Abstract
In winter, dormant cambial cells contain many small vacuoles interspersed throughout the cytoplasm. This differs dramatically from actively growing cambial cells whose structure is dominated by large central vacuoles. Structure reported in studies using conventional chemical fixation and transmission electron microscopy (TEM) conflicts with that described earlier for live cambial cells using light microscopy. In this study, cryofixation (high-pressure freezing/freeze substitution) was used to preserve dormant Pinus contorta fusiform cambial cells, revealing structure more consistent with that in early micrographs of live cambial cells. At the ultrastructural level, the plasmalemma was consistently smooth and tightly associated with the cell wall, contrary to the highly in-folded plasmalemma seen in chemically fixed cambial cells. In addition, both TEM and live-cell confocal microscopy demonstrated that, in some places, dormant cells were partitioned into more numerous, smaller vacuoles than were observed after chemical fixation. Populations of different vacuoles were apparent based on size, shape and membrane staining. Larger vacuoles had prominent tonoplasts and were often present as axially elongated, interconnecting networks with associated microfilament bundles. Endoplasmic reticulum fragmented during rest into numerous vesicular structures similar to small vacuoles, then with the transition to quiescence reformed into the smooth cisternal form.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant dormancy can be defined in various ways. Many plants will cease growth when placed under environmental stress such as drought but resume growth when conditions improve. Different dormancy processes take place during the winter in temperate climates. Winter dormancy consists of two stages: “rest” or “physiological dormancy”, which is maintained by conditions within the plants, followed by “quiescence” or “environmental dormancy”, which is controlled by environmental conditions. The difference between the two stages is that the resting plant will not grow but the quiescent plant will, when placed in ideal environmental conditions (Riding and Little 1984). The main determinant of dormancy is the presence or absence of cell division; even during rest, the cells are not completely inactive. The vacuoles in live dormant cells undergo constant changes in shape and size with cytoplasmic streaming (Bailey 1930). Barnett (1973) and Rensing and Owens (1994) observed active Golgi structures in dormant cambium from trees maintained above freezing. Physiological processes thus continue, albeit at a reduced level compared to actively dividing and expanding cells.
The meristematic vascular cambium is responsible for production of the xylem and phloem, self-maintenance, and signal transmission via translocation of growth regulators. Much of the present knowledge of cambial structure and function, and particularly the concept of the cambial initial, arose from research on dormant cambial cells (Larson 1994). Dormant cell walls are radially thickened and there is an abrupt transition to xylem and phloem because the adjacent last-formed xylem and phloem cells are fully differentiated. At this stage, the lineage of each cell can be easily traced by examination of the wall structure. In addition, dormant fusiform cambial cells are characterized by numerous smaller vacuoles within a cytoplasmic matrix, in contrast to active cambial cells which are mostly large vacuoles with an extremely thin surrounding of cytoplasm. This arrangement can be observed by light microscopy and has been known since the 1930s from live cell preparations (Bailey 1930).
Cambial cells have been extensively studied by transmission electron microscopy (TEM) since the 1960s but images of dormant cambial cells were radically different from those of Bailey (1930). The plasmalemma of chemically fixed cambial cells was typically wrinkled or folded, especially during rest when the membrane was thought to form convoluted “plasmalemma invaginations” (PLI) (Catesson 1994). Also, “paramural bodies” (Barnett 1973) and “pinocytic vesicles” (Sennerby-Forsse 1986) were observed between the plasma membrane and the cell wall. However, it is not known if these structures were artifacts of processing because cambial cells are notoriously difficult to preserve for TEM, and it is well-established that chemical fixation induces extensive physical and chemical changes to native cell structure (Mersey and McCully 1978; Taylor 1988). Without alternative means to prepare the cells, the nature of the true cell structure could not be resolved.
In contrast to chemical fixation, high-pressure freezing (HPF) immobilizes cellular constituents within milliseconds, thereby more accurately preserving their natural form (Kiss and Staehelin 1995). Freeze-substitution can then replace frozen water in the tissues with resin-compatible solvent without placing the cells under osmotic or pH stress. The combination of cryofixation and freeze-substitution of plant tissues thereby preserves transient membrane configurations (Staehelin et al. 1990; Galway et al. 1993) and has revealed ultrastructural features that were not seen following chemical fixation (Staehelin et al. 1990; Samuels et al. 1995; Platt et al. 1997; Thomson and Platt 1997; Bourett et al. 1999).
