Abstract
The Bromeliaceae are a largely Neotropical family originating in open, dry environments. Vriesea Lindl., the third largest genus of the family, is traditionally divided between two sections. About 90% of the species of the genus occur in Brazil, where the centre of diversity is the Atlantic Rainforest. Leaf morphoanatomical studies conducted on bromeliad species have confirmed the importance of structural characters for ecological, and also for systematic purposes. Because of the wide morphological, ecological and taxonomic diversity of Vriesea, and its importance in ecosystems associated with the Atlantic Rainforest, we selected 24 Vriesea species and used anatomical and histochemical analyses to describe the leaf anatomy aiming to identify potential systematic characters, and point out possible traits that responded to environmental conditions during the evolution of the genus. The leaves are hypostomatic with peltate trichomes. They present epidermis with thickened cell walls, with lignin and pectin, covered by cuticle and epicuticular wax. The mechanical hypodermis is usually one-layered. Water-storage parenchyma occurs in both surfaces of the leaf blade. The chlorenchyma is located in the median portion of the blade. Air lacunae are associated with brachiform parenchyma. The vascular bundles are collateral, arranged alternately with the air lacunae and surrounded by a sheath of sclerified and/or parenchyma cells. Extravascular fibres occur in most of the species and are positioned below the mechanical hypodermis on the adaxial surface of the leaf blade. Leaf anatomical analysis can be useful in differential characterisation of small groups of related species but does not seem to reflect species assemblages according to the taxonomic sections or substrate type, but is consistent among the Brazilian analysed species of Vriesea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Bromeliaceae is a largely Neotropical family of flowering plants and displays a remarkable range of diversification and adaptive radiation (Benzing, 2000; Givnish et al., 2011). The development of CAM (crassulacean acid metabolism) photosynthetic metabolism, the formation of foliar rosettes that store water, and the presence of absorbent scales are key features for the success of the family, which inhabits a wide range of environments and grows on a diversity of substrates (Givnish et al., 2007). Among the morphological features linked to the diversification of the family is leaf morphoanatomy. Tomlinson (1969), in a broad survey of the anatomy of the Bromeliaceae, highlighted the evolutionary importance of structural features of the leaves, such as the frequently lignified walls of the epidermal cells, the presence of mechanical hypodermis, the occurrence of water-storage parenchyma, and absorbent peltate trichomes. These features are thought to be adaptations to xeric conditions, thus supporting the hypothesis that Bromeliaceae originated in open and dry environments (Bouchenak-Khelladi et al., 2014). Among the eight subfamilies of Bromeliaceae (sensu Givnish et al., 2011), Tillandsioideae (sensu Barfuss et al., 2016) has the widest geographical distribution and has undergone several evolutionary shifts among different substrates (Givnish et al., 2014). Tillandsioideae is also exceptional among the subfamilies for its extensive morphological variation in both the reproductive (Barfuss et al., 2016) and vegetative axes (Benzing, 2000). The rosettes range in size from only a few centimetres long in the atmospheric Tillandsia L. that weighs a few grams (Benzing, 2000) to large impounding tanks in Alcanatrea (É.Morren ex Mez) Harms, that can hold up to 40 l of water (Versieux et al., 2012), reflecting different strategies for water and nutrient absorption.
With about 200 species, Vriesea Lindl. is the second most species-rich tillandsioid genus (Barfuss et al., 2016; Butcher & Gouda, [cont. updated] 2020). Together with Alcantarea, Stigmatodon Leme, G.K.Br. & Barfuss and Waltillia Leme, Barfuss & Halbritter, it composes the subtribe Vrieseinae of tribe Vrieseeae, in the core Tillandsioideae (Barfuss et al., 2016; Leme et al., 2017). To date, phylogenetic analyses of molecular data (Barfuss et al., 2005, 2016; Machado et al., 2020) and combined morphological and molecular data (Gomes-da-Silva & Souza-Chies, 2017) have been insufficiently informative to reconstruct the evolutionary history of the genus in detail, and infrageneric relationships remain mostly obscure, perhaps due to its likely recent diversification (Kessous et al., 2020). These analyses did strongly support the monophyly of the Brazilian lineage of Vriesea, that has the centre of diversity is in the Paraná sub-region [Atlantic Rainforest and Cerrado domains] (Costa et al., 2014; Barfuss et al. 2016; Gomes-da-Silva & Souza-Chies, 2017), with disjunctions in the Chacoan sub-region (Machado et al., 2020). This disjunct geographical pattern was suggested to be facilitated by the occurrence of islands of humid vegetation in Northeastern Brazil, as relicts of a connection between the Amazon and the Atlantic Forest during the Pleistocene (Melo-Santos et al. 2007; Kessous et al. 2020). However, the analysis of Machado et al. (2020), with increased taxonomic sampling, revealed that extra-Brazilian species from Venezuela, Puerto Rico and Peru, are also present in this clade, suggesting that calling this group of species the “Brazilian lineage” is potentially misleading. The genus Vriesea remains polyphyletic due the inclusion of some species in Stigmatodon and Tillandsia (Barfuss et al., 2016; Gomes-da-Silva & Souza-Chies, 2017; Machado et al. 2020). Besides, some extra-Brazilian species are nested in the Cipuropsis-Mezobromelia clade (Machado et al. 2020). Similarly, none of the phylogenies cited above supported the monophyly of its two sections: Vriesea sect. Vriesea and V. sect. Xiphion (E. Morren) E. Morren, which were based on floral morphology (Mez 1896; Smith & Downs 1977), and are clearly adapted to hummingbird and bat-pollination, respectively.
Most species of Vriesea are epiphytes in the canopy of humid tropical forests, mainly the Atlantic Forest, but terrestrial and rupicolous species (including epilithic and saxicolous) are also common, especially in montane open grassland on rock outcrops (i.e., ‘campos rupestres’ and ‘campos de altitude’), as well as on inselbergs. Within Vrieseinae, the relationship (Vriesea + Stigmatodon) Alcantarea was strongly supported (Barfuss et al., 2016; Leme et al., 2017; Kessous et al., 2020; Machado et al., 2020). It is worth mentioning that Alcantarea and Stigmatodon are endemic to open and montane habitats, while Vriesea is the only genus of the subtribe that invaded the humid forest (Kessous et al., 2020; Machado et al., 2020).
Structural traits contribute importantly to the ecophysiological diversity of Bromeliaceae (Benzing 2000; Males 2016). The evolution of species rich lineages, especially of Bromelioideae and Tillandsioideae, seems to be associated with key innovations, such as epiphytism, avian pollination, and tank habit (Givnish et al. 2014). In this context, Vriesea is remarkable due its high morphological and ecological diversification. In the Atlantic forest it is one of the most species-rich among all vascular epiphytic genera (Martinelli et al. 2008; Ramos et al. 2019). Its flowers are hummingbird or bat-pollinated (e.g. Sazima et al., 1999; Buzato et al., 2000), and its water impounding rosettes are morphologically diverse, ranging from utriculiform to infundibuliform and tubular (Costa et al. 2014). The existing studies of the leaf anatomy of Vriesea were focused on identifying anatomical characters for previously defined taxa (Arruda & Costa 2003, Proença & Sajo 2007; Pereira 2011; Machado 2017), or employed a phylogenetic approach to identify anatomical characters that are synapomorphic for particular clades (Gomes-da-Silva et al. 2012). Such studies included scattered taxa from different environments and morphological groups from both sections. These studies suggested the following characters as putatively informative in the systematics of the genus: the thickness of the mesophyll, the outline of the leaf margin, the presence of the mechanical hypodermis, the size of the air-lacunae, the shape of the water-storage parenchyma cells, and the presence of extravascular fibers (Arruda & Costa 2003; Proença & Sajo 2007; Pereira et al. 2011; Gomes-da-Silva et al. 2012; Machado 2017). However, the cited studies have sampled only about 17% of the species of Vriesea.
Except for Waltillia, which has a narrow and non-impounding rosette (Leme et al. 2017), all genera of Vrieseinae are tank forming and seem to have different water economy strategies. Alcantarea species form large rosettes and leaves with broad air-lacunae (Versieux et al. 2010), while Stigmatodon species have the leaves densely lepidote (Barfuss et al. 2016), but nothing is known about their foliar anatomy. However, the morphological diversity of water impounding rosettes, substrate diversity, and species richness in Vriesea, lead us to hypothesize that leaf anatomy is probably also highly diverse and systematically informative in the genus. Here, we present the most comprehensive survey of the leaf anatomy of Vriesea to date, with structural and histochemical analysis of the leaves of 24 species and comparative data from the literature for another 35 species. We aim assess correlations between leaf anatomy and taxonomic sections and other morphological groups, as well as substrates. We discuss the adaptive and systematic significance of leaf anatomical features.
