous than on those in acid soils (Fig. 3b). Sebacina incrustans was also frequently found and was more abundant on the roots of plants in calcareous than in acid soils (Fig. 3b). A large fraction of the mycorrhizal root tips in acid soil was colonized by an ascomycete morphotype (uncultured Heliotales, Hesp2) whose identity could not be determined unequivocally because in several extraction attempts the samples yielded two ITS sequences (Table S1). It is possible that two species co-colonized the root tips.
We further observed differences in root tip mortality among the beech seedlings in different soil types (Fig. 3c). Under control conditions, the root tips of beech in TS soil showed about 5-fold higher mortality than those from CS, CL, and UL, while those from TN had an intermediate position (Fig. 3c). Drought resulted in strong increases in dead root tips reaching about 65 % mortality in soils from US, TN and TS, whereas the mortality was lower in soils from UL and CL (Fig. 3b).
To find out whether relationships existed between root EM taxon richness, colonization, mortality and performance parameters, we conducted multiple regression analyses (Table 3). Linear regression analysis showed that EM species richness, the degree of root tip colonization and net photosynthesis were strongly correlated (Table 3). Negative relationships were found between EM colonization respective EM species richness and δ13C, as well as between root tip mortality and photosynthesis (Table 3).
Beech N use efficiency and N uptake from 15N-glutamine
Beech seedlings in CS soil contained the highest and those in TS the lowest N contents (Fig. 4a). The effect of drought on beech N content was insignificant (Fig. 4a). Overall, the whole plant N contents were strongly correlated with whole plant biomass (R = 0.909, Table 3).
Nitrogen content (mg N plant−1) (a), nitrogen concentrations of whole plant means (bars) and of leaves (circles) (mg g−1 dry mass) (b), nitrogen concentrations of roots (mg g−1 dry mass) (c) and nitrogen use efficiency (NUE, g biomass g−1 N year−1) (d) of 1.5-year-old beech (Fagus sylvatica) trees raised in different soil types and exposed for 6 weeks to diminished water supply. Abbreviations indicate soil types (CL = Calvörde, clay, Calvörde, sand, UL = Unterlüß, clay, TN = Tuttlingen, calcareous, north exposition, TS = Tuttlingen, calcareous, south exposition) as described in detail in Table 1. Bars indicate means of n = 5 plants per treatment (±SE) of well irrigated controls (black) and drought-treated plants (white). Different letters indicate significant differences with p ≤ 0.05
Beech in CS and CL soils exhibited the highest and those in TN and TS the lowest mean plant N concentrations (Fig. 4b). Drought had no effect on the mean plant N concentrations (Fig. 4b). The foliar concentrations ranged from 21.5 to 28.5 mg N g−1 dry mass of leaves and showed similar patterns as the mean plant N concentrations (Fig. 4b). In contrast to leaves and whole-plant mean N concentrations, the N concentrations in roots from calcareous soils were significantly lower than those from acid soils and declined in response to drought, while no significant drought-induced decline was apparent in roots from the acid soils (Fig. 4c).
We also determined NUE as the amount of biomass produced per unit of N taken up (Fig. 4d). NUE was inversely related to mean plant N concentrations (Table 3), with beech seedlings in CS and CL exhibiting lower NUE than those in UL, TS, and TN soils (Fig. 4d). NUE, mean plant N concentrations and whole plant N contents were correlated among each other (Table 3), but this could be expected because of partial autocorrelation of the values. Notably, there were also significant, negative relationships between the mean plant N concentration and the degree of mycorrhizal root colonization or EM species richness (Table 3).
To investigate the capacity of the plant for N uptake from an organic source, 15N-glutamine was administered (Fig. 5a). Under control conditions, the 15N uptake rate was significantly lower for roots grown in acid soils than for those in the calcareous soils (Fig. 5a). In response to drought, decreases in 15N uptake were observed in roots from all soil types (Fig. 5a). The decline was stronger in roots of seedlings grown in UL, TN, and TS soils than in CS and CL soils (Fig. 5a).
