Abstract
Riparian forests are known to be critical environments for biodiversity maintenance. The leaf anatomy of Sapium glandulosum (L.) Morong., Bauhinia aculeata L., Inga vera Willd., Pithecellobium dulce (Roxb.) Benth., Guazuma ulmifolia Lam. and Cecropia peltata L., growing along an altitudinal gradient (682–800 – 1030 m a.s.l.) on the high basin of the Tocuyo river were studied, in order to evaluate the possible foliar phenotypic plasticity that makes possible their adaptation at this altitudinal range. Leaf blade samples were collected from adult trees growing at two different altitude; these samples were fixed in FAA and processed using classical techniques in optical microscopy. The leaf histology was similar at both altitudes for all taxa, but differences were detected between them on quantitative anatomic characteristics, which varied depending on the species. The features with higher plasticity were: adaxial stomatal density (amphistomatic leaves), trichome density, palisade parenchyma thickness and leaf thickness. I. vera seems to be the taxon in which lower plasticity in the blade’s anatomical characters was evident, while G. ulmifolia was the species with the highest phenotypic plasticity in the altitudinal gradient, showing more heliomorphic characteristics as altitude increased, which confers it adaptive advantages to this species for colonizing riparian forest ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Riparian forests are ecosystems located at riverbanks, normally over river banks and levees. These environments are associated with macro-thermal weather, precipitation regime that may be humid, seasonal or dry, where a high biodiversity is kept, creating ecological corridors with landscape connectivity along the extensive environmental gradients, thus they represent a very important habitat for the preservation of rare and threatened species (Berthelot et al. 2015). Vegetation from those forests is integrated by several floristic and physiognomic associations that are clustered in successional categories according to their morphological, physiological, anatomical and reproductive adaptations (Dimopoulos and Zogaris 2008). The variability of plant formations that grow in river forests largely depends on substrate characteristics and hydric availability; a factor that influences their establishment is their sensibility to quickly changes through time, either in a natural way by environmental conditions or by anthropic intervention; the latter has increased during previous years, resulting in the disappearance of vegetation strips that have been unable to naturally regenerate due to a high pace of erosion (Dimopoulos and Zogaris 2008).
The Tocuyo river basin is the main slope in the Venezuelan Caribbean and is distributed among Lara, Falcon and Trujillo states. This basin is considered a protected area located among mountain landscapes and small valleys (López and Andressen 1996). The growing anthropogenic activity in the river bank puts local ecosystems at risk, mainly due to the conversion of natural areas into agricultural lands, causing a progressive reduction of the forested surfaces and the richness of riverine species (García Yépez and Rodríguez Rojas 2010). According to Alvarado-Álvarez (2010), the biodiversity of the Tocuyo river basin has not been deeply studied, therefore there is not enough information about the adaptive strategies of species that are distributed throughout the altitude gradient of this basin.
The leaf is an extremely adaptable organ and its morphology and anatomy are intimately linked to the environment in which the plant develops (Solereder 1908; Metcalfe and Chalk 1950; Kröber 2015). Plants frequently respond to altitude gradients with anatomical adaptations more so than with morphological ones, however, this depends on the taxa (Jiménez-Noriega et al. 2017). Studies related to the effect of the altitudinal gradient on foliar anatomy of riparian forests species are scarce, despite of the importance of this type of information to understand the adaptive strategies of species from these ecosystems (Akinlabi et al. 2014). Xeromorphic traits have been reported in sun leaves of trees and bushes that grow in riparian environments, which are mainly associated to drought avoidance mechanisms such as hairy surface, thick cuticule, presence of wax or other reflective material on the leaf surface, increased size of leaf veins (Walters et al. 1980) and smaller and more dense stomata (Walters et al. 1980; Pearce et al. 2005).
The aims of this investigation were to study the foliar anatomy of six arboreal eudicotyledonous species that grow along an altitudinal gradient (682–800 – 1030 m a.s.l.) in the high basin of the Tocuyo river, in order to contribute with the existing knowledge regarding its leaf anatomy and to evaluate the possible foliar phenotypic plasticity that allows its adaptation throughout this altitude gradient. Selected species belong to a vegetal association named after Inga vera and Sapium glandulosum, which was proposed by Alvarado (2009) as new for science being named “Sapio glandulosi-Ingetum verae”, typical of riparian environments, which belongs to a phytosociological class of vegetation that has already been described by Borhidi (1996) for Cuba island, where it is called “Guazumo-Ceibetea pentandrae Borhidi” and whose distribution is circunscribed to the low lands of Central America and the Caribbean.
Materials and methods
Study area
The study area is located in the Tocuyo river high basin and comprise three sectors situated in the Moran municipality from the Lara state – Venezuela. In each sector, temperature and relative humidity data were registered, using a HANNA Instruments thermohygrometer, model H18564; the luminous intensity was measured as well with an ACCUPAR radiometer, model LP-80; the average values of these variables are shown in the Table 1. Likewise, in each sector a soil sample was collected for texture, pH and electric conductivity (EC) analysis. The soil texture is franc-sandy in the three sectors; the pH resulted slightly alkaline at 682 m a.s.l. and moderately acid at 800 m a.s.l. and 1030 m a.s.l. and the EC was similar and lower than 1 dS.m−1 in the three sampling sites.