Materials and methods
Taxonomic sampling
Twenty-four species of Vriesea were selected for study to span the morphological diversity of the genus and its occurrence on different substrates. The sample contained 17 species of V. sect. Vriesea and seven species of V. sect. Xiphion as delimited by Costa et al. (2014, 2015). Nineteen of the species were epiphytic, while five were rupicolous. Two species, V. cacuminis and V. sincorana, were collected at the Jardim Botânico do Rio de Janeiro, and the remaining 22 species from natural populations in the Atlantic Rainforest (Table 1). For each species three individuals from the same population were studied, but only a single herbarium voucher was prepared for each species/population. Voucher specimens were deposited in the Herbário do Museu Nacional (R) of the Universidade Federal do Rio de Janeiro (Table 1).
Anatomical observations
Anatomical observations were made from fully developed leaves from adult individuals. All images were processed using Adobe Photoshop 7.0.
For light microscopy, samples from the middle of leaf blades and leaf margins were preserved in 70% aqueous ethanol, and cross sectioned using a Ranvier hand microtome and razor blade. Sections were clarified with sodium hypochlorite, neutralised with acetic water, stained with Safrablau (Bukatsch, 1972) and mounted in glycerine. Histochemical tests were also carried out on the conserved leaf blade samples, to detect the presence of lipophilic compounds, using Sudan III and IV (Jensen, 1962); starch, using Lugol (Langeron, 1949); lignin, using phloroglucin + HCl (Johansen, 1940); cellulose, cutin and suberin, using iodated zinc chloride (Jensen, 1962); and polysaccharides, using a reaction of periodic acid and Schiff’s reagent (Taboga & Vilamaior, 2013). The chemical nature of crystals was determined by differential solubility tests in acetic acid and hydrochloric acid (Maclean & Ivemey-Cook, 1952). Samples of the median portion of sheath blades and sheath margins previously fixed in 4% formaldehyde + 2.5% glutaraldehyde in 0.05 M sodium phosphate buffer, pH 7.2 (Gahan, 1984), were dehydrated in an ethanol series, embedded in Historesin® (Leica, Wetzlar, Germany) following the manufacturer’s recommended procedure, and sectioned with glass knives at 2–5 μm on a RM2255 (Leica) rotary microtome. The sections were stained with toluidine blue O (Feder & O’Brien, 1968). Measurements were obtained using LAS EZ software (version 3.0.0, Leica Microsystems) from photomicrographs made with a Leica ICC50 digital camera attached to a Leica DM500 microscope.
For scanning electron microscopy (SEM), samples from the middle of the leaf blade, previously preserved in 70% aqueous ethanol, were dehydrated in an ethanol series, critical-point dried using a Bal-Tec CPD 030 (Bal-Tec, Pfäffikon, Switzerland) critical-point dryer with CO2, gold-coated using a Denton Vacuum - Desk IV (Denton Vacuum LLC, Moorestown, NJ, USA) vacuum sputter-coater, and examined using a JEOL JSM - 6390LV (JEOL, Tokyo, Japan) microscope.
Results
Leaf blade anatomy
Leaves of all the Vriesea species analysed are hypostomatic (Fig. 1A). In surface view, the stomata have narrowly elliptical (V. eltoniana, V. flexuosa, V. guttata, V. paratiensis, V. philippocoburgii, V. procera, V. sincorana, V. sucrei (Fig. 1B) and V. vagans) to broadly elliptical outlines (V. billbergioides, V. botafogensis, V. cacuminis, V. ensiformis, V. flava, V. gradata, V. hydrophora,V. incurvata, V. jonesiana (Fig. 1C), V. longicaulis, V. pabstii, V. pseudatra, V. psittacina. V. saundersii and V. zildae). The stomata, along with the trichomes, are organised in rows (Fig. 1D), the rows alternating with epidermal bands having neither stomata nor trichomes. This characteristic was not observed in V. pabstii, in which the abaxial leaf surface is densely covered in trichomes.
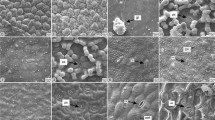
Leaf blades of Vriesea species in cross section (A, E, K–L) and surface view (scanning electron microscopy, SEM, B–D; F–J). A. V. zildae, showing stoma only on abaxial surface (arrows). B. V. sucrei, stomata on abaxial surface. C V. jonesiana, stomata on abaxial surface. D. V. ensiformis, stomata and trichomes organised in linear series on abaxial surface. E. V. eltoniana, trichomes on both adaxial and abaxial surfaces. F. V. eltoniana, trichome on abaxial surface showing shield with four cells. G, H. V. pabstii, adaxial (G) and abaxial (H) surfaces densely covered by trichomes. I, J. V. longicaulis, trichomes remnants (arrows) on adaxial surface (I) and intact trichomes on abaxial surface (J). K, L. Peltate trichome in longitudinal section on abaxial surface showing stalk with five cells (asterisks) in V. botafogensis (K) and with six cells (asterisks) in V. psittacina (L). [Scale bars = 5 μm (C), 10 μm (B), 20 μm (F, K, L), 100 μm (A, D, E, I, J), 250 μm (G, H).]
Peltate trichomes occur on both adaxial and abaxial leaf surfaces (Fig. 1E). In surface view, these comprise a circular shield of four triangular cells (Fig. 1F), surrounded by two concentric series of thin-walled, quadrangular cells: the first series with eight cells and the second with sixteen cells (Fig. 1F). The two series are surrounded by a variable number of radially elongated cells in symmetrical arrangement. Among the species analysed, only V. botafogensis, V. flava and V. pabstii show intact trichomes on both adaxial and abaxial leaf surfaces (Fig. 1G, H). In the other species, intact trichomes occur only on the abaxial surfaces (Fig. 1I, J). In longitudinal section, the trichome stalk has five cells (V. billbergioides, V. botafogensis (Fig. 1K), V. cacuminis, V. ensiformis, V. flava, V. guttata, V. hydrophora, V. philippocoburgii, V. saundersii and V. sincorana) or six cells (V. eltoniana, V.flexuosa V. gradata, V. incurvata, V. jonesiana, V. longicaulis, V. pabstii, V. paratiensis, V. procera, V. pseudatra, V. psittacina (Fig. 1L), V. sucrei, V. vagans and V. zildae).
In surface view, the epidermal cells are transversely oblong and are covered by a smooth cuticle. The anticlinal walls of epidermal cells (Fig. 2A) or only the anticlinal walls parallel to their major axis (V. ensiformis (Fig. 2B) and V. jonesiana) are thickened. In V. gradata and V. psittacina (Fig. 2C), silica bodies occur in the epidermal cells of the abaxial surface. Epicuticular wax occurs on both surfaces of the leaf blade, sometimes hiding the trichomes (V. ensiformis, V. gradata (Fig. 2D) and V. sucrei).
Leaf blade of Vriesea species in surface view (scanning electron microscope, SEM, A–D) and in cross section (E–H). A. Vriesea psittacina, epidermal cells on abaxial surface, showing thickened anticlinal walls (arrows). B. V. ensiformis, epidermal cells on abaxial surface, showing only anticlinal walls parallel to major axis (arrows) thickened. C, D. V. gradata: abaxial surface showing epidermal cells with silica bodies (arrows in C) and peltate trichome covered by epicuticular wax (asterisks in D). E. V. vagans, subjected to the PAS reaction, showing pectin in the anticlinal and inner periclinal walls of epidermal cells. F. V. eltoniana, stomata, abaxial surface. G. V. flava, stomata and substomatic chamber, abaxial surface. H. V. hydrophora, stomata and substomatic chamber, abaxial surface. [Abbreviations: EC = Epidermal Cells, St = Stomata. Scale bars = 10 μm (B, F), 20 μm (C), 50 μm (A, G, H), 100 μm (D, E).]
