Abstract
The interrelationship between phenological events, climatic factors, periodicity of cambial activity and seasonal production of xylem was examined in Dillenia indica L. (Dilleniaceae) growing in sub-tropical wet forest of Meghalaya state, India. The reactivation of cambial activity was seen in the first week of May, 15 days after sprouting of new leaves and buds. The activity of cambium and xylem production gradually declined toward December and ceased from January to April end. There was correlation between leaf fall and cambial dormancy. It was evident from the correlation and regression analysis, the relationship between cambial activity, xylem production with climatic factors, the monthly mean minimum temperature plays an important role for the cambial activity and xylem production rather than influence by rainfall and relative humidity in D. indica L. The data were discussed in the light of cambial activity, xylem production and phenological events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The activity of vascular cambium is not uniform throughout the year and determined by the interaction of internal and external factors (Philipson et al. 1971; Larson 1994; Iqbal 1994; Grotta et al. 2005). The majority of past studies on cambial activity are pertained to plants growing in temperate region with definite seasonal climates (Bailey 1920; Bannan 1955, 1962; Antonova 1996, 1997; Antonova and Stasova 1997; Rensing and Samuel 2004). The seasonal variations of cambial activity and annual rhythm of xylem and phloem differentiation in tropical trees, semi-arid and arid regions have been studied in quite a number of plants (Coster 1927, 1928; Chowdury 1939, 1940, 1941; Koriba 1958; de Alvim 1964; Fahn et al. 1968; Amobi 1974; Ghouse and Hashmi 1978; Denne and Dodds 1981; Dave and Rao 1982; Venugopal 1986; Venugopal and Krishnamurthy 1987; Creber and Chaloner 1990; Larson 1994; Priya and Bhat 1999; Borchert 1999; Rao and Rajput 2001a, b). The effect of genetic and environmental factors on shoot growth and xylem formation has been studied in the West African tropical tree Terminalia superba Engl. and Diels (Combretaceae) (Longman et al. 1979). Periodicity of wood formation in twigs of 11 tropical trees was studied in different ecological areas, such as lowland rainforest, savannah and mangrove swamps in Nigeria (Amobi 1974). Recently, the wood production has been estimated in the natural forest stand in Cameroon by using tree ring analysis (Worbes et al. 2003). Cambial activity and annual rhythm of xylem development in trees and shrubs of desert plants Tamarix aphylla (L.) Karst., T. jordanis var. negevensis, T. gallica L. var. maris-mortui (Gutm.) Zoh., T. jordanis Boiss. var. negevensis Zoh., (Tamaricaceae) have been studied in Israel (Fahn 1958). In the Southeast Asian countries, a sizable number of trees have been studied. The earliest report on the growth of Tectona grandis L.f (Verbenaceae) was studied by Brandis (1856) by measuring ring width (Mariaux 1981). The correlation between cambial growth and rainfall has been assessed in the lowland dipterocarp forest of Peninsular Malaysia (Killmann and Thong 1995). Cambial activity, development of xylem and phenology have been studied in Azardiracta indica L. (Meliaceae) (Rao and Rajput 2001a, b); Tectona grandis L.f (Berlage 1931; Rao and Dave 1981; Venugopal and Krishnamurthy 1987; Rao and Rajput 2001a, b, 1999; Priya and Bhatt 1999); Acacia nilotica (L.) Del., Albizzia lebbeck Benth. (Mimosaceae) (Rao and Rajput 2000, 2001a, b; Venugopal and Krishnamurthy 1987), Dalbergia sissoo Roxb. ex DC (Fabaceae) (Ghouse and Yunus 1974); Venugopal and Krishnamurthy 1987) Polyalthia longifolia Sonn. (Annonaceae) (Ghouse and Hashmi 1978); Mangifera indica L. (Anacardiaceae) (Dave and Rao 1982; Venugopal and Krishnamurthy 1987); Calophyllum inophyllum L. (Clusiaceae), Morinda tinctoria Roxb. (Rubiaceae), Terminalia crenulata Roth. (Combretaceae) (Venugopal and Krishnamurthy 1987).
On the basis of phenological rhythms, cambial activity and climatic conditions, the tropical trees have been classified into four types (evergreen, stem succulent, deciduous and brevideciduous) growing under the same ecological conditions (Borchert 1999). The effect of flood on tree growth in the Amazon forest in relation to phenology and analysis of ring has been studied in detail (Schongart et al. 2002; Dezzeo et al. 2002; Worbes 1999). However, studies on the seasonal activity of vascular cambium and production of xylem in relation to different climatic factors in sub-tropical wet forest are scarce. Therefore, the present study on annual rhythm of cambial activity and differentiation of xylem in relation to phenology as well as the climatic improves our further understanding of tree growth in D. indica L. growing in sub-tropical wet forest of northeast India. Furthermore, this type of study is applicable to estimate tree's productivity and growth as well as evaluating past and present forest environments through tree ring research (Fritts 1976; Worbes 1995, 2002; Schweingruber 1988, 1996).