The objective of this study was to separate chemical fixation artifacts from the undistorted structure using correlative live-cell microscopy and cryofixation/TEM. Preparation of dormant cambial cells from Pinus contorta using these methods has generated results very different from those obtained from chemically fixed cambial cells, leading to new insights into the processes of rest and quiescence in these cells during winter dormancy.
Materials and methods
Eight-month-old container-grown Pinus contorta seedlings were maintained in dormancy by freezer storage at −2°C. The frozen seedlings were placed in a refrigerator at 4°C to induce the progression from rest to quiescence (Van den Driessche 1977; Lavender 1985). To induce resumption of cell division and growth, seedlings were planted ten per box in 15×60 cm planters then placed in a growth chamber at 24°C under constant light.
Seedlings were sampled destructively at regular intervals for examination of the cellular structure by confocal scanning laser microscopy and TEM. Five-centimeter-long stem segments, cut from the seedlings 2 cm below the base of the terminal bud, were bisected radially. Approximately 0.5 mm-thick radial longitudinal slices containing portions of xylem, cambium and phloem were hand-cut from each half. Slices for confocal microscopy and high-pressure-freezing were immediately immersed in 0.2 M sucrose, whereas slices for chemical fixation were immediately immersed in primary fixative.
Chemical fixation
Slices were immersed for 2 h in primary fixative (2.5% glutaraldehyde in 0.05 M phosphate buffer, pH 6.8) at room temperature, rinsed well with buffer, then post-fixed for 2 h with 2% osmium tetroxide in buffer. After rinsing with double distilled water, the slices were dehydrated through an acetone series, then placed on a rotating mixer in capped vials containing 1 ml of acetone. One drop of Spurr’s resin was added to each vial every 5 min until the mixture was approximately 25% resin. The slices were then transferred to 50% resin for 2 h, to 75% resin in open vials for 12 h, and finally to 100% resin with changes two times per day for 3 days. The infiltrated samples were polymerized in fresh Spurr’s resin at 60°C.
High pressure freezing (HPF)
Slices in 0.2 M sucrose (as cryoprotectant) were high-pressure-frozen using a Balzers HPM010 (Balzers Instruments, Balzers, Liechtenstein). Frozen samples were freeze-substituted with 2% osmium tetroxide and 8% dimethoxypropane in acetone for 120 h, using a dry ice-acetone bath which equilibrated at −80°C. The tissues were then warmed to −20°C in a freezer for 4 h, and to 4°C in a refrigerator for 4 h, after which they were brought to room temperature. The tissues were transferred to fresh dry HPLC-grade acetone, then gradually embedded in Spurr’s resin as described above.
Electron microscopy
Half-micron-thick sections of resin-embedded tissue were mounted on glass slides and stained with 1% Toluidine Blue O in 1% sodium borate. Sectioning and fixation quality were assessed by light microscopy; the best blocks were further sectioned at 70 nm for TEM. Sections mounted on Formvar-coated grids were stained with aqueous 2% uranyl acetate for 30 min, followed by Reynold’s lead citrate for 10 min, then observed and photographed by TEM.
Confocal microscopy
The vacuoles of living cells were defined using fluorescein diacetate (FDA), a vital stain that fluoresces in the cytoplasm. The vacuoles appeared as dark unstained regions against the bright fluorescent cytoplasm. Living tissue slices were immersed in 2 μg/ml FDA in 0.2 M aqueous sucrose for 60 min then rinsed for 30 min with 0.2 M sucrose. They were mounted on glass slides in SlowFade (Molecular Probes, Eugene, Ore., USA) with the coverslips sealed by nail polish, and observed using a Bio-Rad 600 confocal scanning laser microscope (Bio-Rad Laboratories Life Science Group, Hercules, Calif.). FDA fluorescence (at 514 nm) was induced using an Argon (blue) laser with an excitation wavelength of 490 nm.
Results
Dramatic differences in the arrangement of vacuoles between dormant and active cambia were visible at the light microscope level. Dormant cambial cells contained dense cytoplasm and small vacuoles (Fig. 1) while active cambial cells were filled with a huge central vacuole and thin parietal layer of cytoplasm (Fig. 2). In dormant cambium prepared by cryofixation (high-pressure freezing/freeze substitution) and viewed with light microscopy, the shapes and arrangements of the vacuoles were different in the rest (Fig. 3) versus the quiescent (Fig. 4) phases of dormancy. During rest, the cell center was dominated by a network of elongated, interconnected vacuoles with peripheral vacuoles of varying sizes. In the quiescent dormant cambial cell, the center of the cell contained more spherical vacuoles and fewer peripheral vacuolar structures.