In cross-section, the leaf blade presents a one-layered epidermis on both surfaces (Fig. 2E). Epidermal cells have thickened anticlinal and inner periclinal walls, both of which present pectin (Appendix 1, Fig. 2E). The stomata are positioned at the same level as the epidermal cells (Fig. 2F). The substomatic chambers are continuous with the air lacunae, being short in V. billbergioides, V. flava (Fig. 2G), V. gradata, V. guttata, V. incurvata, V. jonesiana, V. longicaulis, V. pseudatra, V. psittacina, V. sincorana, V. sucrei, V. vagans and V. zildae, or elongated in V. botafogensis, V. cacuminis, V. eltoniana, V.ensiformis, V. flexuosa, V. hydrophora (Fig. 2H), V. pabstii, V. paratiensis, V. philippocoburgii, V. procera and V. saundersii.
The mechanical hypodermis is usually one-layered and occurs on both leaf surfaces in most species (Fig. 3A), except in V. flava (Fig. 3B) and V. jonesiana in which it is on only the abaxial surface. In V. saundersii there are two layers of mechanical hypodermis on both sides of the leaf blade (Fig. 3C). The hypodermal cells have slightly thickened walls in V. billbergioides, V. eltoniana, V. ensiformis, V. flava, V. gradata (Fig. 3D), V. guttata, V. incurvata, V. jonesiana, V. psittacina, V. sincorana, V. sucrei, V. vagans and V. zildae, and strongly thickened walls in V. botafogensis, V. cacuminis, V. flexuosa, V. hydrophora, V. longicaulis, V. pabstii, V. paratiensis, V. philippocoburgii, V. procera (Fig. 3A), V. pseudatra and V. saundersii. Treatment of samples with iodated zinc chloride (Fig. 3E) and phloroglucin + HCl (Fig. 3F) suggests such thickening is not associated with the presence of lignin. Extravascular fibres (Fig. 3G) are positioned below the mechanical hypodermis on the adaxial side of the leaf blade in all species analysed except V. eltoniana (Fig. 3H), V. flexuosa, V. incurvata, V. jonesiana, V. procera, and V. pseudatra.
Leaf blade in cross-section. A. Vriesea procera note mechanical hypodermis adaxial surface. B. V. flava note hypodermis adaxial surface. C. V. saundersii note two-layered mechanical hypodermis adaxial surface. D. V. gradata hypodermal cells on adaxial surface showing slightly thickened walls (arrow). E. V. ensiformis subjected to the iodated zinc chloride test showing starch grains in chlorenchyma cells thickened-walled epidermal and hypodermal cells as well as extra and perivascular fibres. F. V. botafogensis subjected to the phloroglucinol + HCl test indicating lignin presence in extra and perivascular fibres. G. V. vagans showing extravascular fibres (arrows) positioned below the mechanical hypodermis on the adaxial side of the leaf blade. H. V. eltoniana mesophyll showing the absence of extravascular fibres. I. V. philippocoburgii mesophyll showing water storage parenchyma and air lacunae. J. V. cacuminis mesophyll showing water storage parenchyma. [Abbreviations: AL = Air-Lacuna, Ch = Chlorenchyma, Hy = Hypodermis, MH = Mechanical Hypodermis, WP = Water-storage Parenchyma. Scale bars = 50 μm (A, B, C, D), 100 μm (E, G, H), 200 μm (F, I, J).]
Two to five layers of water-storage parenchyma lie adjacent to the hypodermis and usually have rounded, thin-walled cells. In most species analysed, the number of layers of this tissue is the same in both leaf surfaces (Fig. 3I). In V. flava, V. guttata and V. cacuminis (Fig. 3J), the number of layers is higher in the adaxial surface (3–4) than in the abaxial surface (1–2). The cells of the water-storage parenchyma have concertina-like walls in the abaxial leaf surfaces of V. gradata, V. jonesiana (Fig. 4A) and V. psittacina, (Fig. 4B).
Leaf blade in cross-section. A. Vriesea jonesiana, cells of water-storage parenchyma showing concertina-like walls (arrows). B. V. psittacina, concertina-like walls (arrows) (SEM). C. V. saundersii, vascular bundles sheathed by sclerenchyma fibres (arrows), and chlorenchyma. D. V. pabstii, note chlorenchyma. E. V. cacuminis, diaphragm with compactly arranged arm-cells. F. V. paratiensis, diaphragm with arm-cells widely spaced. G. V. saundersii, note idioblast with raphides in the brachiform parenchyma at arrow. H. V. sucrei, note vascular bundle. I. V. paratiensis, general view of the mesophyll. J. V. sincorana, note sclerenchyma fibres reaching the hypodermis. [Abbreviations: AC = Arm-Cells, Ch = Chlorenchyma, Sc = Sclerenchyma. Scale bars = 50 μm (A-H), 100 μm (J), 200 μm (I)]
In all species examined, the chlorenchyma is located in the median portion of the blade. Air lacunae are associated with brachiform parenchyma. The vascular bundles are collateral, arranged alternately with the air lacunae and surrounded by a sheath of sclerified and/or parenchyma cells (e.g., Fig. 4C). The chlorenchyma is linearly arranged in most species (Fig. 4D) but concave-convex in V. hydrophora, V. pseudatra and V. saundersii (Fig. 4C). The chlorenchyma cells are anticlinally elongated (V. flexuosa, V. hydrophora, V. paratiensis, V. philippocoburgii, V. procera, V. pseudatra, V. saundersii (Fig. 4C) and V. vagans) or periclinally elongated (V. billbergioides, V. botafogensis, V. cacuminis, V. eltoniana, V. ensiformis, V. flava, V. gradata, V. guttata, V. incurvata, V. jonesiana, V. longicaulis, V. pabstii (Fig. 4D), V. psittacina, V. sincorana, V. sucrei and V. zildae).
Air lacunae in the abaxial portion of the mesophyll are traversed by diaphragms with compactly arranged arm-cells in V. billbergioides, V. botafogensis, V. cacuminis (Fig. 4E) V. eltoniana, V. ensiformis, V. flexuosa, V. guttata, V. incurvata, V. jonesiana, V. pabstii, V. pseudatra, V. sincorana, V. sucrei, V. vagans, and V. zildae. The arm-cells are more widely spaced in V. flava, V. gradata, V. hydrophora, V. longicaulis, V. paratiensis, (Fig.4F), V. philippocoburgii, V. procera, V. psittacina, and V. saundersii. Idioblasts with raphides are present in the brachiform parenchyma in V. billbergioides, V. botafogensis, V. cacuminis, V. gradata, V. guttata, V. hydrophora, V. incurvata, V. longicaulis, V. pabstii, V. paratiensis, V. procera var. procera, V. procera var. tenuis, V. saundersii (Fig. 4G), V. sincorana, and V. sucrei. The chlorenchyma cells and arm-cells were found to be rich in starch grains in all species, except for V. cacuminis, V. flava, V. guttata, V. incurvata, V. jonesiana, V. pabstii and V. sincorana (Appendix 1).
The collateral vascular bundles are surrounded by sclerenchyma fibres and parenchyma cells (Fig. 4C) or have clusters of sclerenchyma fibres positioned at their poles and interconnected by parenchyma cells (Fig. 4H). On the adaxial face, the sclerenchyma fibres reach the water-storage parenchyma in V. botafogensis, V. flexuosa, V. guttata, V. paratiensis (Fig. 4I), V. philippocoburgii, V. procera, and V. vagans, and reach the hypodermis in V. cacuminis, V. eltoniana, V. ensiformis, V. gradata, V. incurvata, V. jonesiana, V. longicaulis, V. pabstii, V. pseudatra, V. psittacina, V. sincorana (Fig. 4J), V. sucrei, and V. vagans.
In cross-section, the leaf margins are rounded in V. incurvata, V. pabstii, and V. sucrei (Fig. 5A), oblique in V. pseudatra, V. sincorana, V. flava and V. hydrophora (Fig. 5B), and truncate in V. billbergioides, V. botafogensis, V. eltoniana, V. ensiformis, V. flexuosa, V. gradata, V. guttata, V. jonesiana, V. longicaulis, V. philippocoburgii, V. procera, V. pseudatra, V. psittacina, V. saundersii, and V. zildae (Fig. 5C). The one-layered epidermis has thickened cell walls, except for the outer periclinal wall that is slightly thinner (Fig. 5D). Trichomes (Fig. 5E) and stomata (Fig. 5F) may be present on the leaf margin. The number of mechanical hypodermis layers varies among species: one layer in V. botafogensis (Fig. 5F), V. cacuminis, V. ensiformis, V. flava, V. flexuosa, V. guttata, V. procera, V. sucrei and V. vagans; two layers in V. eltoniana, V. gradata, V. hydrophora, V. incurvata, V. jonesiana, V. longicaulis, V. pabstii, V. paratiensis, V. philippocoburgii (Fig. 5G), V. pseudatra, V. psittacina, V. sincorana and V. zildae; and three layers in V. saundersii (Fig. 5H). Water-storage parenchyma, chlorenchyma and vascular bundles also occur adjacent to the leaf margin. Vascular bundles are surrounded by sclerenchyma fibres that reach the water-storage parenchyma on the adaxial face (Fig. 5I). In V. botafogensis, V. cacuminis, V. flava, V. flexuosa, V. gradata, V. jonesiana, V. longicaulis, V. paratiensis, V. philippocoburgii, V. psittacina, V. sincorana, V. sucrei, V. vagans and V. zildae, the bundle sheath reaches the hypodermis (Fig. 5J), and in V. saundersii, it reaches and accompanies the hypodermis towards the adaxial surface of the leaf blade (Fig. 5H).
