Abstract
With the aim of recognizing the commonest leaf pattern found in the woody flora of the cerrado (the Brazilian savanna) we analyzed the leaf anatomy of 30 representative species. The leaves are mostly dorsiventral and hypostomatic and covered by trichomes and a thick layer of wax and cuticle; the vascular bundles are surrounded by a sheath of fibers. The mesophyll has a developed palisade tissue, dispersed sclerified cells and idioblasts bearing crystals and phenolic compounds. We compared the results with those reported for other species (60 species) from the same biome and for the families that the studied species belong. The present study suggests that the xeromorphism observed for the cerrado leaves is related to the evolutionary history of this biome, since its first floristic elements must have faced deficient water conditions as well as the consequent soil acidity and toxicity. Therefore we may infer that the leaf anatomical pattern here observed was already present in the first elements of the cerrado and was selected to guarantee the survival of those species in the new environment. Furthermore, the xeromorphic features present in those leaves continue nowadays to help the plants protecting themselves from the different biotic and abiotic factors they are subjected to.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerrado is an ancient biome (Durigan et al. 2004) endemic to Brazil and has ecological relationships with the savanna (Ribeiro and Walter 1998). It covers around 23% of Brazil (Ratter et al. 1997; Furley 1999; Durigan et al. 2003a) and, according to Coutinho (2002), presents a physiognomic gradient ranging from “campo limpo” (open grassland), “campo sujo” (grassland with some shrubs), “campo cerrado” (shrub savanna), cerrado sensu stricto (woodland savanna) to “cerradão” (closed woodlands). On the cerrado areas occur the climate “Aw” of the Köppen’s classification, characterized by dry winters and rainy summers (Ribeiro and Walter 1998), although both rainy and dry seasons have similar maximum values of the vapor pressure deficit (Franco 1998; Meinzer et al. 1999). The annual rainfall is about 1,500 mm (Ribeiro and Walter 1998), the temperature is around 22–23°C and the solar radiation is quite intense all over the year (Coutinho 2002). The cerrado’s soils are old, deep, porous, highly leached and of sandy or sandy loam textures (Malavolta and Kliemann 1985; Ratter et al. 2000; Coutinho 2002). They have low contents of organic matter and are quite acid, aluminotoxic and poor in nutrients (Goodland 1971; Malavolta and Kliemann 1985; Ratter et al. 2000; Coutinho 2002). During the dry season, the water potential values of the soil (Ψs) are quite low in the upper layers but get higher bellow 0.85 m downwards (Franco 1998, 2002).
The cerrado vegetation is formed both by an open strata of trees, with twisted branches and trunks, and by a strata of grasses and small shrubs (Rizzini 1976), that covers the soil only during the rainy period (Beiguelman 1963). Its flora comprises around 6,000 angiosperms and 300 pteridophytes (Mendonça et al. 1998) being considered the third largest Brazilian plant formation in terms of species richness, behind the Amazonia and the Atlantic forests (Coutinho 2002).
Although growing on chemically poor soils, the cerrado has undergone an intense devastation (Victor 1975) and only 1.5% of this biome is protected as federal reserves (Ratter et al. 1997).
Most of the studies on the cerrado are of floristic and phytosociological nature (see Leitão Filho 1992; Durigan et al. 1994; Bicudo et al. 1996; Castro and Martins 1999; Castro et al. 1999; Ratter et al. 2000; Ribeiro and Tabarelli 2002; Tannus and Assis 2004), and although with many typical species, the leaf anatomy of its representative is little known. Relevant studies include those of Morretes (1967, 1969) and Morretes and Ferri (1959), who described the leaf anatomy of many species, and more limited works are those of Beiguelman (1962a, b, c, d), Panizza (1967), Handro (1966, 1967) and Paviani and Ferreira (1974). Recently, the leaves of some representatives of some common families in the cerrado were studied with the aim of indicating adaptations found in the representatives of this biome as well as useful features for taxonomic purposes. It includes Vochysiaceae (Sajo and Rudall 2002), Erythroxyllaceae (Bieras and Sajo 2004) and Melastomataceae (Reis et al. 2004, 2005).
The cerrado plants exhibits many of the so called xeromorphic features, such as hairy surface, thick cuticle, papillate epidermal cells and stomata sunken or protected by trichomes (Morretes and Ferri 1959). However, studies describing the leaf surface of its representatives are rare, except for that of Salatino et al. (1986) about 11 woody species.
This paper describes the leaf anatomy of representative woody species of the cerrado with the aim of recognizing their dominant leaf pattern and pointing out possible adaptations related to the abiotic factors that predominate in this biome.
Materials and methods
We selected the 30 most representative woody plant species of the cerrado sensu lato of São Paulo State, using the floristic studies of Durigan et al. (2003b).
The material was collected in places with different cerrado physiognomies (from “campo sujo” to “cerradão”) in the São Paulo State. Vouchers are deposited in the Herbário Rioclarense (HRCB), at the Instituto de Biociências––São Paulo State University, Rio Claro, São Paulo, Brazil. The species was classified according to Soltis et al. (2005), except for the term Fabaceae, which we replaced by Leguminosae: LAURALES: Lauraceae (Ocotea pulchella (Nees) Mez: HRCB 44667 and Bieras 45), Siparunaceae (Siparuna guianensis Aubl.: HRCB 44668 and Bieras 59,73); MAGNOLIALES: Annonaceae (Xylopia aromatica (Lam.) Mart.: HRCB 44666 and Bieras 36, 49, 60); EUDICOTS: PROTEALES: Proteaceae (Roupala montana Aubl.: HRCB 44669 and Bieras 72, 88, 89); ROSIDS: ROSALES: Moraceae (Brosimum gaudichaudii Trécul: HRCB 44677 and Bieras 79, 96), Urticaceae (Cecropia pachystachya Trécul: HRCB 44678 and Bieras 37); FABALES: Leguminosae (Acosmium subelegans (Mohlenbr.) Yakovlev: HRCB 44679 and Bieras 74; Anadenanthera peregrina var. falcata (Benth.) Speg.: HRCB 44680 and Bieras 33, 51; Bauhinia rufa (Bong.) Steud.: HRCB 44681 and Bieras 30, 93; Copaifera langsdorffii Desf.: HRCB 44682 and Bieras 48, 63, 64; Dimorphandra mollis Benth.: HRCB 44683 and Bieras 27,39; Machaerium acutifolium Vogel: HRCB 44684 and Bieras 75, 114; Platypodium elegans Vogel: HRCB 44685 and Bieras 77, 115; Stryphnodendron adstringens (Mart.) Coville: HRCB 44686 and Bieras 34, 55; Stryphnodendron obovatum Benth.: HRCB 44687 and Bieras 90, 120), Polygalaceae (Bredemeyera floribunda Willd.: HRCB 44688 and Bieras 103); MALPIGHIALES: Malpighiaceae (Byrsonima intermedia A. Juss.: HRCB 44689 and Bieras 62, 109), Ochnaceae (Ouratea spectabilis (Mart.) Engl.: HRCB 44690 and Bieras 50), Salicaceae (Casearia sylvestris Sw.: HRCB 44691 and Bieras 41, 66, 81); MALVALES: Malvaceae (Luehea grandiflora Mart.: HRCB 44692 and Bieras 102); SAPINDALES: Anacardiaceae (Tapirira guianensis Aubl.: HRCB 44693 and Bieras 67, 113), Burseraceae (Protium heptaphyllum (Aubl.) Marchand: HRCB 44694 and Bieras 65), Sapindaceae (Matayba elaeagnoides Radlk.: Bieras 122); ASTERIDS: SOLANALES: Solanaceae (Solanum paniculatum L.: HRCB 44670 and Bieras 76, 101); LAMIALES: Bignoniaceae (Tabebuia ochracea (Cham.) Standl.: HRCB 44671 and Bieras 69), Lamiaceae (Aegiphila lhotskiana Cham.: HRCB 44672 and Bieras 68, 121); APIALES: Araliaceae (Schefflera vinosa (Cham. & Schltdl.) Frodin & Fiaschi: HRCB 44673 and Bieras 35, 46); ASTERALES: Asteraceae (Baccharis dracunculifolia DC.: HRCB 44674 and Bieras 104, 112; Gochnatia barrosii Cabrera: HRCB 44675 and Bieras 110, 117; Gochnatia polymorpha (Less.) Cabrera: HRCB 44676 and Bieras 108, 118).