Material and method
Study area and climate
The upland area, where most of the sub-tropical mixed forest exists, was selected for the study in Botanical Survey of India, Experimental Garden, Shillong. (25°34′N and 91°53′E) with an elevation of 1,100 m a.s.l. The elevation above sea level is characterized by mountain climate or wet hill forest climate with low temperature and relatively high precipitation (Lal 2000; Worbes and Junk 1989). As the altitude increases, various climatic elements such as pressure, temperature and precipitation undergo well-defined changes (Lal 2000). The soil is loamy, reddish brown in colour and lateritic in origin. The pH ranges from 5.9 to 6.2 (Singh 1996; Porwal et al. 2000; Mishra et al. 2003, 2004). Climatologically, this study area belongs to the sub-tropical wet climatic region (Champion and Seth 1968). On the basis of variation of temperature, rainfall and wind, the year in the region may be divided into four distinct seasons: (1) winter (December–February), (2) pre-monsoon or summer (March–May), (3) monsoon (June–September) and (4) retreating monsoon (October and November) (Porwal et al. 2000; Dhirendra Singh 2002); Tripathi et al. 2004). This region receives abundant southwest monsoon from June to October. Highest rainfall is recorded in the months of June and July. The mean temperature ranges from 5 to 15°C in winter and from 20 to 25°C during summer. The data on the climatic factors were collected from Central Seismological and Meteorological observatory, Shillong station, Government of India. Monthly mean, mean maximum, mean minimum temperature, rainfall and relative humidity for the years 2002 and 2003 were chosen for this study (Fig. 1).
Field observations
Periodic collection of twigs measuring from 1.5 to 2 cm in diameter was made from ten plants growing in the Botanical Survey of India, Experimental Garden, Shillong. They were fixed in FAA (formalin-aceto-alcohol), Glutaraldehyde and Cornoy's fluid in the field itself at every fortnight interval for two consecutive years 2002 and 2003. The timing of different phenophases such as leaf fall, leaf flush, flowering, fruiting and seed dispersal was recorded during this period (Fig. 8).
Analyses in laboratory
The materials were processed through customary method of dehydration, paraffin embedding and sectioning at a thickness of 8–10 μm. The sections were stained either with tannic acid and ferric chloride counterstained with lacmoid blue (Cheadle et al. 1953) or with toluidine blue O (Feder and O’Brien 1968). For micro measurement, recently formed xylem tissue was carefully teased out and macerated according to Jeffrey's method (Berlyn and Miksche 1976). Total starch content and phenol were localized by using iodine-potassium iodide and Gibb's reagent, respectively (Johansen 1940; Gahan 1984; McCully 1966). All measurement were made on 100 samples of each of the wood elements. The mean length and breadth of fusiform initial were calculated from slides in Transverse Longitudinal Section (TLS) of 100 randomly chosen fusiform initial. Photographs were taken from Nikon E600 microscope.
Data analysis
The mean value and standard deviation were calculated for all the measurement made. Statistical analysis of relationship between climatic factors (monthly mean, maximum and minimum temperature, rainfall, relative humidity) and anatomical variables such as cambial zone width, length of fusiform initial, width of differentiating xylem zone, length of xylem fibre and vessel element were calculated by using Karl Pearson's correlation coefficient. Multiple (partial) regression analysis was done and (t) values were calculated to determine the influence of particular climatic factor on cambial activity and xylem production (Zar 1974; Schweingruber 1988).
Results
Cambial activity in relation to phenology
The first visible indication of dormancy breakage in D. indica was a slight swelling of young vegetative buds in the middle of April (pre-monsoon or summer season), during which, though three to four cambial layers remained, the fusiform initial showed marked radial swelling. By the middle of May, the maximum leaf flushing started and continued up to the end of December (end of pre-monsoon or summer season to the beginning of winter season). Sprouting of new leaves as well as persistent mature leaves were observed from middle of April to January (end of summer or pre-monsoon season to the middle of winter season). The number of cambial layers ranged from five to nine from May to December. Consequently, the cambial zone width also increased (Table 1). Leaf senescence was initiated from the month of February, and the defoliation continued up to the end of March (winter to the pre-monsoon season). The tree was completely barren for a brief period of 15 days; therefore, D. indica belongs to brevideciduous type (Borchert 1999). During winter, the cambium consisted of only three to four layers and the average width of the cambial zone remained more or less the same (Table 1). Flowering was noticed in the months of June and July (monsoon season). Fruiting was observed in the month of August, and mature fruits were borne on tree from September to the last week of October. The dispersal of seeds took place in the months of October–December (retreating monsoon period to the beginning of winter) (Fig. 8).
Cambial activity and xylem differentiation
In D. indica L., the vascular cambium was non-storied with axially elongated fusiform initials and ray initials; the ray initial were unicellular, uniseriate with rectangular cells, while the multiseriate rays were comprised of more or less isodiametric cells. The ray initials were filled with starch grains and phenolic contents (Figs. 2–3). During the active period of vascular cambium in 8 months from the first week of May to the last week of December (pre-monsoon to towards the beginning of winter seasons), the cambial zone was wider consisting of five to nine cell layers (Fig. 3) in each radial file. During the months of July–September (monsoon season), cell walls of both the fusiform and the ray initials were thin, and the beaded appearance of fusiform initials was not much prominent (Fig. 5). In contrast, during the dormant period of 4 months from January to the end of April (winter to the beginning of pre-monsoon seasons), the cambial zone was narrow, consisting of three to four layers only with relatively thick radial walls in transverse section and surrounded by mature xylem and phloem elements (Fig. 2). The initiation of cambial activity was marked by swelling of fusiform initials and the active vacuolation in the month of May (towards the end of pre-monsoon or summer season) and followed by periclinal divisions in the fusiform initials observed from May onwards. Consequently, the number of cells in the cambial zone and the width of cambial zone increased considerably. During the active period, the number of periclinal divisions was more than the number of anticlinal divisions (Fig. 6). The fusiform initials showed two to three nucleate and aseptate. The average data pertaining to the size of the cambial width zone, differentiating xylem zone, fusiform initial, xylem fibre and vessel element are shown for the different months of years 2002–2003 (Table 1). The cambial activity was slowed down from October to the end of December (from retreating monsoon to the beginning of winter season). In the months of November and December, the percentage of anticlinal divisions was more than the percentage of periclinal divisions. The cessation of cambial activity began towards the end of December, and dormancy was imposed from January to the end of April, and the tree was barren for a brief period of 15 days in April. The starch grains and protein bodies were masked by abundant phenolic contents in the ray initials during dormancy only, the raphids were noticed in the unicellular ray initial (Fig. 4) and on the phloem side; on the other hand, starch grains were more in the xylem rays and xylem parenchyma cells.