Leaf margin, in cross-section. A. Vriesea sucrei. B. V. hydrophora. C. V. zildae. D. V. ensiformis, note epidermis with thickened cell walls, except for the outer periclinal wall that is slightly thinner. E. V. flexuosa, note peltate trichome (arrows). F. V. botafogensis, note stomata (arrow). G. V. philippocoburgii. H. V. saundersii. I. V. procera. J. V. paratiensis. [Abbreviations: MH = Mechanical Hypodermis, Sc = Sclerenchyma. Scale bars = 50 μm (D, F), 100 μm (A, B, C, E, G), 200 μm (H, I, J)]
Sheath anatomy
In cross-section, the sheath has a one-layered epidermis (Fig. 6A). The epidermal cells have thick walls, especially the inner periclinal walls. Such parietal thickening is most pronounced in the abaxial leaf surface (Fig. 6A). The epidermal cells of V. billbergioides, V. botafogensis (Fig. 6B) and V. gradata show uniform wall thickness in both leaf surfaces. In the sheath region, the leaf surface is flat (Fig. 6B) or sometimes undulating due to the presence of trichomes. Among the analysed species, surface undulation may occur on both surfaces (Fig. 6C) or on only one surface (Fig. 6D). Peltate trichomes occur on both surfaces in all species analysed. In longitudinal section, the trichome stalk has five cells in V. billbergioides, V. botafogensis, V. cacuminis, V. ensiformis, V. flava, V. guttata (Fig. 6E), V. hydrophora, V. longicaulis, V. philippocoburgii, V. sincorana, and V. sucrei, and six cells in V. eltoniana, V. flexuosa, V. gradata, V. incurvata, V. jonesiana, V. pabstii, V. paratiensis, V. procera, V. pseudatra, V. psittacina, V. saundersii (Fig. 6F), V. vagans, and V. zildae. Stomata do not occur in the sheath.
Sheath, in cross-section. A. Vriesea eltoniana. B. V. botafogensis. C. V. pabstii, note surface undulation on both sides (arrows). D. V. paratiensis, note surface undulation on abaxial side (arrows). E. V. guttata, peltate trichome in longitudinal section showing stalk with five cells (asterisks). F. V. saundersii, peltate trichome in longitudinal section showing stalk with six cells (asterisks). G. V. longicaulis, mesophyll showing mechanical hypodermis only on abaxial surface. H. V. gradata, idioblast containing raphides (arrow) in the mesophyll. I. V. saundersii, detail of an idioblast containing raphides in brachiform parenchyma. J. V. saundersii, detail of the extravascular fibres. K. V. vagans, mesophyll showing vascular bundles and air-lacunae. L. V. sincorana, sheath margin. [Abbreviations: AL = Air-Lacunae; EF = Extravascular Fibres. Hy = Hypodermis; MH = Mechanical Hypodermis. Scale bars = 10 μm (E, I), 20 μm (F), 50 μm (J, L), 100 μm (A, B, G, H), 200 μm (C, D, K)]
The hypodermis is one-layered and occurs on both faces. In V. eltoniana, V. flexuosa, V. guttata, V. hydrophora, V. jonesiana, V. longicaulis (Fig. 6G), V. pabstii, V. procera, V. pseudatra, V. sincorana, V. vagans, and V. zildae, it is mechanical, with the hypodermal cells having thickened walls only on the abaxial face. The mesophyll consists of water-storage parenchyma and brachiform parenchyma, in which idioblasts containing raphides occur (Fig. 6H, I). Extravascular fibres, arranged in groups, occur below the adaxial hypodermis and may reach the water-storage parenchyma in V. botafogensis, V. ensiformis, V. gradata and V. saundersii (Fig. 6J). There is no chlorenchyma.
The collateral vascular bundles are surrounded by sclerenchyma fibres and parenchymatous cells or have clusters of sclerenchyma fibres positioned at their poles and interconnected by parenchyma cells. The air lacunae are associated with brachiform parenchyma and occur between the vascular bundles. The air lacunae are relatively wide in V. billbergioides, V. botafogensis, V. cacuminis, V. ensiformis, V. pabstii, V. philippocoburgii, V. procera, V. pseudatra, V. saundersii, V. sucrei, and V. vagans (Fig. 6K), and very narrow and difficult to discern in V. eltoniana, V. flexuosa, V. incurvata, V. jonesiana, V. longicaulis (Fig. 6G), V. paratiensis, V. psittacina, V. sincorana, and V. zildae.
In cross-section, the sheath margin is thin and composed only of epidermis (Fig. 6L).
Discussion
Leaf anatomy in relation to environment
The 24 Vriesea species analysed have predominantly xeromorphic structural characteristics in the leaves. The term xeromorphic is used for plants that present an anatomy and growth form specially adapted to environmental conditions in which water is frequently scarce (Mauseth, 1988). Shields (1950) listed the following foliar anatomical characters related to xeric environments: thickened cell walls, the presence of water-storage parenchyma and absorbent trichomes, and sparsely-distributed stomata.
The peltate trichomes of Vriesea species are morphologically similar to those found in representatives of several clades of Bromeliaceae, being mentioned in the general description for the family by Tomlinson (1969). Several authors have suggested that such trichomes are a synapomorphy for the family (Tomlinson, 1969; Gilmartin & Brown, 1987; Givnish et al., 2004, 2007). In a developmental study, Mantovani & Iglesias (2005) reported that the cytoplasmic contents of the peltate trichomes of Bromeliaceae are lost by the time the trichome reaches maturity. Benzing (1976) further noted that the wing cells of such trichomes lose their cytoplasmic contents, while simultaneously acquiring a mechanism for water pumping. In the latter, free water from the environment first enters the cells of the shield and then passes on to the cells of the stalk (following a gradient of increasingly negative water potential) and then continues on to the parenchyma of the mesophyll. This specialised mechanism, which also permits the absorption of water vapour (De Santo et al., 1976), was associated with the structural and ultrastructural characteristics of the fully developed trichome by Papini et al. (2009). Recently, Kowalski et al. (2016) reported living wing cells with cytoplasmic content in peltate trichomes of Vriesea species, even at trichome maturity. This condition would presumably prevent capillarity action and consequent water acquisition (Mantovani & Iglesias, 2005). In the current study, cytoplasmic content in wing cells was observed only in trichomes from the leaf sheath. However only leaf sheath samples were fixed; samples of leaf blades were preserved, not fixed, which does not assure the maintenance of the cytoplasmic content of the cells.
In addition to facilitating the absorption of water and associated mineral salts, peltate trichomes may also facilitate harmonic associations with bacteria that promote nutrient absorption (Kleingesinds, 2016) and function to shield stomata from excessive transpiration (Benzing, 2000).