Sampling
The sampling was performed on May 2014. This was made on six arboreal eudicotyledonous species, but all were not found at the three sectors, thus the foliar material of each one was collected at two different altitudes: Bauhinia aculeata (Fabaceae), Cecropia peltata (Cecropiaceae), Guazuma ulmifolia (Malvaceae) and Sapium glandulosum (Euphorbiaceae) were collected at 682 and 1030 m and Inga vera (Fabaceae) and Phitecellobium dulce (Fabaceae) were sampled at 800 m a.s.l. and 1030 m a.s.l. The samples were submitted to the UCOB herbarium. In each species, three adult trees belonging to the upper strata or canopy were randomly selected and in each one of them, six mature leaves located at the fifth node (counting from the apex down to the base) from exposed branches located on the upper strata were sampled. In each specimen a portion of approximately 1 cm2 was taken at the middle third of the blade in the four species with simple leaves; in the case of I. vera and P. dulce the samples were taken in leaflets of the central portion of the leaf.
Processing and characterization of leaf material
The leaf samples were washed with water and fixed in FAA (formaldehyde-acetic acid-ethanol 70 %) until their processing. Afterwards the material was dehydrated in a tertiary butyl alcohol ascendant battery and it was then included in paraffin in order to do transversal sections of 12 – 18 μm using a Leica rotating microtome. Progressive double staining was made with safranin-fast green and then the sections were mounted on Canadian balsam (Johansen 1940). Additionally, transversal sections were manually made in order to perform histochemistry tests to detect starch and tannins, following the protocols proposed by Johansen (1940). For the epidermis study partial macerates were made with commercial sodium hypochlorite; then the obtained portions were stained with aqueous blue toluidine and mounted in a water-glycerin solution.
The microscopic preparations were observed under a Nikon E-200 optical microscope and digital images were taken with an Evolution LC Color camera attached to the microscope. Likewise, with the help of a micrometric ocular the following measurements were taken: adaxial and abaxial epidermis and hypodermis (if present) thickness, palisade parenchyma and spongy parenchyma thickness (with both values, palisade parenchyma/spongy parenchyma radio was calculated) and blade thickness; stomatal and trichomes were also counted in order to determine the density of these structures. Thirty measurements were made for each variable at distinct and randomly selected microscopic preparations. The phenotypic plasticity index (IPF) for each variable was also calculated as the difference between the maximum and minimum mean values divided by the maximum mean value (Valladares et al. 2006) between the two altitudes for the common variables of the six taxa (epidermis thickness, palisade and spongy parenchyma thickness, blade thickness and stomata density in abaxial epidermis).
Statistical analysis
The quantitative anatomical variables were submitted to a variance analysis comparing between the two altitudinal levels in which each species was collected. In the cases in which significative statistical differences were detected between locations along the altitudinal gradient, a mean comparison was made by a Tukey test (at 5 % significance level). The used software was InfoStat (Di Rienzo et al. 2014). For the graphics elaboration, the SIGMAPLOT version 12.5 program was used.
Results and discussion
Anatomical characterization of the leaf blade
All studied species have an adaxial and abaxial single-layered epidermis; on frontal view, the typical epidermal cells of the adaxial epidermis have a very wavy outline in B. aculeata (Fig. 1A, B), wavy outline in P. dulce (Fig. 1G) and S. glandulosum (Fig. 1H), a straight to slightly wavy outline in C. peltata (Fig. 1C) and I. vera (Fig. 1F), and a straight outline in G. ulmifolia (Fig. 1E). The abaxial epidermis cells exhibited a wavy outline in all the taxa but the undulation degree varied depending on the species (Fig. 2); this type of outline is the most common in the abaxial surface of the blade (Roth 1984). In the cross section of the blade the cells are tabular to slightly elliptical shaped in all the taxa (Fig. 3). Regarding these characteristics no differences were observed between the taxa for the two altitudes.