15N uptake rate of roots (μg 15N g−1 dry mass x h−1) (a) and linear regression curve for the relationship between ectomycorrhizal species richness and 15N uptake rate (b) of 1.5-year-old beech (Fagus sylvatica) trees raised in different soil types and exposed for 6 weeks to diminished water supply. 15N was administered as 15N-glutamine to the soil. Rates were calculated after correction for the natural 15N abundance determined in unlabeled controls. Abbreviations indicate soil types (CL = Calvörde, clay, Calvörde, sand, UL = Unterlüß, clay, TN = Tuttlingen, calcareous, north exposition, TS = Tuttlingen, calcareous, south exposition) as described in detail in Table 1. Bars and circles indicate means of n = 5 plants per treatment (±SE) of well irrigated controls (black) and drought-treated plants (white). Different letters indicate significant differences with p ≤ 0.05
The uptake rate of 15N was negatively correlated with the relative abundance of non-mycorrhizal roots tips (P < 0.001, not shown) and with decreasing carbon discrimination (Table 3). Positive relationships were found between the uptake rate of 15N and EM species richness, photosynthesis, mycorrhizal colonization and 15N recovery in fine roots (Table 3). Testing the relationship of 15N uptake with EM species richness, photosynthesis, and mycorrhizal colonization by multiple correlation analyses revealed that species richness was the best and among the three variables the only parameter that remained after stepwise testing and explained 52 % of the variation (Fig. 5b).
Discussion
Soil types structure beech EM fungal assemblages
In agreement with our expectation, beech seedlings were colonized by different EM fungal assemblages when raised in different soil types. Many EM taxa detected here were also present on the roots of mature and young beech trees in the TN and UL forests, for example C. geophilum, Sebacina incrustans, Tomentella sp., Heliotales, and Tuber sp. (Pena et al. 2010; Leberecht et al. 2015) indicating that the roots were colonized by members of the site-specific EM assemblages. Field studies along geographic and environmental gradients indicated that soil chemistry was an important factor for differences in EM species composition (Lilleskov et al. 2002; Kranabetter et al. 2009; Moeller et al. 2014; Walker et al. 2014; Pretzsch et al. 2014; Zavišić et al. 2016). The present results support this conclusion because potentially confounding effects of temperature and soil humidity were excluded. Other potentially interfering factors such as host species identity (Lang et al. 2011), progeny (Dučić et al. 2009), health and productivity (Druebert et al. 2009; Twieg et al. 2009; Pena et al. 2010; Horton et al. 2013; Pena et al. 2013a) were also excluded here because all plants were raised from the same seed lot and did not differ in photosynthesis, leaf area, growth, and biomass, with one exception (TS). These observations are in agreement with field studies showing that beech is generally able to cope with a large pH gradient and different soil nutrient concentrations, maintaining for example similar leaf production rates across edaphic gradients (Leuschner et al. 2006; Meier et al. 2005).
Experimental manipulation of soil pH and Ca content by liming influenced the root tip EM colonization rate (Børja and Nilsen 2009; Dučić et al. 2009; Monfort-Salvador et al. 2015) and the EM communities, leading for instance to decreases in C. geophilum and increases in Sebacina sp. (Rineau and Garbaye 2009; Rineau et al. 2010). In our study C. geophilum was also less abundant on the roots of beech in calcareous soil than on the roots of trees in acid soil, whereas the opposite behavior was observed for S. incrustans. However, in the TN forest C. geophilum, a drought tolerant EM species (Herzog et al. 2013), was the most abundant taxon and S. incrustans rare. Knowledge on the ecology and physiological requirements of Sebacinales is quite limited (Oberwinkler et al. 2013). Our results indicate that the abundance of these fungi is controlled by soil types and other, yet unknown environmental factors.
Unexpectedly, here the beech seedlings in calcareous soil exhibited higher EM taxon richness and also higher root tip colonization than those in the acid soils. Because regression analyses showed that both root colonization and species richness decreased with decreasing photosynthesis, our results suggest that the colonization of the roots of the young trees is critically depending on recently assimilated carbon.