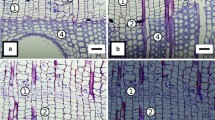
and 3 Transverse sections of Dillenia indica stem showing the dormant and active vascular cambium respectively. Note the cambium is only three layers in the dormant period and six to eight layers in the active cambium (white arrows). Both the active and the dormant ray initials filled with phenolic contents (DX, differentiating xylem zone; DV, differentiating vessel elements; Ph, phloem; bar, 70 μm)
–6 Tangential longitudinal sections (TLS) of vascular cambium in Dillenia indica. 4. Dormant period showing the beaded nature of fusiform initials, note the raphid needles are stored in the unicellular ray initials and phenolic contents in uni/multiseriate ray initials. 5 and 6. Active vascular cambium showing the thin cell walls and beads are not prominent. Note the multinucleate condition of fusiform initials. The ray initials contain starch grains masked by phenolic contents. Periclinal division in the fusiform initials with phragmoblast (white arrows) (R, raphides; Ri, ray initials; Fi, fusiform initials; bar, 15 μm in 4 and 5; bar 20 μm in 6)
Differentiation of xylem tissue
The secondary xylem of D. indica L. consists of libriform fibres, vessel elements and axial parenchyma apotracheal diffuse and paratracheal vasicentric scanty. Details regarding the timing of initiation and the cessation of xylem production as well as its duration and the average width of the cambial zone and the average width of differentiating xylem zone are given (Table 1). Xylem production was noticed for a total period of about 8 months in D. indica L. with the formation of new fibres, vessel elements and xylem rays in the month of May after the formation of new foliage and branches; it was continued up to the last week of December. A comparison was made between the average length of fusiform initials with that of libriform fibres and vessel elements in different months of the year to find out the change in length, if any, during differentiation of fibres and vessel elements (Fig. 7B). The average length of xylem fibres and vessel elements showed the same trend of variation as that of fusiform initials in D. indica L.
Starch grains, polyphenol and tannin contents are the major reserved products found in the ray initials of cambium, xylem rays, and axial parenchyma cells and occasionally in the xylem fibres during dormancy (Figs. 2–3). During the onset of cambial activity, the starch grains were reduced in amount but not totally absent. Phenolic substances do exist in both the dormant and active periods of vascular cambium. The fibres produced during May were thin walled with larger lumen and angled in transverse section, the cell wall thickness ranged from 1.5 to 2 μm (Fig. 3); in contrast, the cell wall thickness of the late wood fibres produced during November ranged from 8 to 10 μm, and the fibres were radially compressed and the lumen was very narrow (Fig. 2). Similarly, the vessel elements produced during the active period had sclariform perforation plates with 50–60 bars, whereas those produced during dormancy had the sclariform perforation plate with 25–30 bars.
Relationship between climatic factors, cambial activity and xylem production
During the onset of cambial reactivation and the differentiation of xylem elements from the first week of May to the last week of December (pre-monsoon to the beginning of winter), a strong positive correlation was shown with monthly minimum ambient temperature and the correlation coefficients were very high (Table 2). The response function and the correlation coefficient of the cambial activity (including cambial zone width, length of fusiform initials) versus the monthly mean minimum temperature (r=+0.85, r=+0.90) was higher than the monthly mean temperature and the mean maximum temperature, respectively (Table 2). Similarly, the width of differentiating xylem zone, length of xylem fibres and vessel elements showed more positive correlation coefficient with the ambient monthly mean minimum temperature (r=+0.88, r=+0.89 and r=+0.82) than precipitation and relative humidity (Table 2).
The multiple (partial) regression analysis has shown that rainfall was statistically significant but had inverse relationship with the cambial zone width (t≥−2.29), fusiform initials length (t≥−2.50), and the differentiating xylem zone width (t≥−2.55). The length of fibres did not show any relationship with any one of the climatic parameters because its t-value is less than 2.26. The length of vessel elements showed statistically significant positive correlation with monthly mean minimum ambient temperature (Table 2). Other climatic parameters were not statistically significant with any one of the quantitative data of the vascular cambium and its derivatives. However, among the mean maximum, mean minimum and mean temperature, the B-value of the mean minimum temperature showed higher value than the rest. Therefore, the mean minimum temperature had some effect on the cambial activity and wood formation. Moreover, during the onset of the cambial reactivation and differentiation of xylem elements during first week of May to the last week of December (pre-monsoon to the beginning of winter season), a positive correlation was shown with monthly minimum ambient air temperature and the correlation coefficients are very high (Table 2). The effect of rainfall and relative humidity on both the cambial activity and differentiations of xylem elements was secondary in nature.