Light micrograph showing structure of dormant cambium in radial section. Dormant cambium has dense cytoplasm and is sandwiched between fully differentiated xylem and phloem
Light micrograph showing structure of active cambium in radial section. Active cambium is primarily vacuolar and grades into xylem and phloem which initially have similar structure. ( C cambium, Ph phloem, X xylem, Bar 40 μm)
Light micrograph showing dormant resting cambium: vacuolar structure. HPF cells sectioned at 0.5 μm and stained with Toluidine Blue (Bar 40 µm)
Light micrograph dormant quiescent cambium: vacuolar structure. HPF cells sectioned at 0.5 μm and stained with Toluidine Blue (Bar 20 µm)
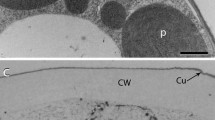
Light micrograph showing dormant cambium: vacuolar structure. Living cells stained with FDA and photographed by confocal microscopy. The vacuolar structure in Figs. 4 and 5 is similar. ( CW cell wall, V vacuole, Bar 20 μm)
The structure of living cambial cells observed by confocal microscopy demonstrated the validity of using cryofixation for preserving native cambial cell structure. In live quiescent cells observed by confocal microscopy, the vacuoles were small, spherical and abundant, appearing as dark spheres in the bright fluorescence of the surrounding cytoplasm (Fig. 5). Sectioned cryofixed cambium (Fig. 4) closely resembled the structure of live cells observed by confocal microscopy (Fig. 5). In contrast, the cellular structure produced by chemical fixation, while identical to that in comparable ultrastructural studies, was significantly different from that of live cells and cryofixed material. In chemically fixed cells (Fig. 6), the vacuoles were rarely spherical and the tonoplast was not smooth as in the cryofixed material (Figs. 7, 8).
Transmission electron micrograph showing resting cambial cells preserved by chemical fixation. Plasma membranes are wrinkled and in-folded. Vacuoles are difficult to delineate due to fracturing of the membranes and the cytoplasm is highly extracted. ( CW cell wall, G Golgi, M mitochondria, Nu nucleus, P plastid, PLI plasmalemma invagination, PM plasma membrane, T tonoplast, Bar 1 μm)
Transmission electron micrograph showing HPF resting cambium. Vacuoles are both large and circular and in elongated interconnecting networks around microfilament bundles. Small vesicular ER lies in the peripheral areas close to the cell walls. Vacuole structure was often different in adjacent cells but was not correlated with cell position in the tissue ( Bar 1 μm)
Transmission electron micrograph showing HPF quiescent cambium. Larger circular vesicles remain, but the interconnecting networks seen in the resting cells are less frequent. Rather, small rounded vacuoles are present in two forms: those with an obvious tonoplast and those without a readily visible tonoplast. ER is present in characteristic smooth cisternal form adjacent to the cell walls. (* small vacuole without visible tonoplast, CW cell wall, ER endoplasmic reticulum, G Golgi, M mitochondria, MF microfilament, Nu nucleus, P plastid, V vacuole, Bar 1 μm)
Cryofixation and TEM showed that dormant cambial cells contained several types of vacuoles. During rest, the cells contained some larger vacuoles, similar to those in active cells, which occupied most of the cell width. These vacuoles were identified in electron micrographs by their elongated shapes, size and darkly stained tonoplast (Fig. 7). In many areas, vacuoles with prominent membranes were present as axially elongated interconnecting networks closely associated with microfilament bundles (Fig. 7). In addition to the clearly membrane-bound vacuoles, the cells contained numerous, small vacuole-like compartments that did not have a visible tonoplast. The membrane delimiting these small vacuoles was either not well-preserved, or not adequately contrasted by osmium tetroxide substitution and uranyl acetate/lead citrate staining. Furthermore, more than one population may comprise these structures because many had obvious contents, while others were apparently vacant. Typical cisternal endoplasmic reticulum (ER) was not observed in resting cells (Fig. 7) but was in quiescent (Fig. 8) and active cells. Attempts to detect ER resident proteins such as the chaperone BIP in resting cells were unsuccessful, although ER in adjacent parenchymatous rays was labeled (data not shown). Concurrent with the re-appearance of smooth cisternal ER, a large population of the small vacuole-like compartments disappeared from the peripheral cytoplasm (Fig. 8). The ER was generally smooth during quiescence and ribosomes were uniformly distributed throughout the cytoplasm in dormant cells. Upon reactivation, rough ER was again observed (not shown).