For the anatomical study, the leaves were fixed in FAA 50 (Johansen 1940) and preserved in 50% alcohol with drops of glycerin. For each species, we analyzed cross-sections of the petioles apex and of the median region of the blade of 3–5 leaves for material. The sections were clarified with sodium hypochlorite 20%, stained with safranin and Astra-Blau (Safrablau) (Bukatsch 1972, modified by Kraus and Arduin 1997) and mounted on glycerin gelatin (Haupt 1930, apud Kraus and Arduin 1997). Astra-Blau was used to detect mucilage and phenolic compounds were evidenced by the utilization of ferric chloride (Johansen 1940). The stomata and papillae types were identified according to Wilkinson classification (Wilkinson 1979). To dissociate the epidermis, fragments of the blade were immersed in pure sodium hypochlorite and each surface was stained with safranin 1% in 100% alcohol and mounted in glycerin gelatin (Haupt 1930, apud Kraus and Arduin 1997).
The micromorphological study was carried out on fragments of 0.5 cm2 of the median third of the blade and on petiole fragments. Each sample was dehydrated in ethanolic series, dried in a critical point dryer, metallized and analyzed in a scanning electronic microscope. The epicuticular wax was classified according to Barthlott et al. (1998) and the trichomes according to Theobald et al. (1979).
Results were recorded on electromicrographs, photomicrographs and stereomicrographs, with the scales projected under the same optical and electronic conditions.
Results
Surface
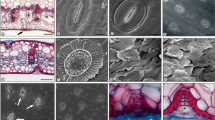
All leaves are completely covered with a dense wax layer covers making visible only the stomatal pore (Figs. 1a, 2d, f). In most of species the epicuticular wax is crustiform (Fig. 1a) both in the petiole and in the blade. However, the wax forms a smooth layer (Fig. 1b), on the petioles of Copaifera langsdorffii, Stryphnodendron obovatum, Ouratea spectabilis, Tabebuia ochracea and Schefflera vinosa and on the blades of Ocotea pulchella, Brosimun gaudichaudii, Matayba elaeagnoides, Aegiphila lhotskiana, and Baccharis dracunculifolia. A smooth wax layer (Fig. 1b) also covers the adaxial surface of the blades of Solanum paniculatum, Tabebuia ochracea and Schefflera vinosa and the abaxial surface of the leaves of Protium heptaphyllum.
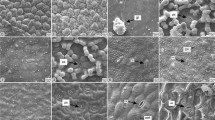
Eletromicrographs of the leaf surface showing epicuticular waxes types. a–f Blade, g Petiole. a Copaifera langsdorffii, crust, b Aegiphila lhotskiana, smooth layer, c Roupala montana, crystalloids in granule shape, d Copaifera langsdorffii, crystalloids in membraneous platelets shape, e Stryphnodendrom adstringens, crystalloids in platelets arranged in rosettes shape, f Matayba elaeagnoides, crystalloids in threads shape, g Xylopia aromatica, epicuticular wax accumulate in sphere shape. Bars a 50 μm, b–f 10 μm, g 100 μm
Eletromicrographs of the leaf surface showing trichomes types. a Brosimum gaudichaudii, simple non-glandular and glandular capitate (arrow), b Bauhinia rufa, simple non-glandular and glandular capitate (arrow), c Aegiphila lhotskiana, non-glandular conical, d Byrsonima intermedia, non-glandular two-armed, e Gochnatia barrosii, non-glandular stellate, f Baccharis dracunculifolia, glandular capitate (arrowhead = papillae). Bars a–b, e 100 μm, c, f 10 μm, d 50 μm
Some species also have crystalloids of wax of different shapes over the smooth layer or crust wax. These crystalloids form granules (Fig. 1c) on the petiole of Roupala montana, Anadenanthera peregrina var. falcata, Copaifera langsdorffii, Machaerium acutifolium, Stryphnodendron obovatum and Ouratea spectabilis and on the adaxial surface of the blades of Roupala montana, Protium heptaphyllum, Matayba elaeagnoides, Solanum paniculatum and Tabebuia ochracea. The crystalloids of wax form membranous platelets (Fig. 1d) on the petiole of Stryphnodendron adstringens, on the adaxial surface of the blades of Luehea grandiflora, on the abaxial surface of the blades of Xylopia aromatica, Copaifera langsdorffii and Dimorphandra mollis and on both surfaces of the leaves of Ocotea pulchella. The crystalloids of wax form platelets arranged in rosette (Fig. 1e) on both surfaces of the blade of Platypodium elegans, Stryphnodendron adstringens and Stryphnodendron obovatum, on the adaxial surface of the leaves of Acosmium subelegans and on the abaxial surface of those of Xylopia aromatica. On the abaxial surface of the Matayba elaeagnoides blade (Fig. 1f), the crystalloids of wax form threads and on the petioles of Xylopia aromatica (Fig. 1g) and of Ocotea pulchella, they accumulates in some points forming spheres.
The blades of Schefflera vinosa and Baccharis dracunculifolia (Fig. 2f) present a striated cuticle under the smooth wax layer.
We could not identify the type of the wax on the petioles of Bauhinia rufa, Solanum paniculatum and Gochnatia polymorpha, and on the abaxial surface of the blades of Cecropia pachystachya, Bauhinia rufa, Luehea grandiflora, Solanum paniculatum, Tabebuia ochracea, Schefflera vinosa, Gochnatia barrosii and Gochnatia polymorpha due to the great amount of trichomes.
Most of studied leaves are hairy (Table 1), although in Anadenanthera peregrina var. falcata, Copaifera langsdorffii, Stryphnodendron obovatum, Casearia sylvestris and Protium heptaphyllum, only the petiole presents trichomes; in Stryphnodendron obovatum they are glandular and simple non-glandular while in the other species there are only simple non-glandular trichomes. Simple non-glandular trichomes (Fig. 2a, b) are also found on the blades of Ocotea pulchella, Xylopia aromatica, Brosimum gaudichaudii, Cecropia pachystachya, Bauhinia rufa, Dimorphandra mollis, Platypodium elegans and Gochnatia polymorpha. Non-glandular conical trichomes (Fig. 2c) occur in Aegiphila lhotskiana, non-glandular two-armed ones (Fig. 2d) in Byrsonima intermedia and Schefflera vinosa and non-galndular stellate (Fig. 2e) in Siparuna guianensis, Solanum paniculatum, Tabebuia ochracea and Gochnatia barrosii. Besides non-glandular trichomes, the leaves of Brosimum gaudichaudii, Bauhinia rufa, Luehea grandiflora, Tabebuia ochracea, Aegiphila lhotskiana and Gochnatia polymorpha also present glandular capitate trichomes (Fig. 2a, b). In Baccharis dracunculifolia (Fig. 2f), the glandular capitate trichomes form scattered groups.