Discussion
The cambial reactivation and xylem differentiation were seen in the month of May (pre-monsoon or summer season), 2 weeks after the onset of bud breaking during the middle of April in D. indica L. at this study site (Fig. 7A; Table 1). The same feature was observed in the sub-tropical climate plants like, Pyrus communis L. and Pyrus malus L. (Rosaceae) (Evert 1961, 1963b), Pinus strobus L. (Pinaceae) (Murmanis 1971) and Quercus boissieri Reut. (Fagaceae), Pistacia alantica Desf. (Anacardiaceae) (Fahn and Werker 1990), as well as the tropical species Tectona grandis L.f (Rao and Dave 1981; Venugopal and Krishnamurthy 1987; Priya and Bhat 1999; Rao and Rajput 2001a, b). Reactivation of the cambium in different months in the same species growing under different local climatic condition is reported in some evergreen species (Zimmerman and Brown 1971), but such comparative studies for the sub-tropical wet forest are lacking.
The cambial activity continues for 8 months from May to December (pre-monsoon to the beginning of winter season), and dormancy from January to the end of April (winter to the pre-monsoon or summer seasons) is imposed strongly by climatic condition. The leaf fall started from February, and complete defoliation resulted in the tree being barren for a brief period of first 15 days during the month of April. Therefore, D. indica L. growing in the sub-tropical climate of northeast India belongs to brevideciduous (William et al. 1997; Borchert 1999). It simulates the phenology of tropical deciduous tree (Philipson et al. 1971; Dave and Rao 1982; Fahn 1982; Liphschitz and Lev-Yadun 1986; Venugopal and Krishnamurthy 1987; Rao and Rajput 2001a, b).
Though D. indica L. growing in the sub-tropical wet climate within a limited habitat shows distinct annual rhythm in the cambial activity, it results in the formation of invisible growth ring annually (Carlquist 1980; Liphschitz et al. 1981; Fahn et al. 1968). In Quercus costaricensis Liebm. (Fagaceae), also growing in mountain sites in Costa Rica, no clear rings with annual periodicity (Worbes and Junk 1989) are formed. Brevideciduous species are confined to microsites with adequate soil water (Borchert 1994; Nepstad et al. 1994). In D. indica L., xylem production was seen only once in a year, i.e. from May to the last week of December. A similar pattern of xylem production is seen in most of the temperate and tropical trees (Philipson et al. 1971; Fritts 1976; Larson 1994; Iqbal 1994; Schweingruber 1996; Worbes 1995, 2002). However, for some trees growing in Israel (Fahn 1958; Liphschitz et al. 1981), Nigeria (Amobi 1974) and peninsular India (Venugopal and Krishnamurthy 1987), two flushes of xylem production in a year are reported. Various anatomical features such as the presences of terminal parenchyma, initial parenchyma, radially compressed fibres and a lower frequency of vessel in the latewood are used to demarcate the growth ring in tropical wood (Chowdhury 1964; Carlquist 1980). In D. indica L., tangentially compressed two to three layers of fibres demarcate the growth ring (Pearson and Brown 1981). In temperate plants over winter, partially differentiated xylem element has been reported in few plants (see also Romberger 1963; Longman and Coutts 1974) in Quercus species, (Timell 1980) in Picea abies L.(Pinaceae), Quercus rubra L. (Fagaceae) (Bannan 1955) in Thuja occidentalis L.(Pinaceae). Such phenomenon has not been encountered in D. indica L.
Shorter element characterized the end of xylem production, while the longer element marked the peak period of the cambial activity and xylem production. Simultaneously, the diameter of wood element was greater during the period when their production was at its peak and minimum during the period when the activity of cambium was the lowest. An analysis of earlier literature reveals that this trend is shown by the largest number of plants studied (see also Taylor 1976), even though the presence of longer late wood fibres has been recorded in certain temperate trees (Bisset and Dadswell 1950; Panshin and De Zeeuw 1980).
Probably starch and crystal of calcium formed the source material for the new cell wall synthesis (e.g. carbohydrates and calcium pectate) when the cambial derivatives are rapidly produced. The maximal and minimal starch content in the xylem of Abies balsamea (L.) Mill. (Abietaceae) associated respectively with the period of cambial dormancy and reactivation (Parker 1960; Sauter 1966; Pomeroy and Siminovitch 1971; Tsuda and Shimaji 1971; see also Riding and Little 1984; Essiamah and Eschrich 1985). When there are two flushes of cambial activity and dormancy, the accumulation and depletion of starch and calcium took place twice a year (Venugopal and Krishnamurthy 1987).
