Abstract
Background
This study investigated the impact of body composition in the arterial stiffness of children with chronic kidney disease (CKD).
Methods
Fat mass (FM), fat tissue index (FTI), fat-free mass (FFM), fat-free tissue index (FFTI), and FFTI/FTI were measured in 26 patients and 25 healthy controls by bio-impedance analysis. Data on patient’s body mass index (BMI) for height-age, serum albumin, glomerular filtration rate (GFR), blood pressure status, and pulse wave velocity (PWV) were collected in patients.
Results
Patients presented lower levels of FM and FFM compared to healthy controls (p = 0.04 and p = 0.055 respectively). In patient group, BMI height-age z-score was positively correlated to FTI (r2 = 0.574, p < 0.001) and FFTI (r2 = 0.338, p = 0.001) and negatively correlated to FFTI/FTI (r2 = 0.263, p = 0.007). Serum albumin was positively correlated only with FFM among body composition data (r2 = 0.169, p = 0.037). PWV z-score was positively correlated to FFTI (r2 = 0.421, p = 0.006) and inversely correlated to FFTI/FTI ≥ 2.5 (r2 = 0.317, p = 0.003). Patients with FFTI/FTI ≥ 2.5 presented lower levels of PWV regardless the need for antihypertensive treatment. Serum albumin ≥ 3.8 mg/dl and FFTI/FTI ≥ 2.5 were independently associated with a lower risk for high PWV, after adjustment for age, sex, and GFR (OR 0.009, 95% CI 0.000–0.729 and OR 0.039, 95% CI 0.002–0.680). All underweight [2 (7.7%)] and overweight [4 (15.4%)] patients presented high PWV. Among normal weight patients, FFTI/FTI ratio ≥ 2.5 was significantly associated with lower PWV z-score (p = 0.013).
Conclusions
Both underweight and overweight are associated with arterial stiffness. Targeting FFTI/FTI ≥ 2.5 could be protective against cardiovascular disease in normal weight children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease represents the main complication of chronic kidney disease (CKD) and accounts for the majority of deaths in end-stage renal disease (ESRD) and renal transplant pediatric patients [1]. Large vessel arteriopathy in CKD leads to arterial stiffness and therefore, loss of vascular compliance and distensibility [2]. Aortic pulse wave velocity (PWV) measurement is currently the most utilized method for assessment of arterial stiffness in pediatric population [3,4,5]. Apart from uremia, multiple risk factors of high PWV have been identified in children with CKD, including hypertension, impaired bone and mineral metabolism, dialysis duration, and high body mass index (BMI) [6,7,8,9,10].
Utilization of BMI as a sole anthropometric marker of nutritional status for cardiovascular risk assessment has been currently put into question. Recent adult studies have emphasized the protective role of lean tissue mass and the negative impact of adipose tissue mass in the CKD related cardiovascular disease [11,12,13]. However, data concerning body composition components in pediatric CKD and their eventual impact on arterial stiffness are currently lacking. The purpose of our study is to detect the impact of body composition indices, measured by bio-impedance analysis, on PWV in children with CKD.
Material and methods
We conducted a case control and cross-sectional study including 26 pediatric patients with CKD followed up at the Pediatric Nephrology Unit of the Hippokratio Hospital of Thessaloniki and 25, stratified by age and sex, healthy controls. The age of participants ranged from 7 to 19 years old. The inclusion criteria for healthy controls included normal estimated glomerular filtration rate (eGFR) (> 90 ml/min/1.73 m2), according to modified Schwartz equation, absence or proteinuria (urine protein to creatinine ratio < 0.2 g/g), and consent to participate in the study [14]. All patients presented eGFR ≤ 80 ml/min/1.73 m2.
Weight and height z-scores were calculated according to World Health Organization (WHO) reference values of healthy children with the same age. BMI was calculated as weight (kg)/height2 (m2). BMI height-age was determined by the age that corresponds to the child’s height when plotted at the 50th percentile on the WHO height for age growth chart. Both BMI and BMI height-age were converted to z-score using Cole’s lambda-mu-sigma method [15]. Patients were classified as underweight, normal weight, and overweight for BMI and BMI height-age if z-score < − 1.65 (5th percentile (perc)), 1.65 ≤ z-score ≤ 1.65 and z-score > 1.65 (95th perc) respectively [16].