Papillate cells (Fig. 2a) occur on the abaxial surface of the leaves of Xylopia aromatica, Brosimum gaudichaudii, Bauhinia rufa, Dimorphandra mollis and Stryphnodendron adstringens.
Blade
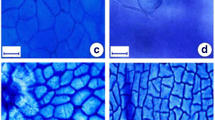
In most of leaves the epidermal cells on the adaxial surface have straight anticlinal walls, in frontal view, as observed for Siparuna guianensis (Fig. 3a). However, these walls are sinuous (Fig. 3b) in Acosmium subelegans, Anadenanthera peregrina var. falcata, Copaifera langsdorffii, Machaerium acutifolium, Byrsomina intermedia, Casearia sylvestris, Tapirira guianensis, Protium heptaphyllum and Aegiphila lhotskiana. The anticlinal walls of the epidermal cells are sinuous (Fig. 3d), on the abaxial surface of the leaves of Siparuna guianensis, Anadenanthera peregrina var. falcata, Copaifera langsdorffii, Byrsonima intermedia, Ouratea spectabilis, Casearia sylvestris, Tapirira guianensis, Protium heptaphyllum, Aegiphila lhotskiana, Schefflera vinosa and Gochnatia polymorpha, and straight (Fig. 3c) in the other species.
Photomicrographs of the leaf surface in frontal view. a, b Adaxial surface: a Siparuma guianensis, with straight anticlinal walls, b Protium heptaphyllum, with sinuous anticlinal walls. c, d Abaxial surface: c Machaerium acutifolium, with straight anticlinal walls and paracytic stomata, d Copaifera langsdorffii, with sinuous anticlinal walls and anomocytics stomata. Bars a, b 70 μm, c, d 30 μm
Most of leaves are hairy, hypostomatic (Figs. 4a, d, e, 5d) and with dorsiventral mesophyll (Figs. 4b–i, 5a–f), (Table 1). Paracytic stomata (Fig. 3c) predominates in the studied species, although anomocytic type (Fig. 3d) occurs in Xylopia aromatica, Anadenanthera peregrina var. falcata, Copaifera langsdorffii, Casearia sylvestris, Protium heptaphyllum, Aegiphila lhotskiana and Schefflera vinosa. The stomata are sunken in Brosimum gaudichaudii, Cecropia pachystachya, Bauhinia rufa and Tabebuia ochracea making impossible to determine their type; we also could not observe the stomata in Solanum paniculatum and Gochnatia polymorpha that have densely hairy leaves.
Photomicrographs of cross-sections of the leaf blade. a Tabebuia ochracea, hypostomatic leaf (arrow = stomata), with two-layered hypodermis and isobilateral mesophyll, b Anadenanthera peregrina var. falcata, amphistomatic leaf (arrow = stomata), with palisade tissue representing 50% of the mesophyll, c Brosimum gaudichaudii, with thick cuticle on the adaxial surface and with coniform papillae (arrowhead) on the abaxial surface, d Gochnatia polymorpha, with thick cuticle on both surface and with prominent stomata (arrow), e Roupala montana, showing epidermal cells with thick outer periclinal wall and sclerified cells (sc) adjacent to the leaf surfaces, f Schefflera vinosa, with one-layered hypodermis of sclerified cell, g Stryphnodendron adstringens, showing thin cuticle and spongy parenchyma compactly arranged, h Siparuna guianensis, showing palisade parenchyma with ibioblasts containing phenolic compounds (pc), i Copaifera langsdorffii, with sclerified cells (sc) associated to the vascular system. Bars a, c, d, h 30 μm, b 35 μm, e–g, i 70 μm
Photomicrographs of cross-sections of the leaf blade. a Luehea grandiflora, with two-layered epidermis on the adaxial surface, b Dimorphandra mollis, showing epidermal cells with mucilage on adaxial surface and cupuliform papillae (arrowhead) on the abaxial surface, c Bauhinia rufa, with phenolic compounds (pc) in the epidermal cells, in the extension of the vascular bundle sheath and in the vascular bundle and with coniform papillae (arrowhead) on the abaxial surface, d Bredemeyera floribunda, showing hypostomatic leaf (arrow = stomata) with dorsiventral mesophyll and with palisade tissue little grown, e Byrsonima intermedia, with spongy parenchyma loosely arranged and idioblast containing crystal (cr), f Xylopia aromatica, with one-layered hypodermis of parenchymatous cells, g Acosmium subelegans, showing blade edge straight and sharp, h Roupala montana, with blade edge rounded; to notice sclerified cells (sc), i Schefflera vinosa, showing blade edge rounded with subepidermic sclerified cells (sc); j Brosimum gaudichaudii, with blade edge slightly revolute and subepidermic collenchyma cells, k Aegiphila lhotskiana, with blade edge slightly revolute, l Dimorphandra mollis, with blade edge rounded. Bars a–d, f 3 μm, e, g–l 10 μm
Thin cuticles (Fig. 4g) predominate on the studied leaves, although some species have a thick cuticle on both surfaces (Figs. 4d, 5e), (Table 1). Some leaves are covered by a thick cuticle on the adaxial surface and by a thin one on the abaxial surface (Fig. 4c, f; Table 1).
Epidermal cells with thin outer periclinal wall occur on both surfaces (Fig. 4g) or only on the abaxial side (Fig. 4d, 5a) in half of the studied leaves (Table 1); the other half have thickened and lignified walls on both surfaces (Figs. 4b, e, i; 5b, d; Table 1).
On the adaxial surface, the epidermal cells have flat periclinal walls (Figs. 4a, c, e, f, i, 5d) in Ocotea pulchella, Siparuna guianensis, Roupala montana, Brosimum gaudichaudii, Cecropia pachystachya, Copaifera langsdorffii, Machaerium acutifolium, Bredemeyera floribunda, Ouratea spectabilis, Tapirira guianensis, Protium heptaphyllum, Matayba elaeagnoides, Solanum paniculatum, Tabebuia ochracea, Aegiphila lhotskiana and Schefflera vinosa. The outer periclinal wall is flatten and the inner is convex (Figs. 4b, 5a, b, e) in Acosmium subelegans, Anadenanthera peregrina var. falcata, Dimorphandra mollis, Platypodium elegans, Byrsonima intermedia, Casearia sylvestris and Luehea grandiflora. The outer periclinal wall is convex and the inner is flat (Fig. 4d) in Stryphnodendron obovatum, Gochnatia barrosii and Gochnatia polymorpha and both periclinal walls are convex (Figs. 4g, 5f) in Xylopia aromatica, Bauhinia rufa, Stryphnodendron adstringens and Baccharis dracunculifolia.
The leaves of Xylopia aromatica (Fig. 5f), Dimorphandra mollis (Fig. 5b) and Stryphnodendron adstringens (Fig. 4g), present cupuliform papillae on the abaxial surface; on those of Brosimum gaudichaudii (Fig. 4c) and Bauhinia rufa (Fig. 5c), such papillae are coniform.
In most studied leaves the stomata are on the same level as the epidermal cells (Figs. 4a, b, e, 5d), although in Cecropia pachystachya, Luehea grandiflora, Solanum paniculatum, Aegiphila lhotskiana, Gochantia barrosii and Gochnatia polymorpha they are prominent (Fig. 4d).
The epidermis is two-layered on the adaxial surface in Luehea grandiflora (Fig. 5a). In Siparuna guianensis (Fig. 4h), Xylopia aromatica (Fig. 5f), Cecropia pachystachya, Matayba elaeagnoides and Schefflera vinosa (Fig. 4f) there is a one-layered hypodermis of sclerified cells in Schefflera vinosa and of parenchymatous cells in the other species. In Tabebuia ochacea (Fig. 4a) the two-layered hypodermis is parenchymatous.