The excellent preservation of the subsets of vacuolar compartments was one area in which cryofixation led to significant improvements over previous TEM preparations but the most startling improvement in cell structure information came from observations of plasma membranes in the dormant cells. In conventional chemically fixed dormant cambial cells, the plasma membrane (plasmalemma) was consistently separated from the cell wall, PLI were extensive, and paramural bodies or pinocytic vesicles were common (Fig. 6). Comparatively, when high-pressure-frozen dormant cells were observed by TEM, the plasmalemma was smooth and tightly appressed to the cell wall (Figs. 7, 8). It was not wrinkled, undulating or folded so PLI were not present; because there was no space between the plasmalemma and the cell wall, vesicles or paramural bodies were not found between the wall and the plasma membrane.
HPF dormant cambial cells also demonstrated remarkable cytoskeletal structure. Though the rare microtubules observed in the dormant cambium were closely associated with the plasmalemma, bundles of microfilaments were readily visible. These longitudinally oriented microfilament bundles were common adjacent to the plasmalemma and in bands of cytoplasm between the vacuoles (Figs. 7, 9). They appeared to provide structure to the inter-vacuolar cytoplasm. Where the bundles were present in narrow cytoplasmic bridges, the tonoplast had a straight, almost angular appearance that was not apparent elsewhere (Fig. 9). This was especially apparent as the vacuolar compartments amalgamated during resumption of active division; re-assembly of the central vacuole was incomplete when activity resumed so the first phragmoplasts initially moved through cytoplasm and the small vacuoles (Fig. 10).
Transmission electron micrograph of the reactivating cambium shows the close relationship between the microfilament bundles and the vacuoles. The microfilament bundles also interact with the nucleus. ( M mitochondria, MF microfilament, Nu nucleus, P plastid, V vacuole, Bar 0.5 μm)
Light micrograph shows phragmoplasts of the first divisions in cellular reactivation advance through the dense cytoplasm and small vacuoles typical of dormant cambial cells. ( Nu nucleus, Ph phragmoplast, V vacuole, Bar 10 μm)
Transmission electron micrograph showing Golgi in the dormant cambium. Golgi show flattened structure with little cis-trans orientation and no staining within the cisternae ( Bar 0.5 μm)
Transmission electron micrograph showing Golgi in the active cambium. The cis-trans orientation is readily apparent from the budding vesicles at the trans face and the progression of staining intensity from cis to trans cisternae. ( M mitochondria. Bar 0.5 μm)
Golgi and plastids were the other organelles that showed differences from those in active cells. Dormant cells contained Golgi with thin, flattened cisternae with little peripheral swelling and few associated vesicles. They typically displayed weak cis-trans orientation (Fig. 11). In active cells, Golgi cisternae were still flattened but the cis-trans orientation was readily apparent, with many vesicles associated with the trans face (Fig. 12). During rest and quiescence, plastids were small and abundant. As meristematic activity resumed, plastids enlarged and starch granules became abundant. With resumption of cell division, starch quickly became depleted in the axial cells but remained for a longer period in the ray cells.
Discussion
Pronounced seasonal changes in cytoplasmic organization occur in cambial cells. Dormant cambial ultrastructure is characterized by small vacuoles surrounded by dense cytoplasm (Bailey 1930; Barnett 1973; Rao and Catesson 1987) while active cells are dominated by large central vacuoles that confine the cytoplasm to narrow peripheral layers. Vacuoles fulfill different roles in dormant and active fusiform cells. In active cells, they are considered to play a major role in cell growth by maintaining high turgor pressure that provides the force necessary for cell expansion (Barnett 1992). Vacuoles almost certainly function in storage of reserves in dormant cells. Vacuolar changes with dormancy may also be related to the achievement of cold hardiness (Niki and Sakai 1981; Kuroda and Sagisaka 1993).