Host nitrogen use is intertwined with mycorrhizal colonization and soil-born stress
The impact of N on EM community structures has often been demonstrated (Talbot and Treseder 2010). The negative relationship between EM species richness respective root colonization and plant N concentrations found here, therefore, suggest that nitrogen also played a major role affecting the establishment of EM host interactions in different soil types. Foliar concentrations of beech plants in the range of 19 to 23 mg g−1 dry mass of leaves, detected for beech plants in UL, TN and TS soils, and of >26 mg g−1 dry mass observed for the plants in CL and CS soils, indicate sufficient and luxurious N supply, respectively (Mellert and Göttlein 2012). At the first glance the high foliar N concentrations were surprising, but the threshold values have been determined for adult trees, whereas here seedlings were analyzed, which owing to their small size have a lower whole-plant N demand than larger trees. Furthermore, the N availability for beech plants in acid soil might have been higher than in the calcareous soils despite lower total soil N concentrations in the acid soils because the N mineralization potential is lower at high than at acid pH (Leuschner 1999; Andrianarisoa et al. 2009). Low plant N availability may require the aid of EM fungi to compete with soil microbes for soil N, but under certain conditions EM fungi can also withhold N from their host plants (Dučić et al. 2009; Näsholm et al. 2013; Pena et al. 2013b). Both mechanisms may increase NUE. Therefore, it is an open question whether lower N availability in calcareous soil of the TS and TN forests fostered higher root colonization to achieve “normal” N nutrition as indicated by the foliar N concentrations of the plants in calcareous soils or whether there was a trade-off with other edaphic stresses that promoted high EM colonization at the expense of lower plant N transfer. The latter stress theory has some support because we found higher root tip mortality despite similar soil humidity in unstressed plants and higher drought sensitivity of EM root tip colonization in calcareous than in acid soils. This stress was apparently stronger for plants in TS than in TN soil because only under these conditions significantly less biomass was produced and the highest root tip mortality was found in absence of drought stress. It is notable that the radial stem growth of the mature beech trees in the TS forest is lower than in the TN forest (Gessler et al. 2001), suggesting that in addition to climatic stresses and low N availability (Gessler et al. 2005), soil properties may have impinged on tree performance.
Drought sensitivity of beech N uptake correlates with EM taxon richness and root tip colonization, but not with whole plant nitrogen use efficiency
The inverse relationships of EM colonization with plant N concentrations and plant N uptake might appear contradictory at the first glance. In line with other studies, N uptake was strongly suppressed by drought (Gessler et al. 2005; Winkler et al. 2010; Danielsen and Polle 2014; Pena and Polle 2014), but the changes in the amounts taken up were too small to affect plant N tissue concentrations or NUE. Whole plant N levels are the result of long-term processes reflecting the conservative nutrient and biomass allocation strategy of beech, which is characterized by a strong increment in N in leaves during the early expansion phase and a subsequent decrease until the leaves are fully expanded (Bauer et al. 1997). Since beech usually forms only one flush at the beginning of the season, the N content of the leaves remains quite stable after leaf maturation and is unaffected by precipitation gradients (Meier et al. 2005; Meier and Leuschner 2014). Correspondingly, the N content, NUE and foliar concentrations were not affected by drought in this pot experiment.
Nitrogen uptake processes at the root level are regulated by root physiology and availability of different N sources in the soil (Rennenberg et al. 2009). In mixtures of inorganic (NH4 +, NO3 −) and organic N (glutamine, arginine) preferential 15N uptake from glutamine was found for beech roots in the TN forest (Dannenmann et al. 2009). Similarly, non-mycorrhizal beech roots also preferred glutamine and suppressed uptake of inorganic N in mixtures (Kreuzwieser et al. 1997; Stoelken et al. 2010; Winkler et al. 2010). Although 15N was derived from glutamine in those experiments as well as in the present one, the main uptake form of N into the roots is not known. In field studies (Finzi and Berthrong 2005; Dannenmann et al. 2009, the present study), fast turn-over processes in the soil cannot be excluded. Application of 13C dual-labelled glutamine showed that even beech roots in artificial soil solutions did not acquire corresponding 13C/15N amounts (Winkler et al. 2010). The reasons could either be rapid degradation of glutamine before root uptake or whole glutamine uptake and respiratory loss of the 13C label (Näsholm et al. 2009).