Surface view of the adaxial epidermis in six dicotyledonous species growing along an altitudinal gradient on the high basin of the Tocuyo river. A-B B. aculeata at 682 m a.s.l. (A), detail indicating with arrow prismatic crystals (cr) in the rib (B). C-D C. peltata at 682 m a.s.l., note glandular trichomes (tg). E G. ulmifolia at 682 m a.s.l. F I. vera at 800 m a.s.l. G P. dulce at 1030 m a.s.l. H S. glandulosum at 682 m a.s.l. (Bars A-H = 30 μm)
Surface view of the abaxial epidermis in six dicotyledonous species growing along an altitudinal gradient on the high basin of the Tocuyo river. A B. aculeata at 1030 m a.s.l. B C. peltata at 1030 m a.s.l. C G. ulmifolia at 682 m a.s.l. D-E I. vera at 1030 m a.s.l., notate different types of trichomes on the leaf surface. F P. dulce at 1030 m a.s.l. G S. glandulosum at 682 m a.s.l. est.: stoma; p: papillae; te: stellate trichome; tf: filiform trichome; tg: glandular trichome; tt: tector trichome. (Bars A-H = 30 μm)
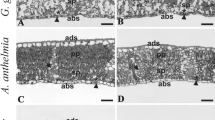
View of the leaf blade cross section in six dicotyledonous species growing along an altitudinal gradient on the high basin of the Tocuyo river. A B. aculeata at 1030 m a.s.l., note crystal in the extension of the vascular bundle. B-C C. peltata at 682 m a.s.l. and 1030 m a.s.l., respectively. D-E G. ulmifolia at 682 and 1030 m a.s.l., respectively. F I. vera at 800 m a.s.l. G P. dulce at 682 m a.s.l. H S. glandulosum at 682 m a.s.l. cr: crystal; h: hypodermis. (Bars A-G = 30 μm, H = 100 μm)
With regard to the stomata no difference was observed between the two altitudes. The leaf is hypostomatic in G. ulmifolia and I. vera and amphistomatic in the remaining species; however, in P. dulce the stomata of the adaxial surface are located mainly in the vicinity of the ribs. Amphistomatous leaves are most commonly found in plants that develop in environments with high luminosity (Haberlandt 1914; Metcalfe and Chalk 1983), however, stomata location on the blade is also a genetic feature (Metcalfe and Chalk 1950). The stomata type is anomocytic in B. aculeata and C. peltata, while in P. dulce, I. vera, S. glandulosum and G. ulmifolia, both the anomocytic and paracytic types were observed. These stomata types have been reported by Metcalfe and Chalk (1983) in C. peltata, G. ulmifolia and I. vera. In P. dulce, Jáuregui (2012) found only anomocytic stomata and Garcia and Torrecilla (1999) reported paracytic ones, however in this study, both types of stomata were found in this species. In S. glandulosum Andrade et al. (2017) observed only paracytic stomata, however we observed anomocytic stomata also in this species. In B. aculeata, the observed stomata type matches the one reported by Albert and Sharma (2013) in Bauhinia racemosa Lam. The stomata types and their location on the blade of each species were the same for both altitudes.
In all the taxa the blade is pubescent, except in S. glandulosum; nevertheless in each species a particular combination of trichomes was observed, as well as differences in their location, which confirms the diagnostical importance of this character in the taxonomic delimitation of dicotyledons (Metcalfe and Chalk 1983). In B. aculeata, tector trichomes were observed on the abaxial epidermis (Fig. 2A), which coincides with what was reported by Duarte-Almeida et al. (2015) in this species. In C. peltata, unicellular tector sting shaped trichomes and glandular trichomes with unicellular pedicel and pluricellular head (Fig. 1D) were observed in both surfaces, while on the abaxial surface there is an intrincated net of very elongated filiform unicellular hairs (Fig. 2B), the latter type was also reported by Gonçalves-Souza and Paiva (2016) in young leaves of Cecropia pachystachya Trécul. In G. ulmifolia, stellate multicellular trichomes were observed in the abaxial surface and glandular trichomes with bicellular pedicel and multicellular head on both surfaces (Fig. 2C); these trichomes were also observed by Scalon et al. (2011) in G. ulmifolia seedling leaves. Regarding I. vera, unicellular tector trichomes with acute apex were found in both surfaces (Fig. 2E) and glandular trichomes with bicellular pedicel and multicellular head were observed on the abaxial surface (Fig. 2D); the first trichome type has also been reported in leaves of I. vera from rainforest, assigning them an important function in the protection against herbivory (Coley et al. 2018); regarding glandular trichomes, their morphology is very similar to the one found by Arambarri et al. (2006) in the species Inga verna Wild. subsp. affinis (D.C.) T.D. Penn. In P. dulce, unicellular papillae were observed on the abaxial surface (Fig. 2F) and acute apex unicellular hairs, in both surfaces, which correspond with the trichomes reported by Garcia and Torrecilla (1999) for this species. No difference was found between specimens of the two altitudes for trichome types.
In C. peltata and G. ulmifolia, a hypodermis constituted by one to two layers of cells was observed below the adaxial epidermis (Fig. 3B-E). In the second species this layer is interrupted in some areas by secretory cavities. Gámez (2013) and Patil and Biradar (2013) studied the foliar anatomy of G. ulmifolia, but they did not mention this tissue, while Barajas et al. (2014) reported a hypodermis similar to the one observed in this study in this species. Regarding C. peltata its foliar anatomy has been very little documented; however, the existence of a hypodermis as characteristic of diagnostic value in this genus was reported by Solereder (1908).