The palisade parenchyma fills almost half of the mesophyll (Figs. 4b, f, 5a, e) in most studied leaves (Table 1); it occupies more than 50% (Figs. 4i, 5b, c, f) in some species and less than 50% (Figs. 4e, g-h, 5d) in others, (Table 1). In Dimorphandra mollis (Fig. 5b), the palisade tissue is one-layered with periclinally elongated cells. In the other species, it is two or three-layered. The cells of the spongy parenchyma are compactly arranged (Figs. 4b, g; 5d), in some of the studied leaves, but have many intercellular spaces in most mesophylls (Figs. 4e, f, h; 5e), (Table 1).
Sclerified cells of variable wall thickening are associated to the vascular system (Figs. 4i, 5c) of most leaves, although in Roupala montana (Fig. 4e) the sclerified cells are adjacent to the surfaces, (Table 1).
The blade edge is slightly revolute (Fig. 5i–l) in most studied leaves and straight (Fig. 5g-h) in Ocotea pulchella, Roupala montana, Acosmium subelegans, Anadenanthera peregrina var. falcata, Copaifera langsdorffii, Stryphnodendron adstringens, Bredemeyera floribunda, Ouratea spectabilis and Baccharis dracunculifolia. It has a rounded shape (Fig. 5h–l) in most leaves and a sharp shape (Fig. 5g) in Siparuna guianensis, Acosmium subelegans, Ouratea spectabilis, Luehea grandiflora, Matayba elaeagnoides and Gochnatia barrosii.
In the leaf edge, the cuticle is usually thin but it is thick (Fig. 5j, k) in Ocotea pulchella, Brosimum gaudichaudii, Copaifera langsdorffii, Platypodium elegans, Byrsonima intermedia, Aegiphila lhotskiana, Schefflera vinosa, Baccharis dracunculifolia and Gochnatia polymorpha. The epidermal cells at the leaf edges have thin outer periclinal walls (Fig. 5j, k) in Siparuna guianensis, Brosimum gaudichaudii, Cecropia pachystachya, Aegiphila lhotskiana, Baccharis dracunculifolia, Gochnatia barrosii and Gochnatia polymorpha; in the other species, the outer periclinal wall is thickened and lignified (Fig. 5g–i, l).
The leaf edges of Ocotea pulchella, Roupala montana (Fig. 5h), Matayba elaeagnoides and Schefflera vinosa (Fig. 5i) presents subepidermic sclerified cells and those of Brosimum gaudichaudii (Fig. 5j) have collenchyma cells. In the other species, this region is parenchymatous (Fig. 5g, k, l).
The midrib has an abaxially dislocated vascular system originating a prominent vein (Fig. 6a, e, h) in Brosimum gaudichaudii, Cecropia pachystachya, Bauhinia rufa, Luehea grandiflora, Matayba elaeagnoides, Solanum paniculatum, Tabebuia ochracea, Gochnatia barrosii and Gochnatia polymorpha, (Table 1). The vascular system is slightly dislocated (Fig. 6b, d, f) in Siparuna guianensis, Roupala montana, Bredemeyera floribunda, Aegiphila lhotskiana and Schefflera vinosa and occupies the same level as the mesophyll (Fig. 6c, g, i), in the other species, (Table 1).
Photomicrographs and stereomicrographs of cross-sections of the leaf blade in the midvein region. a Solanum paniculatum, with main vascular system in continuous arch shape, b Schefflera vinosa, showing main vascular system in continuous arch shape with collateral bundles distributed adjacent to the adaxial surface; to notice secretory cavities (scv) and sclerified cells (sc) in the cortex, c Byrsonima intermedia, showing main vascular system in continuous arch shape with a single amphicribal bundle in an adaxial position, d Siparuna guianensis, showing main vascular system in continuous arch shape with a collateral bundle in an adaxial position; to notice the presence of idioblasts containing phenolic compounds (pc), e Brosimum gaudichaudii, showing main vascular bundle in continuous arch shape with a single amphivasal bundle in an adaxial position, f Roupala montana, with main vascular system in arch interrupted shape, surrounded by sclerified cells and with sclerified cells (sc) in the cortex, g Ouratea spectabilis, with main vascular system in circle interrupted shape, surrounded by sclerified cells, h Tabebuia ochracea, with main vascular system in circle interrupted shape, surrounded by sclerified cells, i Baccharis dracunculifolia, with main vascular system formed by a single collateral bundle. Bars a, c, d 15 μm, b, e, h 200 μm, f, i 70 μm, g 4 μm
The adaxial region of the midrib is convex in most leaves (Fig. 6a–d, g–h); however, it is concave (Fig. 6e, i) in Xylopia aromatica, Brosimum gaudichaudii, Bauhinia rufa, Bredemeyera floribunda and Baccharis dracunculifolia and flat (Fig. 6f) in Ocotea pulchella and Roupala montana.
The vascular system of the midrib is formed by collateral bundles in all species, except for Solanum paniculatum with bicolateral bundles (Fig. 6a). The bundles can form either a continuous arch (Fig. 6a–e) or an arch interrupted by parenchyma (Fig. 6f); they can also be arranged in a circle interrupted or not by parenchyma (Fig. 6g, h), (Table 1). In the leaves of Baccharis dracunculifolia (Fig. 6i), the midrib vein is formed by a single collateral bundle (Table 1).
Besides the main vascular bundles, there are also small isolated bundles in the midrib of various species. Such bundles are collateral and occur adjacent to the adaxial surface (Fig. 6b, d) in Siparuna guianensis, Cecropia pachystachya, Luehea grandiflora, Matayba elaeagnoides, Aegiphila lhotskiana and Schefflera vinosa. There is a single amphivasal bundle in adaxial position in Brosimum gaudichaudii (Fig. 6e) and a single amphicribal in adaxial position in Byrsonima intermedia (Fig. 6c).
For the compound pinnate leaves of the Leguminosae, Anacardiaceae and Burseraceae, the central vascular system of the leaflets is not considered a midvein, since it represents higher orders of ramification.
Except for Solanum paniculatum (Fig. 6a), Brosimum gaudichaudii (Fig. 6e) and Cecropia pachystachya, the midrib vascular system is surrounded by sclerified cells (Table 1), usually forming a thick sheath (Fig. 6f–h). Sclerified cells also appear in the cortical region of the midvein (Fig. 6b, f) of the leaves of Ocotea pulchella, Roupala montana, Byrsonima intermedia, Ouratea spectabillis, Luehea grandiflora, Matayba elaeagnoides and Schefflera vinosa (Table 1).
Petiole
The petioles are usually covered by a thin cuticle, although it is thick in Ocotea pulchella, Siparuna guianensis, Cecropia pachystachya, Bredemeyera floribunda, Solanum paniculatum, Gochnatia barrosii, and Gochnatia polymorpha.
In cross-section, the epidermal cells have thickened walls in most studied species. The outer periclinal wall of these cells is of primary nature in Gochnatia barrosii and Gochnatia polymorpha but it presents a secondary thickening in the petioles of Xylopia aromatica, Brosimum gaudichaudii, Anadenanthera peregrina var. falcata, Bauhinia rufa, Copaifera langsdorffii, Dimorphandra mollis, Machaerium acutiolium, Platypodium elegans, Stryphnodendron adstringens, Casearia sylvestris, Luehea grandiflora, Tabebuia ochracea and Aegiphila lhotskiana. The anticlinal walls of the epidermal cells of some species (Ocotea pulchella, Roupala montana, Acosmium subelegans, Stryphnodendron obovatum, Bredemeyera floribunda, Ouratea spectabilis, Tapirira guianensis, Matayba elaeagnoides and Schefflera vinosa) present secondary thickenings.