In trees, dormancy is defined by lack of cell division, but metabolic activity still occurs when weather conditions permit. The tonoplast has been shown to move with cytoplasmic streaming as the vacuoles undergo constant changes in shape and size even during winter dormancy (Bailey 1930). The vacuolar structure changes from many small and/or elongated vacuoles to fewer rounded vacuoles as cambial cells make the transition from rest to quiescence. Cambial cell division can resume in the spring while the ground and thus the root system is still frozen (Barnett 1973). At those times there is limited capacity for translocation, so the resources for the initiation of cell division must come from within the cells. With resumption of cell division, vacuoles coalesce into larger ones and their contents disappear (Bannan 1955; Robards and Kidwai 1969; Sennerby-Forsse 1986). The first divisions of the cambial cells occur concurrently with vacuolar amalgamation, so that the phragmoplasts of the first cell divisions advance through cytoplasm and small vacuoles. This contrasts with fully active cambial cells where they advance through the central vacuole (Rensing et al. 2002).
In chemically preserved dormant cambial cells, the plasmalemma was conspicuously invaginated. In some cases, the plasmalemma was reported to push into vacuoles, sometimes carrying ER with it (Rao and Catesson 1987). PLI have been most frequently observed in cambial cells during autumn rest (Robards and Kidwai 1969; Rao and Dave 1983; Riding and Little 1984; Rao and Catesson 1987; Kuroda and Sagisaka 1993; Rensing and Owens 1994). These structures were thought to be a result of continued membrane trafficking following the cessation of growth (Rao and Dave 1983; Rao and Catesson 1987) and were hypothesized to function in membrane recycling, or in the vacuolar uptake of apoplastic sugars or ER proteins (Catesson 1994).
Studies that most clearly demonstrated PLI were undertaken using high-molarity fixative buffers. Sucrose or levulose was added to adjust the osmolarity to just below the point of incipient plasmolysis of live cambial cells (Robards and Kidwai 1969; Barnett 1973, 1977; Rao and Catesson 1987). This is the level at which a cell is just able to counteract the osmotic potential of the bathing solution. The fixative buffer is meant to control the outflow of water from the cells during fixation (Bullock 1984). Since the first components with which the fixative interacts are the plasma membrane-bound pumps and proteins responsible for maintaining cellular integrity, the fixative (glutaraldehyde) alters membrane permeability on contact. It is not surprising, therefore, that large-scale changes occur in cells fixed using such methods. Higher molarity buffers would induce greater outflow of water from the cells and increase the likelihood of plasmolytic damage.
Plasmalemma invaginations and wrinkles were absent in HPF dormant cambial cells of Pinus contorta. The plasmalemma was tightly appressed to the cell wall and there was no evidence of PLI or plasmalemma intrusion into the vacuoles. Similar results have been reported in other studies of cryofixed plant cells (Lancelle et al. 1986; Ding et al. 1991). No spaces were present between the plasmalemma and cell wall in cryofixed and substituted tracheids of Cryptomeria japonica and Pinus densiflora (Inomata et al. 1992). Even in highly condensed and folded cells of desiccated Selaginella lepidophylla, the plasmalemma was in close and continuous apposition to the walls (Thomson and Platt 1997). In the present study with Pinus, only chemically fixed cells had wrinkled and invaginated plasma membranes. It is safe to conclude that wrinkles, folds, or invaginations of the plasmalemma are artifacts of chemical fixation.
The question remains as to why such artifactual structures are induced by chemical fixation primarily in resting cambial cells. The rest stage of dormancy is a physiological state wherein the plant cannot resume growth even under favorable conditions. Trees (and seedlings) have a “chilling requirement” that must be fulfilled so that they can make the transition into quiescence (Van den Driessche 1977). They progress through rest most rapidly when the external temperature is in the range of 4–8°C. This is thought to be an adaptation that keeps plants from initiating growth during periods of unseasonably warm winter weather. It is not known what structural or physiological changes occur to alter their response to the environment, but real cellular changes result in the induction of plasma membrane infolding artifacts during chemical fixation; such artifacts are rarely induced in similarly fixed materials from quiescent and active states (Robards and Kidwai 1969; Barnett 1973, 1977; Rao and Catesson 1987).