EM fungi can grow on various organic substances, but glutamine was the only compound efficiently utilized by all fungi tested (Talbot and Treseder 2010). Our results suggest that EM fungi play a major role in 15N acquisition from glutamine and transfer to the host because we observed only a relatively low 15N uptake by roots with low EM colonization and a strong correlation of 15N uptake with EM species richness. The uptake of 15N by diverse EM communities varies with their species composition and environmental constraints (Pena and Polle 2014; Leberecht et al. 2015). The finding that the multiple correlation analyses of 15N uptake with EM taxon richness, mycorrhizal colonization and photosynthesis extracted EM taxon richness as the main explanatory factor further supports that the community structure may have controlled 15N uptake. Apparently, the properties of the EM assemblage were overriding the impact of photosynthesis, which is required for energy supply. Because of the strong co-variation of mycorrhizal colonization and species richness, these effects cannot be separated. The strong drought-induced reductions in N uptake of the plants in calcareous soil at similar levels of soil humidity as in acid soil suggest high lability of N supply of the young plants in these soils. The limitations of N uptake also resulted in small, but significant decreases in root N tissue concentrations under drought in the calcareous soils. The reductions in N uptake were stronger in TS than in TN soil indicating that drought constrained plant N nutrition in this soil type more strongly than in those from the other forests.
In conclusion, we showed that (i) beech roots in different soils were colonized by taxonomically different EM assemblages, (ii) that high EM colonization and species richness correlated with high 15N uptake from glutamine and increased NUE, and that (iii) glutamine-derived 15N uptake was more susceptible to drought in calcareous than in acid soils, whereas NUE was unaffected by drought. It is clear that under field conditions further factors such as high temperatures (Williams et al. 2013), which enhance N mineralization (Gessler et al. 2005) and re-wetting events, which can potentially compensate for N deprivation (He and Dijkstra 2014), affect tree N availability and productivity. Although the results of this relatively short-term pot study cannot be directly applied to explain field observations, our findings enhance our understanding of the mechanisms impinging on beech performance under drought. The interaction of soil type and ecosystem functions of EM communities should be tested in forests along larger geographical gradients because these factors may have important implications for management decisions, when forest ecosystems have to be converted to withstand a future, more arid climate.
Abbreviations
- EM:
-
ectomycorrhizal
- N:
-
nitrogen
- NUE:
-
nitrogen use efficiency
References
Agerer R (2006) Colour atlas of ectomycorrhizae. Einhorn-Verlag, Schwaebisch Gmuend, Germany
Andrianarisoa KS, Zeller B, Dupouey JL, Dambrine E (2009) Comparing indicators of N status of 50 beech stands (Fagus sylvatica L.) in northeastern France. For Ecol Manag 257:2241–2253
Bauer G, Schulze ED, Mund M (1997) Nutrient contents and concentrations in relation to growth of Picea abies and Fagus sylvatica along a European transect. Tree Physiol 17:777—786.