The petiole cortex is parenchymatous in Xylopia aromatica, Roupala montana, Acosmium subelegans, Anadenanthera peregrina var. falcata, Bauhinia rufa, Copaifera langsdorffii, Dimorphandra mollis, Platypodium elegans, Stryphnodendron adstringens, Stryphnodendron obovatum and Matayba elaeagnoides. In the other species, this region is formed by an outer collenchyma and by an inner parenchyma (Fig. 7c). Sclerified cells are common in the petiole cortex (Fig. 7e) of Roupala montana, Stryphnodendron obovatum, Ouratea spectsbilis, Matayba elaeagnoides and Gochnatia polymorpha.
Stereomicrographs of cross-sections of the petiole. a Casearia sylvestris, with main vascular system in continuous arch shape; to notice the presence of idioblasts containing phenolic compounds (pc), b Siparuna guianensis, showing main vascular system in continuous arch shape with two collateral bundles in an adaxial position; to notice the presence of idioblasts containing phenolic compounds (pc), c Solanum paniculatum, showing main vascular system in interrupted arch shape with two amphicribal bundles dislocated towards the adaxial surface, d Gochnatia barrosii, with main vascular system in interrupted arch shape, e Gochnatia polymorpha, showing main vascular system in interrupted arch shape with two collateral bundle in an adaxial position; to notice vascular system surrounded by sclerified cells and the presence of sclerified cells (sc) in the cortex, f Tabebuia ochracea, with main vascular system in continuous circle shape, g Cecropia pachystachya, with main vascular system in interrupted circle shape, surrounded by amphicribal bundles, h Protium heptaphyllum, with main vascular system in continuous triangle shape, surrounded by secretory cavities (scv) involved by phloem elements, i Stryphnodendron adstringens, showing main vascular system in interrupted triangle shape with amphicribal bundles in an adaxial position. Bars a 15 μm, b, e 4 μm, c, d, f, i 50 μm, g 70 μm, h 60 μm
Except for Solanum paniculatum, with bicolateral bundles (Fig. 7c), the vascular system is formed by collateral bundles. These bundles are arranged in a continuous arch (Fig. 7a, b), in some species, and in an arch interrupted by parenchyma (Fig. 7c–e), in others, (Table 1). In Bauhinia rufa and in Luehea grandiflora, this arch is formed by four isolated groups and by three to four vascular groups, respectively. In some species, the vascular tissues are arranged in a continuous circle (Fig. 7f) whereas in others, this circle is interrupted by parenchyma (Fig. 7g), (Table 1). Vascular tissues organized in a continuous (Fig. 7h) or interrupted (Fig. 7i) triangle is also present in some petioles, (Table 1).
Besides the main vascular system, there are some isolated collateral bundles adjacent to the abaxial surface in Bauhinia rufa, and close to the adaxial surface (Fig. 7e) in Siparuna guianensis, Acosmium subelegans, Anadenanthera peregrina var. falcata, Copaifera langsdorffii, Dimorphandra mollis, Platypodium elegans, Stryphnodendron obovatum, Luehea grandiflora, Aegiphila lhotskiana and Gochnatia polymorpha. The isolated bundles are amphivasal and adjacent to the abaxial surface in Roupala montana and are amphicribal and dislocated towards the adaxial surface (Fig. 7c, i) in Solanum paniculatum, Copaifera langsdorffii and Stryphnodendron adstringens. Isolated vascular bundles also appear in the medulla of the petioles of Schefflera vinosa while in Brosimum gaudichaudii, there are groups of phloem cells in this same region. In Cecropia pachystachya, the vascular system of the petioles is surrounded by amphicribal bundles (Fig. 7g) and in Protium heptaphyllum it is surrounded by secretory cavities involved by phloem elements (Fig. 7h).
The vascular system of the petioles is surrounded by sclerified cells, except for Brosimum gaudichaudii, Cecropia pachystachya (Fig. 7g), Bredemeyera floribunda and Solanum paniculatum (Fig. 7c). Such sclerified cells are continuously distributed in most petioles (Fig. 7f), but are sparsely arranged (Fig. 7d, h) in Siparuna guianensis, Bauhinia rufa, Casearia sylvestris, Luehea grandiflora, Tapirira guianensis, Protium heptaphyllum and Aegiphila lhotskiana.
The leaves of Byrsonima intermedia and Baccharis dracunculifolia are semi-sessile.
Secretory sites (Figs. 4i, 5f, 6b, 7h) and epidermal cells with mucilage (Fig. 5b) or with phenolic compounds (Fig. 5c) were observed in few leaves. Idioblasts containing phenolic compounds (Figs. 4h, 5c, 6d, 7a, b) and crystals (Fig. 5e) occur in around 37% of the species (Table 1) being more frequent in the petiole.
Discussion
If we compare the present results (Table 1) with those reported for other regions of cerrado of the Brazil (Table 2), we can suggest that the woody plants of this biome show a leaf anatomical pattern characterized by leaves with: (a) a thick coating and many trichomes; (b) stomata limited to the abaxial surface; (c) dorsiventral mesophyll with developed palisade parenchyma (ca 50% or more of the mesophyll thickness); (d) sclerified cells surrounding the vascular tissues and sparsely distributed in the mesophyll; (e) idioblasts containing phenolic compounds and crystals.
The thick coating that covers most leaves of the cerrado is constituted by epicuticular waxes and a cuticle. The epicuticular waxes, whose synthesis may be induced by the high luminous intensity (Cutter 1986), reduce leaf transpiration (Eglinton and Hamilton 1967; Jeffree 1986; Oliveira et al. 2003) and protect the plant from the excess of radiation (Eglinton and Hamilton 1967; Wilkinson 1979) and from herbivory (Eglinton and Hamilton 1967; Jeffree 1986). As the waxes, the cuticle has also been associated to the reduction of transpiration (Pyykkö 1966; Mortenson 1973; Riederer and Schreiber 2001) and, according to Larcher (2004) and Taiz and Zeiger (2004), plants subjected to water deficiency have leaves with more thickened cuticles. As the waxes too, the cuticle protects the leaves from solar radiation by absorbing the ultraviolet rays (Larcher 2004).
As the epicuticular waxes, the glandular and non-glandular trichomes, usually found in the cerrado leaves (Tables 1, 2), protect the plant from phytophagous animals (Johnson 1975; Theobald et al. 1979); as the waxes and the cuticle, the trichomes also reduce water loss through transpiration since they reflect the solar rays, avoiding leaf heating (Fahn and Cutler 1992; Larcher 2004).
In cerrado habitats, the set waxes + cuticle + trichomes thus seems to protect the leaf from the heating and the excessive radiation due to high luminosity that prevails in this biome. This high luminosity probably induces the synthesis of those thick wax layers, as asserted by Cutter (1986) for plants living in open habitats.
The set waxes + cuticle + trichomes probably also reduces leaf transpiration, since, as mentioned above, the woody plants of the cerrado usually have physiological mechanisms that limit water loss, even when they are not subjected to severe water stress (see Moraes et al. 1989; Perez and Moraes 1991; Mattos et al. 1997; Franco 1998; Moraes and Prado 1998; Meinzer et al. 1999; Prado et al. 2004).
As those of xerophytic plants (Pyykkö 1966; Juniper and Jeffree 1983; Fahn and Cutler 1992), the leaves of the cerrado are usually covered by epidermal cells with thickened and lignified outer periclinal walls, on both surfaces or only on the adaxial side (Tables 1, 2). The fact that such walls are usually flat suggests that they also help to reflect the light because, according to Voguelmann (1993), convex walls increase light capture.