The timing of reactivation, peak activity of cambium and xylem production and mean cell length in D. indica L. were studied in relation to the variation in the climatic factors such as temperature, relative humidity and precipitation. Periodicity of cambium and xylem production is controlled by various environmental and physiological factors (Kramer and Kozlowski 1979; Venugopal 1986; Ajmal and Iqbal 1987; Larson 1994; Rao and Rajput 1999). The monthly mean values of the climatic factors mentioned earlier for all the month of the years 2002 and 2003 are presented (Fig. 1A and B). It was observed that both the reactivation and the peak activity of cambium and xylem production were generally favoured by the mean minimum temperature (15–21°C) in D. indica Linn. The tree showed a positive correlation between the lowest cambial activity and the absence of xylem production with the lowest temperature range (5–12°C) during January–April. Therefore, a rise of 6°C is enough to reactivate cambium as well as induce new buds and foliage in D. indica L. after dormancy. Higher temperature was reported to be conducive for cambial reactivation and xylem production in Picea glauca (Monech) Voss (Pinaceae) (Gregory and Wilson 1968). A similar view was expressed by Kramer and Kolzowski (1979) that temperature was a significant factor for bud break following reactivation and subsequent shoot growth. On the other hand, a rise and fall in temperature was reported to have no effect on the cambial activity in Eucalyptus camaldulensis Dehn. (Myrtaceae) (Waisel et al. 1966) and in Cupressus sempervirens L. (Cupressaceae) (Liphschitz et al. 1981). It appears that temperature factor does not act independently and the law of limiting factor may be in operation (Coile 1936; Keen 1937). The mechanism by which higher temperature promoted the cambial reactivation in many trees is not clear. On the basis of in vitro experiment, the increase in temperature was responsible for the release of auxin reserve from the tissue adjacent to cambium, which, in turn, activated cambium (Wort 1962). On the other hand, temperature had implicated in promoting vacuolation of fusiform initial and may likely be the effect of increased temperature (Catesson 1962). The role of rainfall on cambial behaviour and xylem production under drought condition was studied much more intensively than other factor in the past. Higher rainfall was reported to be conducive to cambial reactivation in several plants growing especially in the tropic and the semi-arid climates (Glock 1955; Reinders-Gouwentek 1965; Roger 1981; Dave and Rao 1982). The present study also shows that the mean rainfall has less correlation than mean minimum temperature in D. indica L. (Table 2). Rainfall probably is an important factor only in the regions where the soil moisture content is dependent on rainfall (Rao and Rajput 2000, 2001a, b). This study has indicated that cambial reactivation, peak activity and xylem production were not limited by rainfall because D. indica L. grows only in limited habitat of sub-tropical wet forest of northeast India, with enough moisture throughout the year. In other words, D. indica is not subjected to physical drought or water stress (Fahn 1959a, b; Amobi 1974). However, the monthly mean precipitation is below 50 mm during December–February (winter season) and the soil type is oxisols where moisture content is 30–40% (Pandey 2004; Porwal et al. 2000; Tripathi 2002; Brady and Well 2002). Moreover, this study site is located 60 km from Cherrapunji, which is the region of highest rainfall (965 cm per year) in the world (Lal 2000; Anonymous 1971, 1972). It was observed that a higher mean minimum temperature generally favoured both cambial reactivation and xylem production in D. indica L. The other factors such as relative humidity and precipitation have little effect on the cambial periodicity and xylem differentiation. In Pinus kesiya Royle. ex. Gordon (Pinaceae), Cedrus deodara Loudon (Pinaceae), Cryptomeria japonica D. Don. (Cupressaceae) growing in Meghalaya at an altitude of 1,500 m a.s.l., mean temperature (15–22°C) played an important role in cambial reactivation and xylem production (Dhirendra Singh 2002). But D. indica L. growing at an altitude of 1,100 m a.s.l. showed response only to mean minimum temperature. The difference of an altitude of 400 m played an important role in the reactivation of cambium and xylem production. It was interesting to observe that the mean temperature at 1,500 m a.s.l. (upper Shillong) is equal to the mean minimum temperature at 1,100 m a.s.l. (15–22°C) at the Botanical Survey of India, Experimental Garden, Shillong. Present study on D. indica growing in the sub-tropical wet forest in the north-eastern hilly region indicate that the trees develop adaptive strategies in response to the altitude and local climate.