All taxa have dorsiventral mesophyll, except P. dulce where it is equifacial (Fig. 3). The palisade and spongy parenchyma strata number was particular and constant at both altitudes in B. aculeata, P. dulce, I. vera, and S. glandulosum. P. dulce exhibited a palisade parenchyma layer in each face of the blade, with longer cells on the adaxial side, and three to five layers of spongy pareynchma between them. B. aculeata had three layers of palisade parenchyma and three layers of spongy parenchyma, and I. vera had two layers of palisade parenchyma and three to five layers of spongy parenchyma with many intercellular spaces. S. glandulosum exhibited a palisade parenchyma layer and seven to eight spongy parenchyma layers. C. peltata and G. ulmifolia were the only taxa whose number of parenchyma layers varied at both altitudes; the first species showed a simple layer of palisade parenchyma at 682 m a.s.l. and two to three layers at 1030 m, while the second species had two strata at 682 m a.s.l. and three at 1030 m; regarding the spongy parenchyma, in C. peltata six to eight layers were observed at both altitudes, while G. ulmifolia exhibited two to four layers at 682 m a.s.l. and seven to eight layers at 1030 m a.s.l. This behavior reflects plasticity in mesophyll structural characteristics of both species, which is more accentuated in G. ulmifolia. Considering that among the measured climatic variables radiation was the only that differed at both altitudes, with a higher value at 1030 m a.s.l., it could be inferred that this factor influenced the increasing number of layers of mesophyll’s parenchyma layers in these taxa, a behavior that has already been observed in leaves from species that grow under high solar radiation environments (Shields 1950; Metcalfe and Chalk 1979; Kröber et al. 2015; Gotoh et al. 2018). Regarding the palisade parenchyma, it has been shown that this tissue can facilitate the penetration of light into deeper mesophyll layers (Eames and Mac Daniels 1925; Xiao et al. 2016). Respect spongy parenchyma, it has been pointed out that a higher development of this tissue may favor the CO2 sheltering capability in the mesophyll, which could reduce of the aperture time of the stomata, thus reducing water loss (Ciccarelli et al. 2009).
The vascular system is composed by closed collateral vascular bundles in each species. In B. aculeata (Fig. 3A), C. peltata (Fig. 3B, C), G. ulmifolia and S. glandulosum, the largest bundles were surrounded by a parenchyma sheath and they often have extensions to both epidermis. I. vera had only a few bundles surrounded by a sclerenchyma sheath, and in P. dulce the vascular bundles had sclerenchyma caps towards the phloem and xylem. In S. glandulosum starch grains were detected in the cells of the parenchyma sheath.
Crystals were observed in all the studied species, mainly in the chlorenchyma, but also in some hypodermic cells in C. peltata and in the parenchyma cells of the vascular bundles in B. aculeata (Fig. 1B, 3A) and G. ulmifolia. The type of crystals did not vary between the specimens from both altitudes; these are prismatic in B. aculeata, G. ulmifolia, I. vera and P. dulce, druse in C. peltata and S. glandulosum, while in B. aculeata both types were evident. The crystal presence and their types are considered to be of diagnostic value (Metcalfe and Chalk 1983). From a functional point of view, the crystals play an important role in the calcium metabolism maintenance, but can also constitute a defense mechanism or a response to stressful conditions (Jáuregui-Zuñiga and Moreno 2004).
Tannins were detected in foliar tissue from several of the studied species; they clustered in the epidermis in P. dulce (Fig. 3G), I. vera (Fig. 3F) and in some cells of the hypodermis in C. peltata (Fig. 3B). In P. dulce Vanitha and Manikandan (2016), also found tannins through phytochemical analysis in aqueous extracts of leaves. It has been pointed out that when these compounds accumulate in the epidermis they may increase ultraviolet light absorption, therefore avoiding its penetration to the internal tissues (Stephanou and Manetas 1997).
Variation in quantitative anatomical characteristics along the altitudinal gradient
Regarding the adaxial epidermis thickness, P. dulce was the only species in which this value was significantly higher as altitude increased, while for I. vera the opposite behavior was observed; in the remaining species there was no statistically significant difference for this variable (Fig. 4A). Regarding the abaxial epidermis, in C. peltata and G. ulmifolia this tissue had a significantly greater thickness at higher altitudes, while for I. vera the opposite behavior was observed; in the other three species there was no statistically significant difference at both altitudes (Fig. 4B). The increase in epidermis thickness at higher altitude has been associated with a high exposition to light intensity (Eames and MacDaniels 1925), which constitutes an advantage since a thick epidermis protects the mesophyll tissues from ultraviolet radiation (Caldwell 1971; Beloved 2012); however, Jiménez-Noriega et al. (2015) did not find an increase in the thickness of this tissue in the Ribes ciliatum Roemes & Schultes specimens growing at an altitudinal gradient in the Tlálo Hill, Sierra Nevada.