The distribution of peltate trichomes on the leaf surfaces of species of Bromeliaceae may be related to environmental conditions (Stefano et al., 2007; Papini et al., 2009). In most genera of Tillandsioideae, the distribution of trichomes is seemingly random on both leaf surfaces, without obvious organisation (Benzing et al., 1978; Adams III and Martin, 1986). The dense trichome layers present in many Tillandsioideae are usually hydrophilic, unlike those of Bromelioideae and Pitcairnioideae. If these trichomes are positioned overlying the stomata, they may hinder gas exchange and will likely restrict CO2 uptake, if water-soaked. Meanwhile, adjacent tillandsioid trichomes that overlap one another present flexible wings, which overlap only when hydrated (wet). When the leaf is dry, the wings are flexed upwards, and the epidermal cells are exposed. Moveable wings are generally associated with higher leaf trichome densities (Pierce et al., 2001), and seem to be present in Vriesea pabstii, a epiphyte species from the Atlantic Rainforest. As reported here for most of the Vriesea species analysed, and in the study of Proença & Sajo (2007), peltate trichomes occur on both the adaxial and abaxial leaf surfaces, although in the mature leaves from most species they remain intact only on the abaxial surface, where they are organized in rows, alternating with the stomata. Strehl (1983) found a similar pattern in species of Guzmania and Kleingesinds et al. (2018) reported a more uniform distribution pattern in leaves of mature-tank plants of Guzmania monostachia (L.) Rusby ex Mez. The linear organisation of the trichomes and spatial separation from the stomata in Vriesea may be advantageous in humid environments if the stomata remain open when the surface is moist. Organisation into rows is also found in the subfamilies Bromelioideae and Pitcairnioideae and is considered a plesiomorphic character within the family (Strehl, 1983).
The presence of epicuticular wax on plant surfaces is related to the sealing of the epidermal surface, reducing the absorption of sunlight and thus reducing heating (Mauseth, 1988; Evert, 2013). It may also cause the leaf surface to be hydrophobic, maintain gas exchange during wet weather, and potentially obstruct pathogens and particulates, aid in self-cleaning (Pierce et al., 2001). Our finding of epicuticular wax in leaves of all the studied species further substantiates the report for Vriesea by Machado (2017). Epicuticular wax has been reported in the several other genera of the Bromeliaceae, including Aechmea, Brocchinia, Catopsis (Benzing et al., 1985; Pierce et al., 2001; Palací et al., 2004), and Alcantarea (Versieux et al., 2010).
The epidermal cells present anticlinal and inner periclinal walls that are thickened with pectin (Appendix 1). Pectin, a branched polysaccharide that adsorbs water molecules, helps maintain hydration at both cell and tissue levels. In all Vriesea species studied to date, the hypodermis has been described as mechanical and with thickened cell walls (Appendix 2). According to Tomlinson (1969), mesophytic bromeliads present hypodermal cells with thin walls that are only slightly lignified and are difficult to distinguish from the water-storage parenchyma cells. Tomlinson (1969) cites species belonging to Canistrum, Catopsis, Fosterella, Guzmania, Pitcairnia, Tillandsia and Vriesea as examples. In the species studied here, the parietal thickening is conspicuous in the epidermal and hypodermal cells but the presence of lignin was not detected histochemically (Appendix 1). Two hypotheses are here proposed to understand the negative result for the acidic phloroglucin test in the epidermal and mechanical hypodermal cells: (1) there was no lignin in the walls of these cells, and (2) there are special chemical characteristics of the lignin that mask the positive result. The first hypothesis is questionable, since safranin staining showed positive results in the same tissues. As to the second hypothesis, phloroglucinol is known to react with aldehydic regions of the lignin polymer by condensation (Ishikawa, 1951 apud Brauns & Brauns, 1960). Safranin, by contrast, is not a specific reagent, staining basophilic, lignified, cutinised, suberised and chitinised substances (Johansen, 1940). The product of phloroglucinol condensation is a compound with characteristic colour, which exhibits conjugated double bonds and delocalised electrons (Pew, 1951). It is possible that, because the leaf surface is subject to intense solar radiation, with the likelihood of photo-oxidation (George et al., 2005), the lignins in the epidermis and hypodermis present few sites of aldehydic nature. This would justify the negative test for phloroglucinol, since it has been proven that purified coniferilic alcohol does not show staining in the test, as well as other compounds of non-aldehydic nature found in lignin (Klason, 1929; Pew, 1951).
The characteristics discussed above (trichomes, wax, epidermal cells walls) probably play essential roles in the maintenance of gas exchange, as well as hydration of the leaf surface and thus of the mesophyll.
In all species analysed, the leaves are hypostomatic, with stomata positioned at the same level as the surrounding epidermal cells. Such characteristics have previously been reported for Vriesea species occurring in different phytogeographical domains such as the Atlantic Rainforest (Arruda & Costa, 2003; Gomes-da-Silva et al., 2012; Machado, 2017) and the Cerrado (Proença & Sajo, 2007; Machado, 2017) and was also described for species of Tillandsia and Aechmea that occur in different vegetation types (Scatena & Segacin, 2005; Proença & Sajo, 2007). Stomata positioned at the same level as the surrounding epidermal cells are mesophytic characteristics of plants that live in humid environments (Fahn & Cutler, 1992). In this specific case, water molecules that exit a stoma, bounce against air molecules repeatedly changing direction but gradually leaving the surface with small probability to bounce back into the stoma (Mauseth, 1988). Hence, we suggest that in Bromeliaceae (and Vriesea) the location and positioning of the stomata in relation to the surrounding epidermal cells are more likely related to shared ancestry than to environmental adaptation.
The parenchyma, present in both surfaces of the leaves analysed, has the function of water storage and presents large cells with thin walls and large expanded vacuoles. These are typical of succulent plants such as many Cactaceae and Euphorbiaceae (Mauseth, 1988). In Vriesea jonesiana and V. psittacina the water-storage parenchyma cells walls are of the concertina type, also found in species of Bromelia, Neoregelia and Quesnelia (Pereira, 2011; Reinert et al., 2011). These flexible concertina walls are able to shorten or lengthen vertically without disrupting cell interconnections in the interior of the leaf as it periodically loses and gains water. Concertina cells may be an anatomical adaptation allowing these leaves to remain evergreen and survive not only for extended periods of drought, but also to store water quickly when it becomes available. Such characteristics have also been described for Cordeauxia edulis Hemsl and Stuhlmannia moavi Taub, Leguminosae species, by Curtis et al. (1996). Vriesea jonesiana and V. psittacina are epiphytes found in the rainforest, and concertina walls may have been one of the adaptive strategies related to their success.
In Vriesea, the chlorenchyma adjacent to the adaxial water-storage parenchyma presents a linear to concave-convex (arch-like) arrangement, perhaps a developmentally constrained feature related to leaf blade narrowing.
The air lacunae, with arm-celled diaphragms, are connected to the substomatic chambers and function to increase the diffusion of oxygen and other respiratory gases, besides giving greater elasticity to the leaf blade (Mauseth, 1988; Evert, 2013). The largest species, with rosettes forming bigger tanks, have broader air lacunae. Starch grains occur in the chlorenchyma cells (including diaphragm arm-cells). Starch is the most abundant carbohydrate in plants. Starch grains are stored temporarily in the chloroplasts when plants are photosynthetically active. Later, they are broken down into sugars and transported to storage cells where they are re-synthesised into starch and accumulated in amyloplasts for more permanent energy storage (Evert, 2013). In an experimental study with plants of Vriesea gigantea Gaudich., Gobara (2015) suggested that the species tolerates water stress by lowering (making more negative) water potential through accumulation of osmoregulatory substances in the leaves, such as glucose and fructose. These are soluble carbohydrates that can originate from the conversion of starch. So, we suggest that the stored starch in the chlorenchyma may be related not only to energy storage but also to osmoregulation and water stress tolerance.
The species studied here have vascular bundles surrounded by parenchymatic and/or sclerenchymatic sheaths, of varied calibres and alternating with the air-lacunae throughout the leaf mesophyll, both in the blade and in the sheath. The lignified sclerenchymatic fibre sheath confers greater rigidity to the leaf blade and also helps protect the vascular bundles. Extravascular fibres, also associated with support, occur in some of the species analysed and are also reported for other species of Vriesea (Arruda & Costa, 2003; Gomes-da-Silva et al., 2012; Machado, 2017). Extravascular fibres are rare in subfamily Tillandsioideae. They are characteristic of the genus Alcantarea (Versieux et al., 2010) and mentioned only for one species of Stigmatodon (Couto, 2017). The presence of extravascular fibres was described for representatives of Aechmea, a genus of subfamily Bromelioideae, by Proença & Sajo (2007). There are no reports of the occurrence of extravascular fibres in subfamily Pitcairnioideae. It is a consensus among the above-mentioned authors that this character is associated with particular taxonomic groups and not with particular environments.
Stomata do not occur in the leaf sheath, as has also been noted by Arruda & Costa (2003) for Vriesea, by Couto (2017) for Stigmatodon, and by Voltolini et al. (2009) for Dyckia distachya Hassler. The absence of stomata is probably related to the location and superposition of the leaves, and the absence of chloroplasts and thus of photosynthesis. Also in the sheath, the water-storage parenchyma is distinctive, with sinuous cell walls—the degree of sinuosity probably depending on the hydration status of the leaf.