References
Ajmal S, Iqbal M (1987) Seasonal rhythm of structure and behaviours of vascular cambium in Ficus rumphii. Ann Bot 60:649–656
Amobi CC (1974) Periodicity of wood formation in twigs of some tropical trees in Nigeria. Ann Bot 38:931–936
Anonymous (1971) Climatological atlas of India. India Meteorological Department, New Delhi
Anonymous (1972) Rain fall atlas of India. India Meteorological Department, New Delhi
Antonova GF (1996) Participation of xyloglucan in the growth of conifers tracheid. In: Proceedings interantional conference “Ecological and physiological aspect of xylogenesis in conifers”, 6–9 August, Krasnoyarks, Russia, pp 4–8
Antonova GF, Stasova VV (1997) Effect of environmental factors on wood formation in larch (Larix sibica Ldb.) stems. Trees 11:462–468
Bailey IW (1920) The cambium and its derivative tissue: II. Size variation of cambial initials in gymnosperms and angiosperms. Am J Bot 7:355–367
Bannan MW (1955) The vascular cambium and radial growth in Thuja occidentalis L. Can J Bot 33:113–138
Bannan MW (1962) The vascular cambium and tree-ring development. In: Kozlowski TT (ed) Tree growth. Ronald Press, New York, USA, pp 3–21
Berlage HP (1931) On the relationship between thickness of tree rings of Djati (teak) trees and rainfall on Java (translated from Dutch). Tectona 24:939–953
Berlyn GP, Miksche JP (1976) Botanical microtechnique and cytochemistry. Iowa State University Press, Ames, Iowa, USA
Bisset IJW, Dadswell HE (1950) Variation in cell length within the growth ring of certain angiosperms and gymnosperms. Aust For 14:17–29
Borchert R (1994) Water status and development in tropical trees during seasonal drought. Trees 8:115–125
Borchert R (1999) Climatic periodicity, phenology and cambium activity in tropical dry forest trees. IAWA J 20:239–247
Brady CN, Well RR (2002) The nature and properties of soils. Pearson Education Pte Ltd., Singapore, pp 86–89
Catesson AM (1962) Modification saisonnieres des vacuoles da la pression osmmptique dans le cambium d Acer pseudoplatanus. C R Acad Sci (Paris) 254:3887–3889
Carlquist S (1980) Further concepts in ecological wood anatomy, with comment on recent work in wood anatomy and evolution. Aliso 9:499–553
Champion HG, Seth SK (1968) Revised survey of the forest types of India. Government of India, New Delhi
Cheadle VI, Gifford Jr EM, Esau K (1953) A staining combination for phloem and contiguous tissues. Stain Technol 28:49–53
Chowdhury KA (1939) The formation of growth rings in Indian trees: Part I. Ind For Rec Util 2:1–39
Chowdhury KA (1940) The formation of growth rings in Indian trees: Part II. Ind For Rec Util 2:41–57
Chowdhury KA (1941) The formation of growth rings in Indian trees: Part III. A study of the effect of locality. Ind For Rec Util 2:59–75
Chowdhury KA (1964) Growth rings in tropical trees and taxonomy. J Ind Bot Soc 43:334–342
Coile TS (1936) The effect of rainfall and temperature on the annual radial growth of pine in the southern United states. Eco Monogr 6:533–562
Coster C (1927) Zur Anatomie and physiologie der zuwachszonen and jahresringbildung in den tropen: I. Ann Jard Bot Buitenzorg 37:49–160
Coster C (1928) Zur anatomie and physiologie der zuwachszonen and jahresringbildung in den tropen: II. Ann Jrd Bot Buitenzorg 38:1–114
Creber GT, Chaloner WG (1990) Environmental influences on cambial activity. In: Iqbal M (ed) The vascular cambium. Research Studies Press, Tauton, UK
Dave YS, Rao KS (1982) Cambial activity in Mangifera indica L. Acta Bot Acad Sci Hung 28:73–79
de Alvim TP (1964) Tree growth periodicity in tropical climates. In: Zimermann MH (ed) The formation of wood in forest trees. Academic Press, New York, pp 479–496
Denne MP, Dodd RS (1981) The environmental control of xylem differentiation. In: Barnett JR (ed) Xylem cell development. Castle House Publications, Tunbridge Wells, England, pp 236–255
Dezzeo N, Worbes M, Ishii I, Herrera R (2002) Growth ring analysis of four tropical species in seasonally flooded forest of mapir river, a tributary of the lower Orinoco river, Venezuala. Plant Ecol (in press)
Dhirendra Singh N (2002) Studies on the environmental information in tree rings of some tree species growing in North-East India. D.Phil. Thesis. North Eastern Hill University, Shillong, India
Essiamah S, Eschrich W (1985) Changes of starch content in the storage tissues of deciduous trees during winter and spring. IAWA Bull 6:97–106
Evert RF (1961) Some aspects of cambial development in Pyrus communis. Am J Bot 50:479–88
Evert RF (1963b) The cambium and seasonal development of the phloem in Pyrus malus. Am J Bot 50:149–59
Fahn A (1958) Xylem structure and annual rhythm of development in trees and shrubs of the desert: I. Tamarix aphylla, T. jordanis var. negevensis, T. gallica var. maris-mortui. Trop Woods 109:81–94
Fahn A (1959a) Xylem structure and annual rhythm of development in trees and shrubs of the desert: II. Acacia tortilis and Acacia raddiana. Bull Res Coun Israel 70:23–28
Fahn A (1959b) Xylem structure and annual rhythm of development in trees and shrubs in Israel. In: Proceedings of the IX international botanical congress, Montreal, Canada, p 110
Fahn A, Waisel Y, Binyamini L (1968) Cambial activity in Acacia radiana. Savi. Ann Bot 32:677–685
Fahn A (1982) Plant anatomy. 3rd edn. Pergamon Press, Oxford