In the cerrado leaves, a 1:1 ratio between palisade and spongy parenchyma (Tables 1, 2) prevails. According to Mortenson (1973), developed palisade parenchyma is common in plants living under satisfactory water conditions, as the woody species of the cerrado. Such plants are usually evergreen (Cole 1986) and use physiological mechanisms, as the fall in stomatal conductance, to guarantee the stability of their leaf water condition, even when water is scarce (see Moraes et al. 1989; Perez and Moraes 1991; Mattos et al. 1997; Franco 1998; Moraes and Prado 1998; Meinzer et al. 1999; Prado et al. 2004). For Voguelmann (1993), a developed palisade parenchyma is important in the case of sun leaves, such as those of the cerrado woody plants, because it distributes light in a uniform way to all layers of this organ.
Secretory cavities as well as idioblasts containing crystals and phenolic compounds are frequent in the cerrado species (Tables 1, 2). According to Varanda et al. (1998), plants growing on poor soils, like those of cerrado, usually deviate the biosynthetic ways to produce defense compounds, like phenolic compounds, due to the high cost to replace the material lost through the action of herbivores. On the other hand, the generalized occurrence of calcium oxalate in the cerrado plants suggests, according to Handro (1966), that they concentrate calcium since this element is found in very small amounts in the soils of this biome.
Revolute leaf edges that, according to Mortenson (1973), are often found in xeromorphic leaves appear in 70% of the studied species although other cerrado species show no predominance of a particular edge type (see Morretes and Ferri 1959; Morretes 1967, 1969; Beiguelman 1962a, b, c, d; Bieras and Sajo 2004).
In most leaves of the cerrado, the vascular system of the petiole and of the midvein is well developed and formed by various bundles arranged in arch, circle or triangle. It usually have the same configuration in the petioles and the midvein, although in some cases the vascular system of the petiole split on originating some isolated vascular bundles adaxially arranged in the midvein region. In most leaves, the midveins are prominent and dislocated towards the abaxial face, due to the grouping of bundles in this region. Although it has not been demonstrated, this great amount of vascular tissues probably provide a quick movement of the water to the entire organ since the cerrado leaves are usually mesophyll size (4,500–18,225 mm2) (Bieras and Sajo unpublished) and present dense venation (see Sajo and Rudall 2002; Bieras and Sajo 2004; Reis et al. 2004).
Sclerified cells in the mesophyll and around the vascular system are common in the cerrado leaves, as observed for xerophytic plants (Pyykkö 1966; Fahn and Cutler 1992). According to Pyykkö (1966), such cells prevent the cellular collapse of the mesophyll when the leaf loses water.
Different theories have been proposed to explain the origin of the cerrado based on climatic, anthropic and pedologic factors (Rizzini 1976). The cerrado seems to have appeared some time in the Quaternary, between the Miocene and the Pleistocene, when the recurrent Neotropical aridity favored the expansion of savannas (Fernandes 2000). With the rainfall reduction and the consequent forests retraction, only the ecotypes of forest widely distributed would have contributed to the diversification of the sclerophyllous vegetations (Rizzini 1997). The scattered rains that then prevailed would have carried away huge amounts of exchangeable bases from the soil surface, increasing its acidity and aluminum concentrations (Sarmiento 1984). During the Holocene, with the return of humidity, the leaching process would have increased, thus favoring the expansion of the cerrado and the retraction of the mesophytic forests (Furley 1999). Due to vicariant mechanisms (Rizzini 1971; Heringer et al. 1977), forest species from the Amazonian and Atlantic basins contributed to form a rich, complex savanna flora with various endemisms (Franco 2002). The species occupying this environment in formation had to adapt first to the humidity reduction and to the soil acidity and toxicity increase and, later, to the increase of the frequent fires (Furley 1999).
It therefore seems consistent to think that the soil participated in the cerrado distribution, as pointed out by Alvim and Araujo (1952) and by Alvin (1954), since its plants grow on acid and poor in exchangeable bases substrata, especially in calcium. In 1958, Arens proposed the theory of the oligotrophic scleromorphism according to which the woody species of the cerrado are not subjected to water stress since their deep roots reach the deep water. So, the plants carry out intense photosynthesis but, due to the lack of nitrogen and phosphorus, they do not consume all the carbon produced, which accumulate as cuticle and thick cell walls, originating scleromorphic organs. According to Goodland (1971), part of this scleromorphism was caused by the toxicity of the aluminum present in high concentrations in the cerrado soils. Studying the relationship between the vegetation and the soil features in different cerrado physiognomies, Lopes and Cox (1977) concluded that, together, the theories of the oligotrophic scleromorphism (Arens 1958) and of the aluminotoxic scleromorphism (Goodland 1971) explain the cerrado occurrence. Also for Aoki and Santos (1979), the cerrado occurrence is more related to edaphic than climatic factors, not only because of its wide distribution on Brazil, where the rainfall and temperature conditions are quite variable, but also because forest and cerrado are found in contact and under the same climate.
According to Veloso (1964), the semideciduous seasonal forest, the cerrado and the caatinga originate from a same floristic trunk and the dominance of some families in a given area results from the higher ability of these plants to support the soil deficiency in water or nutrients.
The fire, a frequent abiotic factor in the cerrado does not interfere with the quality of soils because, according to Coutinho (1990), the ashes improve the nutritional content of the substrata for up to 60 days; however, these nutrients are not leached to the deeper layers and only benefit herbaceous plants and subshrubs, which have superficial roots.
Leaf scleromorphism, reported for other vegetations, has been also interpreted as a result of the soil deficiency. The scleromorphic features of some succulents from deserts have been associated to the lack of nitrogen in the soil (Evenari 1949) and the sclerophyllous plants of the Central America tolerate low phosphorus levels in the soil by reducing their protein synthesis and increasing their quantity of fibers (Loveless 1961). According to Araujo and Mendonça (1998), specimens of Aldina heterophylla (Leguminosae) show a marked trend to scleromorphism in Amazonian regions of “campina aberta”, where the soil is poor, the light is intense and the temperature is high. The scleromorphic structures present in some orchids from the “campinas abertas” of the Amazonian be also related to the low level of soil fertility (Bonates 1993). Studying the vegetation of “bana” in Venezuela, Sobrado and Medina (1980) noted an increase of sclerophylly as a response to the oligotrophic conditions of the soil and to the broad fluctuations in the water levels.
As mentioned above, some xeromorphic features (hairy surfaces, thick coating, stomata limited to the inferior surface, developed palisade parenchyma, sclerified cells in the mesophyll and around the vascular bundles, revolute edges and developed main vascular system) are constant in the leaves of the woody species of the cerrado (Tables 1, 2). On the other hand, such features are not restricted to the representative plants of this biome, but usually appear in the other members of the same families they belong (see Metcalfe and Chalk 1957). This suggests that the xeromorphism observed for the cerrado leaves is related to the evolutionary history of this biome since its first floristic elements must have faced deficient water conditions as well as the consequent soil acidity and toxicity. We may thus infer that the leaf anatomical pattern here observed was already present in the first elements of the cerrado and was selected to guarantee the survival of those species in the new environment. The xeromorphic features present in those leaves continue nowadays to help the plants protecting themselves from the different biotic and abiotic factors they are subjected to.