Leaf Anatomy and Systematic Implications
This study identifies consistent structural features to characterise the leaf anatomy of Brazilian species of Vriesea s.s.(Machado et al., 2020), including: leaves hypostomatic with peltate trichomes; epidermis with thickened cell walls, with lignin and pectin, covered by cuticle and epicuticular wax; mechanical hypodermis usually one-layered; water-storage parenchyma in both surfaces of the leaf blade; chlorenchyma located in the median portion of the blade; air lacunae associated with brachiform parenchyma; collateral vascular bundles arranged alternately with the air lacunae and surrounded by a sheath of sclerified and/or parenchyma cells; extravascular fibres (75% of the analysed species) positioned below the mechanical hypodermis on the adaxial surface of the leaf blade.
The two sections—Vriesea sect. Vriesea and V. sect. Xiphion—were established traditionally, based on floral morphology (Mez, 1896; Smith & Downs, 1977). The current study and data from the literature (Appendix 2) indicate that species of V. sect. Vriesea and of V. sect. Xiphion lack particularly distinctive leaf anatomical features, but some prevailing character states (more than 50% of the species from each section) are worth mentioning, including: the presence of mechanical hypodermis on both sides of the leaf blade; the same number of layers of water-storage parenchyma in both leaf surfaces; the presence of extravascular fibres and the chlorenchyma arranged linearly. In relation to clorenchyma cells and arm-cells, the majority of species from V. sect. Vriesea have chlorenchyma cells anticlinally elongated and arm-cells compactly arranged, while the majority of the species from V. sect. Xiphion have chlorenchyma cells periclinally elongated and the arm-cells widely spaced. On this basis, it is clear that exclusive character states do not occur in these two groupings. Nevertheless, when smaller groups of species are addressed (e.g. species pairs) leaf anatomical analysis can be useful in differential characterisation.
Characters for diagnosing species.—Vriesea botafogensis and V. saundersii are closely related (Clade IV; Machado et al., 2020) rupicolous species of V. sect. Vriesea that are endemic to coastal inselbergs in the city of Rio de Janeiro. These species have commonly been confused because of their morphological similarity and occurrence in the same geographic space (Leme & Costa, 1994). Nevertheless, their leaf anatomies show marked differences, for example the mechanical hypodermis has two layers in both leaf surfaces in V. saundersii, but only one layer in V. botafogensis. This character state seems to be related to the greater flexibility of the leaves of V. botafogensis, which are thinner and strongly spiralled along their longitudinal axes. Leaves of V. saundersii have a greater number of chlorenchyma layers (3–6) compared with the leaves of V. botafogensis (2–3). This difference may be related to the occurrence of V. saundersii in rocky outcrop bases, with greater protection from arboreal vegetation, while V. botafogensis occurs on the southern faces of outcrops and is more exposed to wind, sun and rain. Another good example concerns the species V. vagans and V. philippocoburgii, both belonging to V. sect. Vriesea and to Clade I (Machado et al., 2020). Vriesea vagans was first described as a variety of V. philippocoburgii. Both species are epiphytic (V. philippocoburgii is rarely rupicolous) and form large populations in open forest canopies, in ravines and on roadsides. Vriesea vagans has stolons and is slightly smaller. The leaf blade anatomies of these species also differ significantly in: the number of trichome stalk cells (six in V. vagans vs. five in V. philippocoburgii); the extension of the substomatic chamber (short in V. vagans vs. elongated in V. philippocoburgii); the presence of extravascular fibres (absent in V. vagans vs. present in V. philippocoburgii); and the arrangement of the arm-cells (compact in V. vagans vs. with large intercellular spaces in V. philippocoburgii).
Vriesea neoglutinosa and V. procera, both belonging to V. sect. Vriesea and to Clade X (Machado et al., 2020), species of the Brazilian Atlantic Rainforest domain, and may occur in sympatry (Uribbe, 2014). Vriesea neoglutinosa and V. procera present morphological similarities. The leaf blade anatomy differentiates the two species by the number of trichomes stalk cells (five in V. neoglutinosa vs. six in V. procera); the presence of extravascular fibres (present in V. neoglutinosa vs. absent in V. procera); the number of layers of water-storage parenchyma in each leaf surface (higher in the adaxial surface in V. neoglutinosa vs. the same in both leaf surfaces in V. procera); and the arrangement of the chlorenchyma (concave-convex in V. neoglutinosa vs. linear in V. procera).
Vriesea bituminosa is a species with wide distribution in the Atlantic Rainforest. Due to its morphological similarity with other species of V. sect. Xiphion the name V. bituminosa has been widely and erroneously used in floristic surveys (Moura, 2011). Vriesea zildae belongs to the same section but differs from V. bituminosa by the smaller dimensions of the floral bracts and narrower leaf blades. Although the species belong to the same morphological group, their leaf blade anatomy is quite distinct, with differences in the equivalence of the number of layers of water-storage parenchyma on each leaf surface (higher in the adaxial surface in V. bituminosa vs. the same in both leaf surfaces in V. zildae); the presence of extravascular fibres (present in V. bituminosa vs. absent in V. zildae); the arrangement of chlorenchyma (concave-convex in V. bituminosa vs. linear in V. zildae); and the arrangement of the arm-cells (compact in V. bituminosa vs. with large intercellular spaces in V. zildae).
Vriesea hydrophora and V. pabstii belong to V. sect. Xiphion and are very similar in the morphology of their rosettes and inflorescences. However, V. hydrophora has more robust inflorescences with a higher number of branches (Costa et al., 2007, Costa & Wendt, 2007). Both species occur in rainforest areas with intense rainfall and mist. Vriesea hydrophora differs from V. pabstii in robustness and in leaf blade characteristics such as: trichomes with shorter stalks; equivalence (vs. inequivalence) of the number of layers of water-storage parenchyma on both leaf surfaces; chlorenchyma with concave-convex outlines and with cells anticlinally elongated (vs. chlorenchyma linearly arranged and with cells periclinally elongated), and arm-cells with large spaces between (vs compactly arranged arm-cells).
Conclusion
The leaves of the Brazilian species of Vriesea s.s. analysed here show mainly xeromorphic structural characters, which include thickened cell walls, the presence of water-storage parenchyma, air-lacunae and absorbent trichomes. However, they also show some mesomorphic characters such as the location and position of the stomata. Thus, the obtained results on leaf anatomy seem to reflect not only ancestry (dry habitats) but also the recent arrival of the group in rainforests (wetter habitats). It is also possible that these species present different physiological strategies for coping with water stress and for the effective maintenance of gas exchange in humid environments. As such, we consider that leaf anatomy can act as a tool for diagnosing species, and also provide traits related to both ancestry and environmental adaptation.
Differences in leaf anatomy do not seem to correlate with the taxonomic sections of Vriesea or groups of Vriesea species occupying different substrate types. However, conserved aspects of leaf anatomy are quite helpful for characterising the Brazilian species of the genus, including: leaves hypostomatic with peltate trichomes; epidermis with thickened cell walls, with lignin and pectin, covered by cuticle and epicuticular wax; mechanical hypodermis usually one-layered; water-storage parenchyma in both surfaces of the leaf blade; chlorenchyma located in the median portion of the blade; air lacunae associated with brachiform parenchyma; collateral vascular bundles arranged alternately with the air lacunae and surrounded by a sheath of sclerified and/or parenchymatous cells; and extravascular fibres (in most species) positioned below the mechanical hypodermis on the adaxial surface of the leaf blade.
Literature cited
Adams III W. W. & C. E. Martin. 1986. Morphological changes accompanying the transition from juvenile (atmospheric) to adult (tank) forms in the Mexican epiphyte Tillandsia deppeana (Bromeliaceae). American Journal of Botany 73: 1207–1214.
Arruda, R. C. O. & A. F. Costa. 2003. Foliar anatomy of five Vriesea sect. Xiphion (Bromeliaceae) species. Selbyana 24: 180–189.
Barfuss, M. H. J., R. Samuel, W. Till & T. F. Stuessy. 2005. Phylogenetic relationships in subfamily Tillandsioideae (Bromeliaceae) based on DNA sequence data from seven plastid regions. American Journal of Botany 92: 337–351.