Bolte A, Czajkowski T, Kompa T (2007) The north-eastern distribution range of European beech - a review. Forestry 80:413–429
Børja I, Nilsen P (2009) Long term effect of liming and fertilization on ectomycorrhizal colonization and tree growth in old scots pine (Pinus sylvestris L.) stands. Plant Soil 314:109–119
Carsjens C, Nguyen QN, Guzy J, Knutzen F, Meier IC, Müller M, Finkeldey R, Leuschner C, Polle A (2014) Intra-specific variations of stress-related genes in beech progenies are stronger than drought-induced responses. Tree Physiol 34:1348–1361
Ciais P, Reichstein M, Viovy N, Granier A, Ogée J, Allard V, Buchmann N, Aubinet M, Chr B, Carrara A, Chevallier F, De Noblet N, Friend A, Friedlingstein P, Gobron N, Grünwald T, Heinesch B, Keronen P, Knohl A, Krinner G, Loustau D, Manca G, Matteucci G, Miglietta F, Ourcival JM, Pilegaard K, Rambal S, Seufert G, Soussana JF, Sanz MJ, Schulze ED, Vesala T, Valentini R (2005) Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437:529–533
Clemmensen KE, Sorensen PL, Michelsen A, Jonasson S, Lena Ström L (2008) Site-dependent N uptake from N-form mixtures by arctic plants, soil microbes and ectomycorrhizal fungi. Oecologia 155:771–783
Danielsen L, Polle A (2014) Poplar nutrition under drought as affected by ectomycorrhizal colonization. Environ Exp Bot 108:89–98
Dannenmann M, Simon J, Gasche R, Holst J, Naumann PS, Kögel-Knabner I, Knicker H, Mayer H, Schloter M, Pena R, Polle A, Rennenberg H, Papen H (2009) Tree girdling provides insight in the role of labile carbon in the competitive balance of N partitioning between soil microorganisms and adult European beech. Soil Biol Biochem 41:1622–1631
Druebert C, Lang C, Valtanen K, Polle A (2009) Beech carbon productivity as driver of ectomycorrhizal abundance and diversity. Plant Cell Environ 32:992–1003
Dučić T, Berthold D, Langenfeld-Heyser R, Beese F, Polle A (2009) Mycorrhizal communities in relation to biomass production and nutrient use efficiency in two varieties of Douglas fir (Pseudotsuga menziesii var. menziesii and Var. glauca) in different forest soils. Soil Biol Biochem 41:742–753
Ellenberg H (2009) Vegetation ecology of Central Europe. Cambridge University Press, Cambridge, p. 756
Finlay RD, Frostegard A, Sonnerfeldt AM (1992) Utilization of organic and inorganic nitrogen sources by ectomycorrhizal fungi in pure culture and in symbiosis with Pinus contorta Dougl. Ex loud. New Phytol 120:105–115
Finzi AC, Berthrong ST (2005) The uptake of amino acids by microbes and trees in three cold-temperate forests. Ecology 86:3345–3353
Finzi AC, Norby RJ, Calfapietra C, Gallet-Budyneka A, Gielen B, Holmes WE, Hoosbeek MR, Iverseng CM, Jackson RB, Kubiske ME, Ledford J, Liberloo M, Oren R, Polle A, Pritchard S, Zak DR, Schlesinger WH, Ceulemans R (2007) Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc Natl Acad Sci U S A 104:14014–14019
Gessler A, Schrempp S, Matzarakis A, Mayer H, Rennenberg H, Adams MA (2001) Radiation modifies the effect of water availability on the carbon isotope composition of beech (Fagus sylvatica L.). New Phytol 50:653–664
Gessler A, Jung K, Gasche R, Papen H, Heidenfelder A, Börner E, Metzler B, Augustin S, Hildebrand E, Rennenberg H (2005) Climate and forest management influence nitrogen balance of European beech forests: microbial N transformations and inorganic N net uptake capacity of mycorrhizal roots. Eur J For Res 124:95–111
Gessler A, Keitel C, Kreuzwieser J, Matyssek R, Seiler W, Rennenberg H (2007) Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees Struct Funct 21:1–11
Granier A, Reichstein M, Bréda N, Janssens IA, Falge E, Ciais P, Grünwald T, Aubinet M, Berbigier P, Bernhofer C, Buchmann N, Facini O, Grassi G, Heinesch B, Ilvesniemi H, Keronen P, Knohl A, Köstner B, Lagergren F, Lindroth A, Longdoz B, Loustau D, Mateus J, Montagnani L, Nys C, Moors E, Papale D, Peiffer M, Pilegaard K, Pita G, Pumpanen J, Rambal S, Rebmann C, Rodrigues A, Seufert G, Tenhunen J, Vesala I, Wang Q (2007) Evidence for soil water control on carbon and water dynamics in European forests during the extremely dry year: 2003. Agric For Meteorol 143:123–145