Fahn A, Werker E (1990) Seasonal cambial activity. In: Iqbal M (ed) The vascular cambium. Research Studies Press, Tauton, Somersset, pp 139–157
Feder N, O’Brien TP (1968) Plant microtechnique: some principles and new methods. Am J Bot 55:123–142
Fritts HC (1976) Tree rings and climate. Academic Press, New York, USA
Gahan PB (1984) Plant histochemistry and cytochemistry: an introduction. Academic Press, Florida
Ghouse AKM, Hashmi S (1978) Seasonal cycle of vascular differentiation in Polyalthia longifolia (Annonaceae). Beitr Biol Pflanzen 54:375–380
Ghouse AKM, Yunus M (1974) Cambial structure in Dalbergia. Phytomorphology 24(3, 4):152–158
Glock WS (1955) Tree growth: II. Growth rings and climate. Bot Rev 21:73–188
Gregory RA, Wilson BF (1968) A comparison of cambial activity of white spruce in Alaska and New England. Can J Bot 46:733–734
Grotta AT, Gartner BL, Radosevich SR, Huso M (2005) Influence of ed alder competition on cambial phenology and latewood formation in Douglas-fir. IAWA J 26(3):309–324
Iqbal M (1994) Structural and operational specializations of the vascular cambium of seed plants. In: Iqbal M (ed) Growth patterns in vascular plants. Dioscorides Press, Portland, Oregon, USA, pp 211–271
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Keen FP (1937) Climate cycles in the eastern Oregon as indicated by tree rings. Mon Weather Rev 65:175–188
Killmann W, Thong HL (1995) The periodicity of growth in tropical trees with special reference to Dipterocarpaceae: a review. IAWA Bull 16(4):329–335
Koriba K (1958) On the periodicity of tree growth in the tropics, with reference to the mode of branching, the leaf fall and the formation of the resting bud. Gar Bull Straits Settlements 17:11–81
Kramer PJ, Kozlowski TT (1979) Physiology of woody plants. Academic Press, New York
Lal DS (2000) Climatology. Sharda Pustak Bhavan, Allahabad, India
Larson PR (1994) The vascular cambium, development and structure. Springer, Berlin Heidelberg New York
Liphschitz N, Lev-Yadun S, Waisel Y (1981) The annual rhythm of activity of the lateral meristem (cambium and phellogen) in Cupressus sempervirens. Ann Bot 47:485–496
Liphschitz N, Lev-Yadun S (1986) Cambial activity of evergreen and seasonal dimorphics around the Mediterranean. Ann Bot 47:485–496
Longman KA, Leakey RRB, Denne MP (1979) Genetic and environmental effects on shoot growth and xylem formation in a tropical tree. Ann Bot 44:377–380
Mariaux A (1981) Past efforts in measuring age and annual growth in tropical trees. In: Bormann FH, Berlyn G (eds) Age and growth rate of tropical trees: new directions for research. Yale University, School of Forestry and Environmental Studies, Bulletin No. 94, pp 20–31
McCully ME (1966) Histological studies on genus Fucus: I. Light microscopy of mature vegetative plant. Protoplasma 62:287–305
Mishra BP, Tripathi RS, Tripathi OP, Pandey HN (2003) Effect of disturbance on the regeneration of four dominant and economically important woody species in a subtropical wet hill forest of Meghalaya, north-east India. Curr Sci 84(11):1449–1453
Mishra BP, Tripathi OP, Tripathi RS, Pandey HN (2004) Effect of anthropogenic disturbances on plant diversity and community structure of a sacred grooved in Meghalaya, north-east India. Biodivers Conserv 13:421–436
Nepstad DC, de Carvalho CR, Davidson EA, Jipp Ph, Lefebvre GH, Negreiros SE, Trumbore SE, Vieira S (1994) The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372:666–669.
Longman KA, Coutts MP (1974) Physiology of the oak tree. In: Morres Mg, Preng F H (eds) The British oak. BSBI/EW. Classy Ltd., UK, pp 193–221
Murmanis L (1971) Structural changes in the vascular cambium of Pinus strobus during an annual cycle. Ann Bot 35:133–141
Pandey HN (2004) Ecological analysis of selected agroforestry system in Meghalaya. Final Technical Report No. 5 (8)/98SW/DF, Indian Council of Agricultural Research, New Delhi
Panshin AJ, De Zeeuw C (1980) Textbook of wood technology. 4th edn. McGraw-Hill, New York
Parker J (1960) Seasonal changes in the physical nature of the bark parenchyma cells of Pinus strobus. Protoplasma 52:223–229
Pearson RS, Brown HP (1981) Commercial timbers of India. vol I. A.J. Reprints Agency, pp 1–10
Philipson WR, Ward JM, Butterfield BG (1971) The vascular cambium, its development and activity. Chapman and Hall, London
Pomeroy MK, Siminovitch D (1971) Seasonal cytological changes in secondary phloem parenchyma cells in Robinia pseudoacacia in relation to cold hardiness. Can J Bot 49:787–795
Porwal MC, Talukdar G, Singh H, Triparthi OP, Tripathi RS, Roy PS (2000) Biodiversity characterization at landscape level using remote sensing and geospatial modeling in Meghalaya (India). In: Roy PS, Singh S, Toxopeus AG (eds) Biodiversity and enviroment. Indian Institute of Remote Sensing, Dehradun, pp 206–219
Priya PB, Bhat KM (1999) Influence of rainfall, irrigation and age on the growth periodicity and wood structure in Teak (Tectona grandis). IAWA J 20(2):181–192
Rao KS, Dave YS (1981) Seasonal variation in the cambial anatomy of Tectona grandis (Verbenaceae). Nord J Bot 1:535–542
Rao KS, Rajput KS (1999) Seasonal behaviour of vascular cambium in Teak (Tectona grandis L.f) growing in moist deciduous and dry deciduous forest of Gujarat. IAWA J 20:85–93
Rao KS, Rajput KS (2000) Cambial activity and development of wood in Acacia nilotica (L.) Del. Growing in different forest of Gujarat state. Flora 165–171