Barfuss, M. H. J., W. Till, E. M. C. Leme, J. P. Pinzón, J. M. Manzanares, H. Halbritter, R. Samuel & G. K. Brown. 2016. Taxonomic revision of Bromeliaceae subfam. Tillandsioideae based on a multi-locus DNA sequence phylogeny and morphology. Phytotaxa 279: 1–97.
Benzing, D. H., K. Henderson, B. Kessel & J. Sulak. 1976. The absorptive capacities of bromeliad trichomes. American Journal of Botany 63: 1009–1014.
Benzing D. H., J. Seemann & A. Renfrow. 1978. The foliar epidermis in Tillandsioideae (Bromeliaceae) and its role in habitat selection. American Journal of Botany 65: 359–365.
Benzing, D. H., T. J. Givnish & D. Bermudes. 1985. Absorptive trichomes in Brocchinia reducta (Bromeliaceae) and their evolutionary and systematic significance. Systematic Botany 10: 81–91.
Benzing, D. H. 2000. Bromeliaceae: profile of an adaptive radiation. Cambridge University Press, London.
Bouchenak-Khelladi, Y., A. M. Musaya & H. P. Linder. 2014. A revised evolutionary history of Poales: origins and diversification. Botanical Journal of the Linnean Society 175: 4–16.
Brauns, F. E. & D. A. Brauns. 1960. The chemistry of lignin covering the literature for the 1949–1958. Academic Press, San Diego.
Bukatsch, F. 1972. Bemerkungen zur doppel farburng Astrablau-Safranin. Mikrokosmos 6: 255.
Butcher, D. & E. J. Gouda. 2020. The new bromeliad taxon list. http://botu07.bio.uu.nl/bcg/taxonList.php.
Buzato, S., M. Sazima & I. Sazima. 2000. Hummingbird-pollinated floras at three Atlantic Forest sites. Biotropica 32: 824–841.
Costa, A. F., M. G. L. Wanderley & R. L. Moura. 2007. Vriesea (Bromeliaceae). Pp. 126–155. In: T. S. Melhem, M. G. L. Wanderley, S. E. Martins, S. L. Jung-Mendaçolli, G. J. Shepherd & M. Kirizawa (eds), Flora Fanerogâmica do Estado de São Paulo, FAPESP, São Paulo.
Costa, A. F. & T. Wendt. 2007. Bromeliaceae na região de Macaé de Cima, Nova Friburgo, Rio de Janeiro, Brazil. Rodriguésia 58: 905–939.
Costa, A. F., J. Gomes-da-Silva & M. G. L. Wanderley. 2014. Vriesea (Bromeliaceae, Tillandsioideae): taxonomic history, and morphology of the Brazilian lineage. Journal of the Torrey Botanical Society 141: 338–352.
Costa, A. F., J. Gomes-da-Silva & M. G. L. Wanderley. 2015. Vriesea (Bromeliaceae, Tillandsioideae): a cladistic analysis of eastern Brazilian species based on morphological characters. Rodriguésia 66: 429–440.
Couto, D. R. 2017. Revisão taxonômica e filogenia de Stigmatodon Leme, G.K. Br. & Barfuss (Bromeliaceae - Tillandsioideae): um grupo especialista de faces verticais dos inselbergs do leste do Brasil. PhD thesis, Universidade Federal do Rio de Janeiro, Brazil.
Curtis, J. D., N. R. Lersten & G.P. Lewis. 1996. Leaf anatomy, emphasizing unusual “concertina” mesophyll cells, of two East African legumes (Caesalpinoideae, Leguminosae). Annals of Botany (Oxford) 78: 55–59.
De Santo, A. V., A. Alfani & P. De Luca. 1976. Water vapour uptake from the atmosphere by some Tillandsia species. Annals of Botany (Oxford) 40: 391–394.
Evert, R. F. 2013. Anatomia das plantas de Esau, 1st ed. Blucher, São Paulo.
Fahn, A. & D. F. Cutler. 1992. Xerophytes. Encyclopedia of Plant Anatomy, Band XIII Teil 3, Gebrüder Borntraeger, Stuttgart.
Feder, N. & T. P. O’Brien. 1968. Plant microtechnique: some principles and new methods. American Journal of Botany 55: 123–142.
Gahan, P. B. 1984. Plant histochemistry and cytochemistry—an introduction. Academic Press Inc., London.
George, B., E. Suttie, A. Merlin & X. Deglise. 2005. Photodegradation and photostabilisation of wood—the state of the art. Degradation and Stability 88(2): 268–274.
Gilmartin, A. J. & G. K. Brown. 1987. Bromeliales, related monocots, and resolution of relationships among Bromeliaceae subfamilies. Systematic Botany 12: 494–500.
Givnish, T. J., K. C. Millan, T. M. Evans, J. Hall, J. C. Pires, P. E. Berry & K. J. Sytsma. 2004. Ancient vicariance or recent long-distance dispersal? Inferences about phylogeny and South American-African disjunctions in Rapateaceae and Bromeliaceae based on ndhF sequence data. International Journal of Plant Science 135(4 Suppl.): S35–S54.
Givnish, T. J., K. C. Millam, P. E. Berry & K. J. Sytsma. 2007. Phylogeny, adaptive radiation, and historical biogeography of Bromeliaceae inferred from ndhF sequence data. Pp. 3–26. In: J. T. Columbus, E. A. Friar, J. M. Porter, L. M. Prince, M. G. Simpson (eds.), Monocots: comparative biology and evolution—Poales. Rancho Santa Ana Botanic Garden, Claremont.
Givnish, T. J., M. H. J. Barfuss, B. V. Ee, R. Riina, K. Schulte, R. Horres, P. A. Gonsiska, R. S. Jabaily, D. M. Crayn, A. C. Smith, K. Winter, G. K. Brown, T. M. Evans, B. K. Holst, H. Luther, W. Till, G. Zizka, P. E. Berry & K. J. Sytsma. 2011. Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: insights from an eight-locus plastid phylogeny. American Journal of Botany 98: 872–895.
Givnish, T. J., M. H. J. Barfuss, B. V. Ee, R. Riina, K. Schulte, R. Horres, P. A. Gonsiska, R. S. Jabaily, D. M. Crayn, A. C. Smith, K. Winter, G. K. Brown, T. M. Evans, B. K. Holst, H. Luther, W. Till, G. Zizka, P. E. Berry & K. J. Sytsma. 2014. Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Molecular Phylogenetics and Evolution 71: 55–78.
Gobara, B. N. K. 2015. Caracterização da capacidade de indução ao CAM em plantas de Vriesea gigantea (Bromeliaceae) sob déficit hídrico. MSc thesis, Universidade de São Paulo, Brazil.
Gomes-da-Silva, J., F. A. C. Vargens, R. C. O. Arruda & A. F. Costa. 2012. A morphological cladistic analysis of the Vriesea corcovadensis group (Bromeliaceae: Tillandsiodeae), with anatomical descriptions: new evidence of the non-monophyly of the genus. Systematic Botany 37: 641–654.
Gomes-da-Silva, J. & T. T. Souza-Chies. 2017. What actually is Vriesea? A total evidence approach in a polyphyletic genus of Tillandsioideae (Bromeliaceae, Poales). Cladistics 2017: 1–19.
Jensen, W. A. 1962. Botanical histochemistry: principles and pratice. W. H. Freeman & Co., San Francisco.
Johansen, D. A. 1940. Plant microtechnique. MacGraw-Hill Comp. Book Inc., London.
Kessous, I. M., B. Neves, D. R. Couto, B. Paixão-Souza, L. C. Pederneiras, R. L. Moura, M. H. J. Barfuss, F. Salgueiro & A. F. Costa. 2020. Historical biogeography of a Brazilian lineage of Tillandsioideae (subtribe Vrieseinae, Bromeliaceae): the Paranaean Sea hypothesized as the main vicariant event. Botanical Journal of the Linnean Society 192: 625–641.
Klason, P. 1929. Beiträge zur Konstitution des Fichtenholz-Lignins. Berichte der deutschen chemischen Gesellschaft 62: 2523–2526.
Kleingesinds, C. K. 2016. Bactérias diazotróficas em Guzmania monostachia (Bromeliaceae): identificação, sinalização e colonização dos tecidos foliares. PhD thesis, Universidade de São Paulo, Brazil.
Kleingesinds, C. K., B. N. K. Gobara, D. Mancilha, M. A. Rodrigues, D. Demarco & H. Mercier. 2018. Impact of tank formation on distribution and cellular organization of trichomes within Guzmania monostachia rosette. Flora–Morphology, Distribution, Functional Ecology of Plants 2431: 11–18.