Grelet GA, Meharg AA, Duff EI, Anderson IC, Alexander IJ (2009) Small genetic differences between ericoid mycorrhizal fungi affect nitrogen uptake by Vaccinium. New Phytol 181:708–718
Hammer O, Harper DAT, Ryan PD (2001) PAST: palaeontological statistics software package for education and data analysis. Palaeontol Electron 4:9–17
He MZ, Dijkstra FA (2014) Drought effect on plant nitrogen and phosphorus: a metaanalysis. New Phytol 204:924–931
Heinrichs R, Brumsack H, Loftfield N, Konig N (1986) Improved pressure digestion system for biological and inorganic materials. J Plant Nutr Soil Sci 149:350–353
Herzog C, Peter M, Pritsch K, Günthardt-Goerg MS, Egli S (2013) Drought and air warming affects abundance and exoenzyme profiles of Cenococcum geophilum associated with Quercus robur, Q. petraea and Q. pubescens. Plant Biol 15:230–237
Hobbie EA, Högberg P (2012) Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol 196:367–382
Horton BM, Glen M, Davidson NJ, Ratkowsky D, Close DC, Wardlaw TJ, Mohammed C (2013) Temperate eucalypt forest decline is linked to altered ectomycorrhizal communities mediated by soil chemistry. For Ecol Manag 302:329–337
Keller G (1996) Utilization of inorganic and organic nitrogen sources by high-subalpine ectomycorrhizal fungi of Pinus cembra in pure culture. Mycol Res 100:989–998
Kranabetter JM, Durall DM, MacKenzie WH (2009) Diversity and species distribution of ectomycorrhizal fungi along productivity gradients of a southern boreal forest. Mycorrhiza 19:99–111
Kranabetter JM, Hawkins BJ, Jones MD, Robbins S, Dyer T, Li T (2015) Species turnover (β-diversity) in ectomycorrhizal fungi linked to NH4 + uptake capacity. Mol Ecol 24:5992–6005
Kreuzwieser J, Gessler A (2010) Global climate change and tree nutrition: influence of water availability. Tree Physiol 30:1221–1234
Kreuzwieser J, Herschbach C, Stulen I, Wiersema P, Vaalburg W, Rennenberg H (1997) Interactions of NH4 + and L-glutamate with NO3 − transport processes of non-mycorrhizal Fagus sylvatica roots. J Exp Bot 48:1431–1438
Lang C, Seven J, Polle A (2011) Host preferences and differential contributions of deciduous tree species shape mycorrhizal species richness in a mixed central European forest. Mycorrhiza 21:297–308
Leberecht M, Dannenmann M, Gschwendtner S, Bilela S, Meier R, Simon J, Rennenberg H, Schloter M, Polle A (2015) Ectomycorrhizal communities on the roots of two beech (Fagus sylvatica) populations from contrasting climate differ in nitrogen acquisition in a common environment. Appl Environ Microbiol 81:5957–5967
Leuschner C (1999) Zur Abhängigkeit der Baum- und Krautschicht mitteleuropäischer Waldgesellschaften von der Nährstoffversorgung des Bodens. Ber d ReinhTüxen-Ges 11:109–131
Leuschner C, Meier IC, Hertel D (2006) On the niche breadth of Fagus sylvatica: soil nutrient status in 50 central European beech stands on a broad range of bedrock types. Ann For Sci 63:355–368
Lilleskov EA, Fahey TJ, Horton TR, Lovett GM (2002) Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 83:104–115
Meier IC, Leuschner C (2008) Leaf size and leaf area index in Fagus sylvatica forests: competing effects of precipitation, temperature, and nitrogen availability. Ecosystems 11:655–669
Meier IC, Leuschner C (2014) Nutrient dynamics along a precipitation gradient in European beech forests. Biogeochemistry 120:51–69
Meier IC, Leuschner C, Hertel D (2005) Nutrient return with leaf litter fall in Fagus sylvatica forests across a soil fertility gradient. Plant Ecol 177:99–112