In chemically fixed cambial cells, ER tended to be ribosome-studded and in cisternal form during active periods, but smooth and more vesicular during dormancy (Srivastava and O’Brien 1966; Kidwai and Robards 1969; Robards and Kidwai 1969; Murmanis 1971; Barnett 1973; Rao and Dave 1983; Catesson 1994). In HPF cells, contrast of ER membranes was low; ER was identified by its characteristic arrangement and, when present, the ribosomes arrayed along its periphery. Characteristic cisternal ER was present in HPF quiescent and active cambial cells, in smooth and rough forms respectively, but ER was not readily apparent in resting cells. Significantly, as the cells progressed into quiescence, cisternal smooth ER re-appeared with a concomitant disappearance of the smallest of the vacuole-like compartments from the peripheral cytoplasm. Since the smooth ER reappeared in the region of the cytoplasm previously occupied by the small vacuole-like compartments, the small vacuole-like compartments most likely represented a domain of ER that fragments during rest, then reforms during quiescence. Using freeze-fracture techniques, Fujikawa and Takabe (1996) demonstrated that ER was vesiculated during early winter dormancy.
In Pinus cambial cells, axial bundles of microfilaments were seen both at the cell tips and running through the cytoplasm to the nucleus. The bundles were seen to contact the nuclear envelope. Microfilaments have been shown to be crucial to nuclear placement during interphase (Shibaoka 1993; Valster and Hepler 1997; Grolig 1998). The longitudinal microfilament bundles may suspend the nucleus in the center of the cambial cells. In Arabidopsis mutants with defective actin strands (i.e., microfilament bundles) and in cells treated with latrunculin B, which interferes with actin polymerization, the normal vacuolar structure was disrupted (Mathur et al. 2003). The distortions of tonoplasts and the presence of elongated vacuoles adjacent to microfilament bundles may reflect interactions between the vacuolar system and the cytoskeleton. This could be important in reforming the large central vacuole during reactivation.
Conclusions
Dormant cambium in cryofixed cells displayed smooth plasmalemma with no intrusions or invaginations as described for chemically fixed cells, thus PLI and paramural bodies are fixation artifacts.
The vacuoles of dormant cells are an intricate system of membrane networks and small vacuoles associated with microfilament bundles.
ER was fragmented into small vesicular structures during rest but reformed into smooth cisternal form as the cells graded into quiescence.
At least two populations of small numerous vacuoles (distinct from vesicular ER) were observed in dormant cells.
References
Bailey IW (1930) The cambium and its derivative tissues. V. A reconnaissance of the vacuome in living cells. Z Zellforsch Microsk Anat 10:651–682
Bannan MW (1955) The vascular cambium and radial growth in Thuja occidentalis. Can J Bot 33:113–138
Barnett JR (1973) Seasonal variation in the ultrastructure of the cambium in New Zealand grown Pinus radiata D. Don. Ann Bot 37:1005–1115
Barnett JR (1977) Tracheid differentiation in Pinus radiata. Wood Sci Technol 11:83–92
Barnett JR (1992) Reactivation of the cambium in Aesculus hippocastanum L.: a transmission electron microscope study. Ann Bot 70:169–177
Bourett TM, Czymmek KJ, Howard RJ (1999) Ultrastructure of chloroplast protuberances in rice leaves preserved by high-pressure freezing. Planta 208:472–479
Bullock GR (1984) The current status of fixation for electron microscopy: a review. J Microsc 133:1–15
Catesson A-M (1994) Cambial ultrastructure and biochemistry: changes in relation to vascular tissue differentiation and the seasonal cycle. Int J Plant Sci 155:251–261
Ding B, Turgeon R, Parthasarathy MV (1991) Routine cryofixation of plant tissue by propane jet freezing for freeze substitution. J Electron Microsc Techn 19:107–117
Fujikawa S, Takabe K (1996) Formation of multiplex lamellae by equilibrium slow freezing of cortical parenchyma cells of mulberry and its possible relationship to freezing tolerance. Protoplasma 190:189–203
Galway ME, Rennie PJ, Fowke LC (1993) Ultrastructure of the endocytotic pathway in glutaraldehyde-fixed and high-pressure frozen/freeze-substituted protoplasts of white spruce ( Picea glauca). J Cell Sci 106:847–858
Grolig F (1998) Nuclear centering in Spirogyra: force integration by microfilaments along microtubules. Planta 204:54–63