Kowalski, V., P. P. D. A. Pereira, F. M. C. Oliveira, M. E. Costa & R. C. Tardivo. 2016. Are the wing’s cells alive? Study case in Vriesea trichomes. Rodriguesia 67: 427–435.
Leme, E. M. C. & A. F. Costa. 1994. Vriesea botafogensis e Vriesea saundersii, duas espécies distintas. Bromélia 1: 11–18.
Leme, E. M., H. Halbritter & M. H. Barfuss. 2017. Waltillia, a new monotypic genus in Tillandsioideae (Bromeliaceae) arises from a rediscovered, allegedly extinct species from Brazil. Phytotaxa 299: 1–35.
Langeron, M. 1949. Précis de microscopie. Masson et Cie. Ed., Paris.
Machado, T. M. 2017. Aplicação de next generation sequence na filogenia da subfamília Tillandsioideae e estudos taxonômicos no complexo Vriesea itatiaiae. PhD thesis, Universidade Federal de Minas Gerais, Brazil.
Machado, T. M., O. Loiseau, M. Paris, A. Weigand, L. M. Versieux, J. R. Stehmann, J. R C. Lexer & N. Salamin. 2020. Systematics of Vriesea (Bromeliaceae): phylogenetic relationships based on nuclear gene and partial plastome sequences. Botanical Journal of the Linnean Society, 192: 656–674.
Maclean, R. C. & W. R. Ivemey-Cook. 1952. Textbook of practical botany. Longmans Greenands Co., London.
Males, J. 2016. Think tank: water relations of Bromeliaceae in their evolutionary context. Botanical Journal of the Linnean Society, 181: 415–440.
Mantovani, A. & R. R. Iglesias. 2005. Quando aparece a primeira escama? Estudo comparativo sobre o surgimento de escamas de absorção em três espécies de bromélias terrestres de restinga. Rodriguésia 73–84.
Martinelli, G., C. M. Vieira, M. Gonzalez, P. Leitman, A. Piratininga, A. F. Costa & R. C. Forzza. 2008. Bromeliaceae da Mata Atlântica brasileira: lista de espécies, distribuição e conservação. Rodriguésia, 59: 209–258.
Mauseth, J. D. 1988. Plant anatomy. The Benjamin/Cummings Publishing Company, California.
Melo Santos, A. M., D. R. Cavalcanti, J. M. C. D. Silva & M. Tabarelli. 2007. Biogeographical relationships among tropical forests in north-eastern Brazil. Journal of Biogeography, 34: 437-446.
Mez, C. 1896. Monographiae phanerogamarum. Prodomi Nunc Continuatio, Nunc Revisio, editore et proparte auctore Casimiro de Candolle, 1–990.
Moura, R. L. 2011. Revisão taxonônica do grupo Vriesea platynema Gaudich. (Bromeliaceae). PhD thesis, Universidade Federal do Rio de Janeiro, Brazil.
Palací A., G. K. Brown & D. E. Tuthill. 2004. Vegetative morphology and leaf anatomy of Catopsis (Tillandsioideae: Bromeliaceae). Selbyana 25: 138–150.
Papini, A., G. Tani, P. Di Falco & L. Brighigna. 2009. The ultrastructure of the development of Tillandsia (Bromeliaceae) trichome. Flora–Morphology, Distribution, Functional Ecology of Plants 205: 94–100.
Pereira, T. A. R. 2011. Anatomia foliar de Bromeliaceae Juss. do Parque Estadual do Itacolomi, Minas Gerais, Brasil. MSc thesis, Universidade Federal de Viçosa, Brazil.
Pew, J. C. 1951 Structural aspects of the color reaction of lignin with phenols. Journal of the American Chemical Society 73: 1678–1685.
Pierce, S., K. Maxwell, H. Griffiths & K. Winter. 2001. Hydrophobic trichome layers and epicuticular wax powders in Bromeliaceae. American Journal of Botany 88: 1371–1389.
Proença, S. L. & M. G. Sajo. 2007. Anatomia foliar de bromélias ocorrentes em áreas de cerrado do estado de São Paulo, Brazil. Acta Botanica Brasilica 21: 657–673.
Reinert, F., M. V. Leal-Costa, N. E. Junqueira & E. S. Tavares. 2011. Are sun- and shade-type anatomy required for the acclimation of Neoregelia cruenta? Anais da Academia Brasileira de Ciências 85: 561–573.
Sazima, M., S. Buzato & I. Sazima. 1999. Bat-pollinated flower assemblages and bat visitors at two Atlantic Forest sites in Brazil. Annals of Botany 83: 705–712.
Scatena, V. L. & S. Segecin. 2005. Anatomia foliar de Tillandsia L. (Bromeliaceae) dos Campos Gerais, Paraná, Brasil. Brazilian Journal of Botany 28: 635–649.
Silva, A. S. 2013. Anatomia foliar de Vriesea Lindl. (Tillandsioideae, Bromeliaceae). Monography, Universidade do Estado do Rio de Janeiro, Brazil.
Shields, L. M. 1950. Leaf xeromorphy as related to physiological and structural influences. Botanical Review 16: 399–447.
Smith, L. B. & R. J. Downs. 1977. Tillandsioideae (Bromeliaceae). Flora Neotropica Monograph 14: 663–1492.
Stefano, M., A. Papini & L. Brighigna. 2007. A new quantitative classification of ecological types in the bromeliad genus Tillandsia (Bromeliaceae) based on trichomes. Revista de Biologia Tropical 56: 191–203.
Strehl, T. 1983. Forma, distribuição e flexibilidade dos tricomas foliares usados na filogenia de Bromeliáceas. Iheringia Serie Botanica 31: 105–119.
Taboga, S. R. & P. S. L. Vilamaior. 2013. Citoquímica. Pp. 42–50. In: H. F. Carvalho & S. M. Recco-Pimentel (eds.), A Célula. Editora Manole, Barueri.
Tomlinson, P. B. 1969. Comelinales–Zingiberales. Pp. 193–294. In: C. R. Metcalfe (ed.), Anatomy of the monocotyledons: III. Claredon Press, Oxford.
Uribbe, F. P. 2014. Variação morfológica em Vriesea procera (Mart. ex Schult. & Schult. f.) Wittm. (Bromeliaceae,Tillandsioideae). MSc thesis, Universidade Federal do Rio de Janeiro, Brazil.
Versieux, L. M., P. M. Elbl, M. G. L. Wanderley & N. L. Menezes. 2010. Alcantarea (Bromeliaceae) leaf anatomical characterization and its systematic implications. Nordic Journal of Botany 28: 385–397.
Versieux, L. M., T. Barbará, M. G. L. Wanderley, A. Calvente, M. F. Fay & C. Lexer. 2012. Molecular phylogenetics of the Brazilian giant bromeliads (Alcantarea, Bromeliaceae): implications for morphological evolution and biogeography. Molecular Phylogenetics and Evolution, 64: 177–189.
Voltolini, C. H., A. Reis & M. Santos. 2009. Leaf morphoanatomy of the rheophyte Dyckia distachya Hassler (Bromeliaceae). Revista Brasileira de Biociências 7: 335–343.
Acknowledgements
We thank the staff of the Laboratory of Bromeliaceae Systematics and Laboratory of Plant Anatomy at the Museu Nacional of the Universidade Federal do Rio de Janeiro for assistance with field work and structural analyses, respectively. This study forms part of the master’s thesis of C.G.F., which was carried out in the Programa de Pós-graduação em Ciências Biológicas (Botânica), Museu Nacional, Universidade Federal do Rio de Janeiro (UFRJ), and was supported by funds from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). A.S.S. was supported by a scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Proc. 106916/2012-2). A.F.C. was supported by a productivity grant from CNPq (Proc. 305704/2018-4) and research grant from the Ministério da Ciência, Tecnologia, Inovações e Comunicações (MCTIC)/CNPq/Ministério da Educação (MEC)/CAPES, Programa de Capacitação em Taxonomia (PROTAX) (Proc. 562187/2010-3). B.S.H. was supported by research grant from the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (Proc. E-26/200.088/2019).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Faria, C.G., Silva, A.S., de Melo, R.K.P. et al. Leaf anatomy of Vriesea (Tillandsioideae–Bromeliaceae). Brittonia 73, 27–52 (2021). https://doi.org/10.1007/s12228-020-09645-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12228-020-09645-6