Average values of adaxial (A) and abaxial (B) epidermis thickness, palisade (C) and spongy parenchyma (D) thickness, palisade/spongy parenchyma ratio (E) and leaf thickness (F) in six dicotyledonous species growing along an altitudinal gradient on the high basin of the Tocuyo river. Equal lowercase letters above the bars do not differ from each other by the Tukey test at 5 % error probability. Vertical bars represent the value of the standard deviation
In C. peltata, the average thickness of hypodermis was 18.2 μm at 682 m a.s.l. and 15.2 μm at 1030 m a.s.l. (with no significant difference between both values), while in G. ulmifolia it was 16.7 μm at the first altitude and 28.4 μm at the second one, resulting in a significant statistical increase (p < 0.05). This tissue has already been described for in G. ulmifolia by Barajas et al. (2014), but there is no available information in existing literature regarding its behavior in response to the altitudinal gradient in riparian forests. The increment in the thickness of the hypodermis with altitude is probably a way to protect the foliar tissue against radiation increase, since this tissue is associated with the capability of storing water, and also with internal tissues, protection against heating and microorganisms attack (Eames and Mac Daniels 1925; Torres-Boeger et al. 2010).
The thickness of the palisade parenchyma significantly increase with altitude in all taxa, except in S. glandulosum and I. vera, but the magnitude of this increase was considerably higher in G. ulmifolia (Fig. 4C). Regarding the thickness of spongy parenchyma, it only significantly increased with altitude in G. ulmifolia while the opposite was observed in S. glandulosum; in the remaining taxa this variable was similar at both altitudes (Fig. 4D). The ratio palisade parenchyma/spongy parenchyma ratio decreased in G. ulmifolia, which indicates that in this taxon, the proportion of spongy parenchyma increased more than palisade parenchyma at higher altitude; in P. dulce, the inverse was observed, and in the remaining species this ratio kept a similar value at both altitudes (Fig. 4E). The increase in the development of palisade parenchyma observed in four of the studied taxa may be linked to an increase in luminous intensity with altitude, as it has been pointed out that this tissue is very sensitive to changes in this climatic variable due to its important role in capturing light (Eames and Mac Daniels 1925; Shields 1950; Metcalfe and Chalk 1983).
The total thickness of the lamina significantly increased with altitude only in G. ulmifolia and B. aculeata, but the magnitude of this increase was remarkably higher in the former, where this increase was produced with the contribution of all tissues, except the adaxial epidermis, while in the second species this was due basically to an increase in the proportion of palisade parenchyma. In S. glandulosum and I. vera the thickness of the lamina significantly reduced and in the remaining species there were no differences in this variable at both altitudes (Fig. 4F). The increase in leaf thickness in plants that grow at a higher altitude and under conditions of higher radiation conditions has been widely supported since the time of first plant anatomists (Eames and Mac Daniels 1925; Shields 1950; Roth 1984) and also in more recent studies (Gratani 2014; Vieira et al. 2014; Gotoh et al. 2018), however, this behavior was only observed in two of the studied taxa, which shows that the species have different responses to changing environment factors (Solereder 1908; Metcalfe and Chalk 1983; Akinlabi et al. 2014). Leaf anatomy suggests that foliage is primarily adapted for photosynthesis rather than for control of transpirational water loss, as it was described by Hlwatika and Bhat (2002) in tree species growing in an undisturbed forest and the adjoining fynbos (Africa).
In all species with amphistomatic leaves, the stomata density in the adaxial epidermis significantly increased with altitude (Fig. 5A); while in the abaxial epidermis this behavior was only observed in C. peltata, S. glandulosum and I. vera; in the remaining species this characteristic was similar at both altitudes (Fig. 5B). The increase in stomata number per area unit as altitude increases has been associated with solar radiation increase (Arambarri et al. 2012; Jiménez-Noriega et al. 2015), since it allows a more effective gas exchange in periods when water availability favors the opening of the stomata (Hanson 1917; Eames and Mac Daniels 1925; Valerio et al. 2013). Similar to what was observed in this study, Pearce et al. (2005) found that the stomata frequency in riparian species varied more in the adaxial epidermis than in the abaxial one. Akinlabi et al. (2014) found that on Chromolaena odorata (L.) King & Robinson, stomata density decreased with altitude, attributing this to the increase of atmospheric carbon dioxide. These opposed responses should be analyzed in detail, considering the magnitudes of environmental variables which might be the responsible ones.
Average values of adaxial (A) and abaxial (B) stomatal density and adaxial (C) and abaxial (D) trichomes density, in six dicotyledonous species growing along an altitudinal gradient on the high basin of the Tocuyo river. Equal lowercase letters above the bars do not differ from each other by the Tukey test at 5% error probability. Vertical bars represent the value of the standard deviation
Regarding trichomes density, on the adaxial epidermis it decreased with altitude in G. ulmifolia and C. peltata and kept a similar value in I. vera and P. dulce (Fig. 5C), while in the abaxial epidermis, the trichomes frequency decreased at a higher altitude in G. ulmifolia and P. dulce and kept a similar value in the remaining species (Fig. 5D). In P. dulce the papillae number mm−2 was 563 at 800 m a.s.l. and 1032 at 1030 m a.s.l., which indicates that this species doubled its papillae density at higher altitude; this behavior follows the results of Garcia and Lapp (2001) in P. unguis-cati (L.) Bentham; it is assumed that this response is associated to stomata protection in order to avoid excessive water loss as the environment gets dryer. It has been indicated that the increase of trichomes density at higher altitude is an important strategy to protect foliar tissues against intense radiation and the action of the wind, as well as contributing with light reflection, which prevents leaf overheating and excessive transpiration, however an opposite trend has also been reported by Filella and Peñuelas (1999), who found that in Quercus ilex L., trichome density in leaves was higher at lower altitude.