Inomata F, Takabe K, Saiki H (1992) Cell formation of conifer tracheid as revealed by rapid-freeze and substitution method. J Electron Microsc 41:369–374
Kidwai P, Robards AW (1969) On the ultrastructure of resting cambium of Fagus sylvatica L. Planta 89:361–368
Kiss JZ, Staehelin LA (1995) High pressure freezing. In: Severs NJ, Shotton DM (eds) Rapid freezing, freeze fracture and deep etching. Wiley-Liss, New York, pp 89–104
Kuroda H, Sagisaka S (1993) Ultrastructural changes in cortical cells of apple ( Malus pumila Mill.) associated with cold hardiness. Plant Cell Physiol 34:357–365
Lancelle SA, Callaham DA, Hepler PK (1986) A method for rapid freeze fixation of plant cells. Protoplasma 131:153–165
Larson PR (1994) The vascular cambium: development and structure. Springer, Berlin Heidelberg, New York
Lavender DP (1985) Bud dormancy. In: Duryea ML (ed) Evaluating seedling quality: principles, procedures, and predictive abilities of major tests. Oregon State University, Corvallis, pp 7–15
Mathur J, Mathur N, Kernebeck B, Hülskamp (2003) Mutations in actin-related proteins 2 and 3 affect cell shape development in Arabidopsis. Plant Cell 15:1632–1645
Mersey B, McCully ME (1978) Monitoring the course of fixation of plant cells. J Microsc 114:49–76
Murmanis L (1971) Particles and microtubules in vascular cells of Pinus strobus L. during cell wall formation. New Phytol 70:1089–1093
Niki T, Sakai A (1981) Ultrastructural changes related to frost hardiness in the cortical parenchyma cells from mulberry Morus bombycis cultivar Gorogi twigs. Plant Cell Physiol 22:171–184
Platt KA, Oliver MJ, Thomson WW (1997) Importance of the fixative for reliable ultrastructural preservation of poikilohydric plant tissues. Observations on dry, partially, and fully hydrated tissues of Selaginella lepidophylla. Ann Bot 80: 599–610
Rao KS, Catesson A-M (1987) Changes in the membrane components of nondividing cambial cells. Can J Bot 65:246–254
Rao KS, Dave YS (1983) Ultrastructure of active and dormant cambial cells in Teak ( Tectona grandis L. f). New Phytol 93:447–456
Rensing KH, Owens JN (1994) Bud and cambial phenology of lateral branches from Douglas-fir ( Pseudotsuga menziesii) seedlings. Can J For Res 24:286–296
Rensing KH, Samuels AL, Savidge RA (2002) Ultrastructure of vascular cambial cell cytokinesis in pine seedlings preserved by cryofixation and substitution. Protoplasma 220:39–49
Riding RT, Little CHA (1984) Anatomy and histochemistry of Abies balsamea cambial zone cells during the onset and breaking of dormancy. Can J Bot 62:2570–2579
Robards AW, Kidwai P (1969) A comparative study of the ultrastructure of resting and active cambium of Salix fragilis L. Planta 84:239–249
Samuels AL, Giddings TH, Staehelin LA (1995). Cytokinesis in Tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol 130: 1345–1357
Sennerby-Forsse L (1986) Seasonal variation in the ultrastructure of the cambium in young stems of willow ( Salix viminalis) in relation to phenology. Physiol Plant 67:529–537
Shibaoka H (1993) The use of tobacco BY-2 cells for the studies of the plant cytoskeleton. J Plant Res (Special Issue) 3:3–15
Srivastava LM, O’Brien TP (1966) On the ultrastructure of cambium and its vascular derivatives. I. Cambium of Pinus strobes L. Protoplasma 61:257–276
Staehelin LA, Giddings TH, Kiss JZ, Sack FD (1990) Macromolecular differentiation of Golgi stacks in root tips of Arabidopsis and Nicotiana seedlings as visualized by high pressure frozen and freeze-substituted samples. Protoplasma 157:75–91
Taylor DP (1988) Direct measurement of the osmotic effects of buffers and fixatives in Nitella flexilis. J Microsc 150:71–80
Thomson WW, Platt KA (1997) Conservation of cell order in desiccated mesophyll of Selaginella lepidophylla ([Hook and Grev.] Spring). Ann Bot 79:439–447
Valster AH, Hepler PK (1997) Caffeine inhibition of cytokinesis: effect on the phragmoplast cytoskeleton in living Tradescantia stamen hair cells. Protoplasma 196:155–166
Van den Driessche R (1977) Survival of coastal and interior Douglas-fir seedlings after storage at different temperatures, and effectiveness of cold storage in satisfying chilling requirements. Can J For Res 7:125–131
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rensing, K.H., Samuels, A.L. Cellular changes associated with rest and quiescence in winter-dormant vascular cambium of Pinus contorta . Trees 18, 373–380 (2004). https://doi.org/10.1007/s00468-003-0314-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-003-0314-7