Rao KS, Rajput KS (2001a) Xylem structure and annual rhythm of development in the twigs of Acacia nilotica (L.) Del. growing in different forest of Gujarat state (India). Phyton 41:1–12
Rao KS, Rajput KS (2001b) Relationship between seasonal cambial activity, development of xylem and phenology in Azadirachta indica growing in different forest of Gujarat state. Ann For Sci 58:691–698
Reinders-Gowentek CA (1965) Physiology of cambium and other secondary meristem of the shoot. In: Rhuland W (ed) Encyclopedia of plant physiology: XV, vol 1. Springer-Verlag, Berlin, pp 1077–1105
Rensing KH, Samuel AL (2004) Cellular changes associated with rest and quiescence in winter-dormant vascular cambium of Pinus contorta. Trees 18:373–380
Riding RT, Little CHA (1984) Anatomy and histochemistry of Abies balsamea cambial zone cells during the onset and breaking of dormancy. Can J Bot 62:2570–2580
Romberger JA (1963) Meristems, growth and development in woody plants. US Department Agriculture, Technical Bulletin No. 1293
Roger S (1981) Seasonal variation in radial growth and phloem activity of Terminalia ivorensis Chev A. Ann Bot 47:603–610
Sauter JJ (1966) Untersuchungen zur physiologie der pappelholzstrahlen: I. Jahrespereodischer verlauf der starkespeicherung in holzstrahlparenchym. Z Pflanzenphysiol 55:246–258
Schongart, Piedade J, Ludwigshausen MFT, Horna S, Worbes M (2002) Phenology and stem growth periodicity of tree species in Amazonian floodplain forest. J Trop Eco 18:581–597
Schweingruber FH (1988) Tree rings. Reidel, Dordrecht
Schweingruber FH (1996) Tree rings and environment. In: Dendrochronology. Paul Haupt, Berne, Switzerland
Singh S (1996) Technical report on delineation of geoecological zones in Meghalaya. Report No. GE/UGC/SR (1) SS. Department of Geography, North Eastern Hill University
Taylor FW (1976) Fibre length variation within growth rings of certain angiosperms. Wood Fibre 8:116–119
Timell TE (1980) Organization and ultra-structure of the dormant cambial zone in compression wood of Picea abies. Wood Sci Technol 14:161–179
Tripathi OP (2002) Study of distribution pattern and ecological analysis of major forest types of Meghalaya. North Eastern Hill University, Shillong, India
Tripathi OP, Pandey HN, Tripathi RS (2004) Distribution, community characteristic and tree population structure of subtropical pine forest of Meghalaya, northeast India. Int J Ecol and Environ 29:207–213
Tsuda M, Shimaji K (1971) Seasonal changes of cambial activity and starch content of Pinus densiflora Seib. J Jpn For Soc 53:103–107
Venugopal N (1986) Some studies on the vascular cambium and its derivatives in some tropical plants. D.Phil. Thesis, University of Madras, India
Venugopal N, Krishnamurthy KV (1987) Seasonal production of secondary xylem in the twigs of certain tropical trees. IAWA Bull 8:31–40
Waisel Y, Noah I, Fahn A (1966) Cambial activity in Eucalyptus camaldulensis Dehn: I. The relation to extension growth in young sampling. La-Yearen 16:59–73
Williams RJ, Myers BA, Muller WJ, Duff GA, Eamus D (1997) Leaf phenology of woody species in a Northern Australian tropical savanna. Ecology 78:2542–2558
Worbes M (1995) How to measure growth dynamic in tropical trees. A review. IAWA J 16:337–351
Worbes M, Junk WJ (1989) Dating tropical trees by means of 14 C from bomb test. Ecology 70(2):503–507
Worbes M (1999) Annual growth rings, rainfall dependent growth and long term growth pattern of tropical trees from forest reserves caporal in Venezuala. J Ecol 87:391–403
Worbes M (2002) One hundred years of tree ring research in the tropics—a brief history and an outlook to future challenges. Dendrodhronologia 20(1–2):217–231
Worbes M, Staschel R, Roloff A, Junk WJ (2003) Tree ring analysis reveals age structure, dynamic and wood production of natural forest stand in Cameroon. For Ecol Manag (in press)
Wort DJ (1962) Physiology of cambial activity. In: Kozlowski TT (ed) Tree growth. Ronal Press, New York, pp 89–95
Zar JH (1974) Biostatistical analysis. Prentice Hall, Englewood Cliffs, NJ
Zimmermann MH, Brown CL (1971) Trees: structure and function. Springer-Verlag, New York, USA
Acknowledgements
We would like to thank Prof. N.K. Chrungoo for providing facilities. Our sincere thanks to Botanical Survey of India, Experimental Garden, Shillong for collection of materials. One of us (MGL) expressed gratitude to the Council of Scientific and Industrial Research, New Delhi, for granting Senior Research Fellowship (Sanction No. 9/347(146)/2K2/EMR-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Buckeridge
Rights and permissions
About this article
Cite this article
Venugopal, N., Liangkuwang, M.G. Cambial activity and annual rhythm of xylem production of elephant apple tree (Dillenia indica Linn.) in relation to phenology and climatic factor growing in sub-tropical wet forest of northeast India. Trees 21, 101–110 (2007). https://doi.org/10.1007/s00468-006-0101-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-006-0101-3








 ) width of cambial (–○–) and differentiating xylem zone (-□-). B Showing the mean length of fusiform initial (–○–), mean length of vessel element (-□-) and mean length of xylem fibre (–▵–)
) width of cambial (–○–) and differentiating xylem zone (-□-). B Showing the mean length of fusiform initial (–○–), mean length of vessel element (-□-) and mean length of xylem fibre (–▵–)