Mellert KH, Göttlein A (2012) Comparison of new foliar nutrient thresholds derived from van den Burg’s literature compilation with established central European references. Eur J For Res 131:1461–1472
Moeller HV, Peay KG, Fukami T (2014) Ectomycorrhizal fungal traits reflect environmental conditions along a coastal California edaphic gradient. FEMS Microbiol Ecol 87:797–806
Monfort-Salvador I, García-Montero LG, Grande MA (2015) Impact of calcium associated to calcareous amendments on ectomycorrhizae in forests: a review. J Soil Sci Plant Nutr 15:217–231
Müller-Haubold H, Hertel D, Seidel D, Knutzen F, Leuschner C (2013) Climate responses of aboveground productivity and allocation in Fagus sylvatica: a transect study in mature forests. Ecosystems 16:1498–1516
Näsholm T, Persson J (2001) Organic nitrogen acquisition by boreal forest plants. Physiol Plant 111:419–442
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48
Näsholm T, Högberg P, Franklin O, Metcalfe D, Keel SG, Campbell C, Hurry V, Linder S, Högberg MN (2013) Are ectomycorrhizal fungi alleviating or aggravating nitrogen limitation of tree growth in boreal forests? New Phytol 198:214–221
Oberwinkler F, Riess K, Bauer R, Selosse MA, Weiß M, Garnica S, Zuccaro A (2013) Enigmatic Sebacinales. Mycol Prog 12:1–27
Pena R, Polle A (2014) Attributing functions to ectomycorrhizal fungal identities in assemblages for nitrogen acquisition under stress. ISME J 8:321–330
Pena R, Offermann C, Simon J, Naumann PS, Geßler A, Holst J, Dannenmann M, Mayer H, Kögel-Knabner I, Rennenberg H, Polle A (2010) Girdling affects ectomycorrhizal diversity and reveal functional differences of EM community composition in a mature beech forest (Fagus sylvatica). Appl Environ Microbiol 76:1831–1841
Pena R, Tejedor J, Zeller B, Dannenmann M, Polle A (2013a) Interspecific temporal and spatial differences for the acquisition of litter-derived nitrogen of ectomycorrhizal fungal assemblages. New Phytol 199:520–528
Pena R, Simon J, Rennenberg H, Polle A (2013b) Ectomycorrhiza affect architecture and nitrogen partitioning of beech (Fagus sylvatica L.) seedlings under shade and drought. Environ Exp Bot 87:207–217
Persson J, Högberg P, Ekblad A, Högberg MN, Nordgren A, Näsholm T (2003) Nitrogen acquisition from inorganic and organic sources by boreal forest plants in the field. Oecologia 137:252–257
Plassard C, Bonafos B, Touraine B (2000) Differential effects of mineral and organic N sources, and of ectomycorrhizal infection by Hebeloma cylindrosporum, on growth and N utilization in Pinus pinaster. Plant Cell Environ 23:1195–1205
Pretzsch H, Rotzer T, Matyssek R, Grams TEE, Häberle KH, Pritsch K, Kerner R, Munch JC (2014) Mixed Norway spruce (Picea abies [L.] Karst) and European beech (Fagus sylvatica [L.]) stands under drought: from reaction pattern to mechanism. Trees Struct Funct 28:1305–1321
Rennenberg H, Dannenmann M, Gessler A, Kreuzwieser J, Simon J, Papen H (2009) Nitrogen balance in forest soils: nutritional limitation of plants under climate change stresses. Plant Biol 11:4–23
Rineau F, Garbaye J (2009) Does forest liming impact the enzymatic profiles of ectomycorrhizal communities through specialized fungal symbionts? Mycorrhiza 19:493–500
Rineau F, Maurice JM, Nys C, Voiry H, Garbaye J (2010) Forest liming durably impact the communities of ectomycorrhizas and fungal epigeous fruiting bodies. Ann For Sci 67:110
Ruehr NK, Offermann CA, Gessler A, Winkler JB, Ferrio JP, Buchmann N, Barnard RL (2009) Drought effects on allocation of recent carbon: from beech leaves to soil CO2 efflux. New Phytol 184:950–961
Sardans J, Penuelas J (2012) The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol 160:1741–1761
Schmidt S, Handley LL, Sangtiean T (2006) Effects of nitrogen source and ectomycorrhizal association on growth and delta N-15 of two subtropical eucalyptus species from contrasting ecosystems. Funct Plant Biol 33:367–379