The IPF values for the quantitative anatomical characteristics that were common among the six species are shown in Table 2, where we can observe that the highest IPF value corresponds to G. ulmifolia, while in the five remaining species the IPF was low, which indicates that this taxon was the one that exhibited higher phenotypical plasticity in the considered altitude gradient. The thickness of the spongy and palisade parenchyma, along with the blade thickness, were the characteristics that contributed the most to its average IPF. This suggests that the mesophyll structure plays an important role in the adaptation of G. ulmifolia to environmental changes associated with the studied riparian forest altitude gradient, particularly to the luminous intensity increase associated with higher altitude; nonetheless, it has been indicated that this species is sensitive to high irradiance during its early developmental stages (Contin et al. 2014).
The species with the lowest IPF were C. peltata, followed by B. aculeata, P. dulce and I. vera; however, we must consider that in the first three species there were changes in quantitative variables which were not included in the average IPF. In P. dulce papillae density doubled with altitude, in C. peltata, the strata number of the mesophyll parenchyma increased at the highest altitude, and in B. aculeata the adaxial stomata density significantly increased with altitude. These changes are also indicators of structural plasticity and make evident the individual differences of these taxa regarding their adjustment to the altitude gradient. I. vera seems to be the taxon in which lower plasticity in the anatomical characters of the leaf blade was evident. It is possible that the species that showed a low average IPF adjust to environmental changes by another morphoanatomic or physiologic characters not included in this study, or that they activate modifications in another organs, to be able to adapt to environmental variations that occur with altitude. Pereira et al. (2018) studied simultaneously anatomical and physiological plasticity in leaves from two arboreal tropical species growing under different irradiance levels and found a higher phenotypical plasticity in the physiological rather than the anatomical characteristics. Gratani (2014) has indicated that species with higher phenotypical plasticity have better survival capability when environmental conditions change quickly; it has also been pointed out that those taxa showing high levels of phenotypical plasticity through altitude gradients may have a higher capability to respond to global change (Read et al. 2014). The six studied taxa show similarity in their qualitative foliar structure, but not in terms of quantitative leaf anatomical characteristics where a variable plasticity degree was observed, depending on the taxon. The more plastic characteristics in the altitude gradient were: stomata density on the adaxial epidermis (amphistomatic leaves), trichome density, palisade parenchyma thickness, and leaf thickness. G. ulmifolia was the taxon with the highest leaf anatomical plasticity in the altitude gradient from the studied riparian forest which confers it an adaptive advantages for colonizing riparian ecosystems. However, in the remaining taxa the presence of certain responses revealed some degree of leaf plasticity, which indicates that their adaptive capability is influenced by genotypic characteristics of each species. Taking in account that relative humidity and temperature are similar at the two sampling sites, the plasticity responses observed in the studied arboreal species seem to be related to the increase of luminous intensity with altitude, which induces the development of characters mainly associated with heliomorphism.
References
Akinlabi A, Jimoh M, Saheed S (2014) Effects of altitudinal gradients on morphoanatomical characters of Chromoloaena odorata (L.) King & Robinson. FUTA J Res Sci 10:150–156
Albert S, Sharma B (2013) Comparative foliar micromorphological studies of some Bauhinia (Leguminosae) species. Turk J Bot 37:276–281
Alvarado H (2009) Flora y vegetación ribereña de la cuenca del río Tocuyo, estados Lara y Falcón (Venezuela). Universidad de Alicante, España, Tesis doctoral
Alvarado-Álvarez H (2010) Caracterización estructural y florística de un bosque ribereño de la cuenca del río Tocuyo (Tocuyo Occidental), estado Lara, Venezuela. Ernstia 20:1–20
Andrade EA, Folquitto DG, Luz LEC, Paludo KS, Farago PV, Budel JM (2017) Anatomy and histochemistry of leaves and stems of Sapium glandulosum. Rev Bras Farmacogn 27:282–289