References
Alvim P, Araujo WA (1952) El suelo como factor ecológico em el desarrollo de la vegetación en el centro-oeste del Brasil. Turrialba 2:153–160
Alvin PT (1954) Teoria sobre a formação dos campos cerrados. Rev Bras Geogr 4:496–498
Aoki H, Santos JR (1979) Fatores ambientais dos cerrados e imagens orbitais. Bol Tec Inst Florest 31:1–69
Araujo MGP, Mendonça MS (1998) Escleromorfismo foliar de Aldina heterophylla Spruce ex Benth (Leguminosae: Papilionoideae) em três campinas da amazônia central. Acta Amazon 28:353–371
Arens K (1958) O cerrado como vegetação oligotrófica. Bol Bot USP 15:59–77
Barthlott W, Neinhuis C, Cutler DF, Ditsch F, Meusel I, Theisen I, Wilhelmi H (1998) Classification and terminology of plant epicuticular waxes. Bot J Linn Soc 126:237–260
Beiguelman B (1962a) Contribuição para o estudo anatômico das plantas do cerrado: I. Anatomia da folha e do caule de Erythroxylum suberosum St Hil. Rev Biol 3:97–110
Beiguelman B (1962b) Contribuição para o estudo anatômico das plantas do cerrado: II. Anatomia da folha e do caule de Byrsomina coccolobifolia kth. Rev Biol 3:111–123
Beiguelman B (1962c) Contribuição para o estudo anatômico das plantas do cerrado: III. Anatomia da folha e do caule de Annona coriacea Mart. Rev Biol 4:1–12
Beiguelman B (1962d) Contribuição para o estudo anatômico das plantas do cerrado: I. Anatomia da folha e do caule de Ouratea spectabilis (Mart.). Engl. Rev Biol 4:13–26
Beiguelman B (1963) Considerações sobre a vegetação dos cerrados. Cienc Cult 15:39–44
Bicudo LRH, Cesar O, Monteiro R (1996) Florística comparativa de uma área de cerrado no município de Botucatu, SP (Brasil). Braz Arch Biol Technol 39:685–691
Bieras AC, Sajo MG (2004) Anatomia foliar de Erythroxylum P. Browne (Erythroxylaceae) do Cerrado do estado de São Paulo. Acta Bot Bras 18:601–612. doi:10.1590/S0102-33062004000300018
Bonates LCM (1993) Estudos ecofisiológicos de Orchidaceae da Amazônia. II–Anatomia ecológica foliar de espécies com metabolismo CAM de uma campina na Amazônia central. Acta Amazon 23:315–348
Castro AAJF, Martins FR (1999) Cerrados do Brasil e do nordeste: caracterização, área de ocupação e considerações sobre a sua fitodiversidade. Pesqui Foco 7:147–178
Castro AAJF, Martins FR, Tamashiro JY, Shepherd GJ (1999) How rich is the flora of brazilian cerrados? Ann Mo Bot Gard 86:192–224. doi:10.2307/2666220
Cole MM (1986) The savannas: biogeography and geobotany. Academic Press, London
Coutinho LM (1990) O cerrado e a ecologia do fogo. Cienc Hoje 12:23–30
Coutinho LM (2002) O bioma cerrado. In: Klein AL (org) Eugen Warming e o cerrado brasileiro: um século depois. Editora da Unesp, São Paulo, pp 77–91
Cutter EG (1986) Anatomia Vegetal, Parte I: células e tecidos. Editora Roca, São Paulo
Durigan G, Leitão Filho HF, Rodrigues RR (1994) Phytosociology and structure of a frequently burnt cerrado vegetation in SE-Brasil. Flora 189:153–160
Durigan G, Ratter JA, Bridgewater S, Siqueira MFS, Franco GADC (2003a) Padrões fitogeográficos do cerrado paulista sob uma perspectiva regional. Hoehnia 30:39–51
Durigan G, Siqueira MF, Franco GADC, Bridgewater S, Ratter JA (2003b) The vegetation of priority areas for cerrado conservation in São Paulo state, Brazil. Edinb J Bot 60:217–241. doi:10.1017/S0960428603000155
Durigan G, Baitello JB, Franco GADC, Siqueira MF (2004) Plantas do Cerrado Paulista: Imagens de uma paisagem ameaçada. Páginas & Letras Editora e Gráfica, São Paulo
Eglinton G, Hamilton RJ (1967) Leaf epicuticular waxes. Science 156:1322–1335. doi:10.1126/science.156.3780.1322
Evenari M (1949) Ecologia de las plantas de desierto. Rev Arg Agron 16:121–148
Fahn A, Cutler DF (1992) Xerophytes. Gebrüder Borntraeger, Berlin
Fernandes A (2000) Fitogeografia brasileira. Multigraf, Fortaleza
Franco AC (1998) Seasonal patterns of gas exchange, water relations and growth of Roupala montana an evergreen savanna species. Plant Ecol 136:69–76. doi:10.1023/A:1009763328808
Franco AC (2002) Ecophysiology of woody plants. In: Oliveira PS, Marquis RJ (eds) The cerrado of Brazil: ecology and natural history of a neotropical savanna. Columbia University Press, New York, pp 178–197
Furley PA (1999) The nature and diversity of neotropical savanna vegetation with particular reference to the Brazilian cerrados. Glob Ecol Biogeogr 8:223–241
Goodland R (1971) Oligotrofismo e aluminio no cerrado. In: Ferri MG (coord) III Simpósio sobre o cerrado. Editora da USP, São Paulo, pp 44–60
Handro W (1966) Escleromorfismo foliar e nutrição mineral de Gomphrena prostata (Mart.). An Acad Bras Cienc 38:225–242
Handro W (1967) Contribuição ao estudo da venação e anatomia foliar das amarantáceas dos cerrados. II- Gênero Pfaffia. An Acad Bras Cienc 39:495–506
Heringer EP, Barroso GM, Rizzo JA, Rizzini CT (1977) A flora do cerrado. In: Ferri MG (coord) IV Simpósio sobre o cerrado. EDUSP, São Paulo, pp 211–232
Jeffree CE (1986) The cuticle, epicuticular waxe and trichomes of plants, with reference to their structure, functions and evolution. In: Juniper SR, Southwood SR (eds) Insects and the plant surface. Edward Arnold, London, pp 23–64
Johansen DA (1940) Plant microtechnique. Mac Graw-Hill, New York
Johnson HB (1975) Plant pubescence: an ecological perspective. Bot Rev 41:233–258. doi:10.1007/BF02860838
Juniper BE, Jeffree CE (1983) Plant surfaces. Edward Arnold, London
Kraus J, Arduin M (1997) Manual básico de métodos em morfologia vegetal. EDUR, Rio de Janeiro
Larcher W (2004) Ecofisiologia Vegetal. RiMa, São Carlos
Leitão Filho HF (1992) A flora arbórea dos cerrados do estado de São Paulo. Hoehnea 19:151–163
Lopes AS, Cox FR (1977) Cerrado vegetation in Brazil: an edaphic gradient. Agron J 69:828–831
Loveless AR (1961) A nutricional interpretation of sclerophylly based on differences in the chemical composition of sclerophyllous and mesophytic leaves. Ann Bot (Lond) 25:168–184
Malavolta E, Kliemann HJ (1985) Desordens nutricionais no cerrado. POTAFOS, Piracicaba
Mattos EA, Reinert F, Moraes JAPV (1997) Comparison of carbon isotope discrimination and CO2 and H2O gas exchange between the dry and the wet season in leaves of several cerrado woody species. Braz J Plant Physiol 9:77–82
Meinzer FC, Goldstein G, Franco AC, Bustamante M, Igler E, Jackson P, Caldas L, Rundel PW (1999) Atmospheric and hydraulic limitations on transpiration in brazilian cerrado woody species. Funct Ecol 13:273–282. doi:10.1046/j.1365-2435.1999.00313.x