Shi L, Guttenberger M, Kottke I, Hampp R (2002) The effect of drought on mycorrhizas of beech (Fagus sylvatica L.): changes in community structure, and the content of carbohydrates and nitrogen storage bodies of the fungi. Mycorrhiza 12:303–311
Stoelken G, Simon J, Ehlting B, Rennenberg H (2010) The presence of amino acids affects inorganic N uptake in non-mycorrhizal seedlings of European beech (Fagus sylvatica). Tree Physiol 30:1118–1128
Talbot JM, Treseder KK (2010) Controls over mycorrhizal uptake of organic nitrogen. Pedobiologia 53:169–179
Turnbull MH, Goodall R, Stewart GR (1995) The impact of mycorrhizal colonisation upon nitrogen source utilization and metabolism in seedlings of Eucalyptus grandis hill ex maiden and Eucalyptis maculata hook. Plant Cell Environ 18:1386–1394
Twieg BD, Durall DM, Simard SW, Jones MD (2009) Influence of soil nutrients on ectomycorrhizal communities in a chronosequence of mixed temperate forests. Mycorrhiza 19:305–316
Valtanen K, Eissfeller V, Beyer F, Hertel D, Scheu S, Polle A (2014) Carbon and nitrogen fluxes between beech and their ectomycorrhizal assemblage. Mycorrhiza 24:645–650
van der Heijden MGA, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423
Walker JKM, Phillips LA, Jones MD (2014) Ectomycorrhizal fungal hyphae communities vary more along a pH and nitrogen gradient than between decayed wood and mineral soil microsites. Botany-Botanique 92:453–463
Williams AP, Craig D, Allen CD, Macalady AK, Griffin D, Woodhouse CA, Meko DM, Swetnam TW, Rauscher SR, Seager R, Grissino-Mayer HD, Dean JS, Cook ER, Gangodagamage C, Cai M, McDowell NG (2013) Temperature as a potent driver of regional forest drought stress and tree mortality. Nat Clim Chang 3:292–297
Winkler JB, Dannenmann M, Simon J, Pena R, Offermann C, Sternad W, Clemenz C, Naumann PS, Gasche R, Kögel-Knabner I, Gessler A, Rennenberg H, Polle A (2010) Carbon and nitrogen balance in beech roots under competitive pressure of soil-borne microorganisms induced by girdling, drought and glucose application. Funct Plant Biol 37:879–889
Zavišić A, Nassal P, Yang N, Heuck C, Spohn M, Marhan S, Pena R, Kandeler E, Polle A (2016) Phosphorus availabilities in beech (Fagus sylvatica L.) forests impose habitat filtering on ectomycorrhizal communities and impact on tree nutrition. Soil Biol Biochem 98:127–137
Zhang J, Taniguchi T, Tateno RXM, Du S, Liu GB, Yamanaka N (2013) Ectomycorrhizal fungal communities of Quercus liaotungensis along local slopes in the temperate oak forests on the loess plateau, China. Ecol Res 28:297–305
Acknowledgments
We are grateful to M. Smiatacz and to T. Klein (Laboratory for Radio-Isotopes, Universität Göttingen) for plant maintenance and excellent technical support. We thank Dr. R. Pena for critical reading and comments on the manuscript. Soil nutrients were determined in the Central Laboratory of the Department for Ökopedologie der gemäßigten Zonen (Büsgen Institut, Universität Göttingen). 15N analyses were conducted in the Kompetenzzentrum für Stabile Isotope (KOSI) (Universität Göttingen) under the supervision of Dr. J. Dyckmans. The research was funded by the Deutsche Forschungsgemeinschaft (Po362/19-1) and partly by the DFG Priority Program 1374 “Infrastructure-Biodiversity-Exploratories” (Po362/18-3). J. T. acknowledges a postdoctoral fellowship awarded by the Chinese Science Council (CSC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Tatsuhiro Ezawa.
Electronic supplementary material
ESM 1
(PDF 80.4 kb)
Rights and permissions
About this article
Cite this article
Leberecht, M., Tu, J. & Polle, A. Acid and calcareous soils affect nitrogen nutrition and organic nitrogen uptake by beech seedlings (Fagus sylvatica L.) under drought, and their ectomycorrhizal community structure. Plant Soil 409, 143–157 (2016). https://doi.org/10.1007/s11104-016-2956-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2956-4