Arambarri A, Monti C, Bayón N, Hernández M, Novoa MC, Colares M (2012) Ecoanatomía foliar de arbustos y árboles del distrito chaqueño oriental de la Argentina. Bonplandia 5–26
Arambarri AM, Freire SE, Colares MN, Bayón ND, Novoa MC, Monti C, Stenglein SA (2006) Leaf anatomy of medicinal shrubs and trees from gallery forests of the Paranaense Province (Argentina). Part 1. Bol Soc Argen Bot 41:233–268
Barajas L, Herreño N, Mejía A, Borrego P, Pombo L (2014) Guásimo, Guazuma ulmifolia. Departamento de Ciencias Básicas, Fundación Universitaria Juan N. Corpas, Escuela de Medicina, Bogotá
Beloved M (2012) Leaf anatomical variation in relation to stress tolerance among some woody species on the Accra plains of Ghana. J Plant Develop 19:13–22
Berthelot J-S, Saint-Laurent D, Gervais-Beaulac V, Présent A (2015) A comparison of the composition and diversity of tree populations along a hydrological gradient in floodplains (southern Québec, Canada). Forests 6:929–956
Borhidi A (1996) Phytogeography and vegetation ecology of Cuba. Hungarian Academy of Sciences y Hungarian National Research Fund, Budapest 923
Caldwell MM (1971) Solar UV irradiance and the growth and development of higher plants. In: Giese AC (ed) Photophysiology, vol VI. Academic Press, New York-London, pp 131–177
Ciccarelli D, Forino LMC, Balestri M, Pagni AM (2009) Leaf anatomical adaptations of Calystegia soldanella, Euphorbia paralias and Otanthus maritimus to the ecological conditions of coastal sand dune systems. Caryologia 62:142–151
Coley PD, Endara M-J, Kursar TA (2018) Consequences of interspecific variation in defenses and herbivore host choice for the ecology and evolution of Inga, a speciose rainforest tree. Oecologia 187:361–376
Contin DR, Soriani HH, Hernández I, Furriel RP, Munné-Bosch S, Martinez CA (2014) Antioxidant and photoprotective defenses in response to gradual water stress under low and high irradiance in two Malvaceae tree species used for tropical forest restoration. Trees 28:1705–1722
Di Rienzo J, Casanoves F, Balzarini M, Gonzalez L, Tablada M, Robledo C (2014) InfoStat versión 2013. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina http://www.infostat.com.ar Accessed 15 July 2014
Dimopoulos P, Zogaris S (2008) Vegetation and flora of riparian zones. In: Arizpe D, Mendes A, Rabaça JE (eds) Sustainable riparian zones: a management guide. Generalitat Valenciana, Valencia, pp 66–83
Duarte-Almeida JM, Clemente MS, Arruda RC, Vaz AM, Salatino A (2015) Glands on the foliar surfaces of tribe Cercideae (Caesapiniodeae, Leguminosae): distribution and taxonomic significance. An Acad Bras Ciên 87:787–796
Eames AJ, MacDaniels S (1925) Introduction to plant anatomy. Mc Graw Hill, New York
Filella I, Peñuelas J (1999) Altitudinal differences in UV absorbance, UV reflectance and related morphological traits of Quercus ilex and Rhododendron ferrugineum in the Mediterranean region. J Plant Ecol 145:157–165
Gámez L (2013) Estudio ecoanatómico de cuatro especies arbóreas de Malvaceae en la Estación Experimental Caparo, estado Barinas (Venezuela). Pittieria 37:41–51
Garcia M, Lapp M (2001) Morfoanatomía foliar en tres biotipos de Pithecellobium unguis-cati (L.) Bentham creciendo en distintas comunidades vegetales. Phyton 2001:147–158
Garcia M, Torrecilla P (1999) Anatomia foliar de algunos taxones representativos de las secciones del género Pithecellobium Martius s. str. (Leguminosae: Mimosoideae: Ingeae) en Venezuela. Ernstia 9:1–19
García Yépez J, Rodríguez Rojas P (2010) El Tocuyo: región histórica. Terra Nueva Etapa 26:40
Gonçalves-Souza P, Paiva EAS (2016) Food bodies of Cecropia pachystachya (Cecropiaceae) leaves: structural and functional features suggesting complementary role to Müllerian bodies. New Zeal J Bot 54:323–334
Gotoh E, Suetsugu N, Higa T, Matsushita T, Tsukaya H, Wada M (2018) Palisade cell shape affects the light-induced chloroplast movements and leaf photosynthesis. Sci Rep 8:1472
Gratani L (2014) Plant phenotypic plasticity in response to environmental factors. Adv Bot 2014:1–17
Haberlandt G (1914) Physiological plant anatomy. Mac Millan, London. England
Hanson HC (1917) Leaf-structure as related to environment. Am J Bot 4:533–560
Hlwatika C, Bhat R (2002) An ecological interpretation of the difference in leaf anatomy and its plasticity in contrasting tree species in Orange Kloof, Table Mountain, South Africa. Ann Bot 89:109–114
Jáuregui-Zuñiga D, Moreno A (2004) La biomineralización del oxalato de calcio en plantas: retos y potencial. REB 23:18–23
Jáuregui D (2012) Guía ilustrada de las epidermis foliares de Angiospermas presentes en Venezuela. Colección Estudios, Consejo de Desarrollo Científico y Humanístico, Universidad Central de Venezuela, Caracas