Mendonça RC, Felfili JM, Walter BMT, Silva Junior MC, Rezende AV, Filgueiras TS, Nogueira PE (1998) Flora vascular do cerrado. In: Sano SM, Almeida SP (coords) Cerrado: ambiente e flora. EMBRAPA-CPAC, Planaltina, pp 289–556
Metcalfe CR, Chalk L (1957) Anatomy of the dicotyledons––leaves, stem and wood in relation to taxonomy with notes on economic uses. Clarendon Press, Oxford
Moraes JAPV, Perez SCJGA, Carvalho LF (1989) Curso diário e sazonal do potencial da água e da resistência estomática em plantas de um Cerradão. Ann Mo Bot Gard 27:13–23
Moraes JAPV, Prado CHBA (1998) Photosynthesis and water relations in Cerrado vegetation. In: Scarano FR, Franco AC (eds) Ecophysiological strategies of xerophytic and amphibious plants in the neotropics. Serie Oecologia Brasiliensis vol IV. PPGE-UFRJ, Rio de Janeiro, pp 45–63
Morretes BL (1967) Contribuição ao estudo da anatomia das folhas de plantas do cerrado II. Bol Bot USP 22:209–244
Morretes BL (1969) Contribuição ao estudo da anatomia das folhas de plantas do cerrado III. Bol Bot USP 24:7–32
Morretes BL, Ferri MG (1959) Contribuição ao estudo da anatomia das folhas de plantas do cerrado. Bol Bot USP 16:7–70
Mortenson TH (1973) Ecological variation in the leaf anatomy of selected species of Cercocarpus. Aliso 8:19–48
Oliveira AFM, Meirelles ST, Salatino A (2003) Epicuticular waxes from caating and cerrado species and their efficiency against water loss. An Acad Bras Cienc 75:431–439. doi:10.1590/S0001-37652003000400003
Panizza S (1967) Contribuição ao estudo morfológico e anatômico de Jacaranda caroba (Velloso) D.C. Bignoniaceae. Revta Fac Farm Bioq USP 5:93–106
Paviani TI, Ferreira ML (1974) Anatomia foliar de Plathymenia reticulata Benth. Braz J Biol 34:159–176
Perez SCJG, Moraes JAPV (1991) Curso diário e sazonal do potencial de água e da condutância estomática em espécies de cerradão. Braz J Biol 51:801–804
Prado CHDA, Wenhui Z, Rojas MHC, Souza GM (2004) Seasonal lesf gas exchange and water potencial in a woody cerrado species community. Braz J Plant Physiol 16:7–16. doi:10.1590/S1677-04202004000100002
Pyykkö M (1966) The leaf anatomy of East Patagonian xeromorphic plants. Ann Bot Fenn 3:453–622
Ratter JA, Ribeiro JF, Bridgewater S (1997) The brazilian cerrado vegetation and threats to its biodiversity. Ann Bot (Lond) 80:223–230. doi:10.1006/anbo.1997.0469
Ratter JA, Bridgewater S, Ribeiro JF, Dias TAB, Silva MR (2000) Estudo preliminar da distribuição das espécies lenhosas da fitofisionomia cerrado sentido restrito nos Estados compreendidos pelo bioma cerrado. Bol Herb Ezechias Paulo Heringer 5:5–43
Reis C, Proença SL, Sajo MG (2004) Vascularização foliar e anatomia do pecíolo de Melastomataceae do cerrado do estado de São Paulo, Brasil. Acta Bot Bras 18:987–999. doi:10.1590/S0102-33062004000400029
Reis C, Bieras AC, Sajo MG (2005) Anatomia foliar das Melastomataceae do cerrado do estado de São Paulo. Braz J Bot 28:451–466
Ribeiro JF, Walter BMT (1998) Fitofisionomias do Bioma Cerrado. In: Sano SM, Almeida SP (coords) Cerrado: ambiente e flora. EMBRAPA-CPAC, Planaltina, pp 89–166
Ribeiro LF, Tabarelli M (2002) A structural gradient in cerrado vegetation of Brazil: changes in woody plant density, species richnes, life history and plant composition. J Trop Ecol 18:775–794. doi:10.1017/S026646740200250X
Riederer M, Schreiber L (2001) Protecting against water loss: analysis of the barrier properties of plant cuticles. J Exp Bot 52:2023–2032. doi:10.1093/jexbot/52.363.2023 Plants under Stress Special Issue
Rizzini CT (1971) A flora do cerrado. In: Ferri MG (coord) III Simpósio sobre o cerrado. EDUSP, São Paulo, pp 107–153
Rizzini CT (1976) Tratado de fitogeografia do Brasil. EDUSP, São Paulo
Rizzini CT (1997) Tratado de fitogeografia do Brasil: aspectos ecológicos, sociológicos e florísticos. Âmbito Cultural, Rio de Janeiro
Sajo MG, Rudall PJ (2002) Leaf and stem anatomy of Vochysiaceae in relation to subfamilial and suprafamilial systematics. Bot J Linn Soc 138:339–364. doi:10.1046/j.1095-8339.2002.00025.x
Salatino A, Montenegro G, Salatino MLF (1986) Microscopia eletrônica de varredura de superfícies foliares de espécies lenhosas do cerrado. Braz J Bot 9:117–124
Sarmiento G (1984) The ecology of neotropical savannas. Harvard University Press, Cambridge
Sobrado MA, Medina E (1980) General morphology, anatomical structure, and nutrient content of sclerophyllous leaves of the “Bana” vegetation of Amazonas. Oecologya 45:341–345. doi:10.1007/BF00540202
Soltis DE, Soltis OS, Endress PK, Chase MW (2005) Phylogeny and evolution of angiosperms. Sinauer Associates, Massachussetts
Taiz L, Zeiger E (2004) Fisiologia Vegetal. Artmed, Porto Alegre
Tannus JLS, Assis MA (2004) Composição de espécies vasculares de campo sujo e campo úmido em área de cerrado, Itirapina––SP, Brasil. Braz J Bot 27:489–506
Theobald WL, Krahulik JL, Rollins RC (1979) tricome description and classification. In: Metcalfe CR, Chalk L (eds) Anatomy of the dicotyledons. New York Claredon Press, Oxford, pp 40–53
Varanda EM, Ricci CV, Brasil IM (1998) Espécies congenéricas da mata e do cerrado: teor de proteínas e compostos fenólicos. Bol Bot USP 17:25–30
Veloso HP (1964) Contribuição à fitogeografia do Brasil; a flora através dos tempos. Anu Bras Econ Florest 16:19–42
Victor MAM (1975) A devastação florestal. Sociedade Brasileira de Silvicultura, São Paulo
Voguelmann TC (1993) Plant tissue optics. Annu Rev Plant Physiol Plant Mol Biol 44:231–251. doi:10.1146/annurev.pp.44.060193.001311
Wilkinson HP (1979) The plant surface (Mainly leaf). In: Metcalfe CR, Chalk L (eds) Anatomy of the dicotyledons. New York Claredon Press, Oxford, pp 97–165
Acknowledgments
This study that represents part of the doctoral thesis of Angela C. Bieras was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP, Brazil (Process: no. 03/04365-1 and Biota/FAPESP no. 2000/12469-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Buckeridge.
Rights and permissions
About this article
Cite this article
Bieras, A.C., Sajo, M.d.G. Leaf structure of the cerrado (Brazilian savanna) woody plants. Trees 23, 451–471 (2009). https://doi.org/10.1007/s00468-008-0295-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-008-0295-7