Jiménez-Noriega PMS, Terrazas T, López-Mata L, Sánchez-González A, Vibrans H (2017) Anatomical variation of five plant species along an elevation gradient in Mexico City basin within the trans-Mexican Volcanic Belt, Mexico. J Mt Sci 14:2182–2199
Jiménez-Noriega MS, Terrazas T, López-Mata L (2015) Variación morfo-anatómica de Ribes ciliatum a lo largo de un gradiente altitudinal en el norte de la Sierra Nevada, México. Bot Sci 93:23–32
Johansen DA (1940) Plant microtechnique. Mc. Graw-Hill Book Co, Inc, New York and London
Kröber W, Heklau H, Bruelheide H (2015) Leaf morphology of 40 evergreen and deciduous broadleaved subtropical tree species and relationships to functional ecophysiological traits. Plant Biol 17:373–383
López M, Andressen R (1996) Caracterización climática de las cuencas de los ríos Yacambú y Tocuyo en el ramal andino de la región centro occidental de Venezuela. Bioagro 8:87–95
Metcalfe CR, Chalk L (1950) Anatomy of the dicotyledons. The Clarendon Press, Oxford
Metcalfe CR, Chalk L (1979) Anatomy of the dicotyledons. The Clarendon Press, Oxford
Metcalfe CR, Chalk L (1983) Anatomy of the Dicotyledons. The Clarendon Press, Oxford
Patil JU, Biradar S (2013) Pharmacognostic study of Guazuma ulmifolia. International Res J Pharm 4:130–131
Pearce DW, Millard S, Bray DF, Rood SB (2005) Stomatal characteristics of riparian poplar species in a semi-arid environment. Tree Physiol 26:211–218
Pereira S, Frosi G, Oliveira MT, Lustosa BM, Arruda EP, Santos MG (2018) Changes in phenotypic variability of two tropical woody species due to short and long-term exposure to different irradiances. Bragantia 77:429–439
Read QD, Moorhead LC, Swenson NG, Bailey JK, Sanders NJ (2014) Convergent effects of elevation on functional leaf traits within and among species. Funct Ecol 28:37–45
Roth I (1984) Stratification of tropical forests as seen in leaf structure.W. junk Publ. The Netherlands, Boston
Scalon SP, Pereira HH, Glaeser DF, Silva JJ, Betoni R, Mussury RM (2011) Physio-anatomic aspects on the initial growth of Guazuma ulmifolia lam. Seedlings (Sterculiaceae). An Acad Bras Ciênc 83:695–703
Shields LM (1950) Leaf xeromorphy as related to physiological and structural influences. Bot Rev 16:399–447
Solereder H (1908) Systematic anatomy of the dicotyledons: a handbook for laboratories of pure and applied botany. Clarendon Press, Oxford
Stephanou M, Manetas Y (1997) The effects of seasons, exposure, enhanced UV-B radiation, and water stress on leaf epicuticular and internal UV-B absorbing capacity of Cistus creticus: a Mediterranean field study. J Exp Bot 48:1977–1985
Torres-Boeger MR, Soffiatti P, Gomes-Souto MA, Budchen M, Bagatini KP, Dal Forno M (2010) Functional morphology of two Lepismium species (Rhipsalideae, Cactaceae). Rev Mex Biodiv 81:393–400
Valerio R, Franco-Salazar V, Véliz J (2013) Adaptaciones epidérmicas foliares de cuatro especies siempreverdes, Isla la Tortuga, Venezuela. Acta Biol Venez 36:39–59
Valladares F, Sanchez-Gomez D, Zavala MA (2006) Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J Ecol 94:1103–1116
Vanitha V, Manikandan K (2016) Bio-activity guided determination of active compounds in the leaves of Pithecellobium dulce. Rasayan J Chem 9:471–477
Vieira WL, Boeger MRT, Cosmo NL, Coan AI (2014) Leaf morphological plasticity of tree species from two developmental stages in araucaria forest. Braz Arch Biol Technol 57:476–485
Walters A, Teskey R, Hinckey T (1980) Impact of water level changes on woody riparian and wet land communities. Biological Services Program, Washington
Xiao Y, Tholen D, Zhu X-G (2016) The influence of leaf anatomy on the internal light environment and photosynthetic electron transport rate: exploration with a new leaf ray tracing model. J Exp Bot 67:6021–6035
Author information
Authors and Affiliations
Contributions
GA, HA and MG conducted field trips for collection of samples. HA made the identification of the species in the riparian forest. GA prepared the foliar samples in the laboratory to obtain microscopic preparations. GA, MG and DJ realized the descriptions of the tissues under the microscope. GA, obtained microscopic measures of quantitative anatomical variables in the lab. FZ did the statistical analysis of data and made the graphics. MG, GA and DJ wrote the manuscript. All authors read and approved the final manuscript for its publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alvarado, G., García, M., Jáuregui, D. et al. Leaf anatomy of six arboreal eudicotyledons species growing along an altitudinal gradient on the high basin of the Tocuyo river, Venezuela. Biologia 75, 523–533 (2020). https://doi.org/10.2478/s11756-020-00427-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-020-00427-9