Body composition was assessed using a portable multi-frequency bio-impedance analyzer (Bodystat Quadscan 4000, Bodystat, Beaconsfield, UK). The participants were instructed to refrain from eating and drinking 3 h before measurement. For patients on peritoneal dialysis, body composition was assessed 3 h post dialysis session. None of the patients had a day-time dwell that could have affected the accuracy of the test. Electrodes were placed on the left hand and foot of the body with the patient in the supine position. Input variables included patient’s age, sex, weight, height, and blood pressure (BP). The body composition was calculated by analyzing the electrical responses at 4 frequencies between 5 and 200 kHz. Fat-free tissue mass (FFM), including lean tissue mass and total body water, and fat tissue mass (FM) were derived from the impedance data, which they were also expressed as FFTI (fat-free tissue mass/height2) and FTI (fat tissue mass/height2) respectively. Moreover, a ratio of FFTI to FTI (FFTI/FTI) was calculated for each participant. For patients in peritoneal dialysis, bio-impedance analysis was performed at their dry weight, estimated by an experienced nephrologist, based on the percent monthly change in body weight and BP and clinical evaluation.
Arterial stiffness was evaluated by measurement of carotid-femoral (cf) PWV. The investigation took place in a quiet, semi-darkened, temperature-controlled room (21 °C). Before the examination, all participants rested in recumbent position for about 15 min. The participants were instructed to refrain from eating, smoking, and drinking beverages containing caffeine 3 h and also from drinking alcohol 10 h before measurement. All measurements were performed by the same experienced investigator, within 2 days from bio-impedance analysis. The Complior System (Colson, Les Lilas, France) was used to assess PWV. The investigator assessing PWV was blinded to the results of the bio-impedance analysis. PWV was calculated according to the equation PWV = (0.8 × D(m)/t (s), where t denotes the transit time of the arterial pulse along the distance, and D the distance assimilated to the surface between the recording sites [17]. D was measured directly using a centimeter tape, whereas t was obtained by the Complior System automatically. The transit time was quantified as the time delay between the feet of the two waveforms that were recorded simultaneously at the right common carotid artery and the right femoral artery (“foot-to-foot” method) using mechano-transducers directly applied on the skin. PWV levels were expressed as z-scores, using Cole’s lambda-mu-sigma method, according to age and height in healthy children; 1.65 z-score was considered as the cut-off between normal and high levels [18].
Average systolic and diastolic BP were measured by oscillometry using appropriate-sized cuffs (Ombron 705IT). BP z-scores were calculated according to age and height. The antihypertensive treatment of each patient was recorded.
Finally, we collected data of the patients’ serum albumin (mg/dl), serum calcium (Ca), and serum phosphorus (P). We took into consideration the average level for each variable of 6 months prior to the vascular and body composition assessment. For serum albumin, a cut-off of 3.8 mg/dl was used as a marker of adequate nutritional status as already reported in previous studies [16].
Data were expressed as median values and ranges. All statistical tests were realized with SPSS Statistics software for Windows. Linear and quadratic regression analysis was used to assess the correlations between the on-study parameters. Mann-Whitney test, Kruskal Wallis, and Fisher’s exact test were used to define associations between the on-study parameters. Logistic regression was performed to detect independent risk factors of high PWV after adjustment for possible confounders (age, sex, eGFR, and serum phosphorus). Finally, general linear model multivariate analysis was used to assess differences between patient groups according to nutritional parameters after adjustments for Bonferroni multiple comparisons. A p value of < 0.05 was considered statistically significant.
Results
Twenty-six patients (16 boys and 10 girls) of Caucasian origin, with a median age of 14 years (range 7–17 years) and a median eGFR of 30 ml/min/1.73 m2 (range 7–80) were included in this study (Table 1). eGFR was > 60 and ≤ 80 ml/min/1.73 m2 in 7 (26.9%) patients, > 30 and ≤ 60 in 6 (23.1%), > 15 and ≤ 30 in 4 (15.4%), and ≤ 15 in 9 (34.6%) patients respectively. Primary kidney disease was congenital abnormalities of kidney and urinary tract (CAKUT) in 10 (38.5%), hemolytic uremic syndrome in 4 (15.4%), polycystic kidney disease in 4 (15.4%), tubulo-interstitial nephritis in 2 (7.7%), Alport syndrome in 1 (3.8%), nephronophthisis in 1 (3.8%), primary glomerulopathy in 1 (3.8%), focal segmental glomerulosclerosis in 1 (3.8%), and unknown reason in 2 (7.7%) patients. All patients with stage 5 of CKD were on automated peritoneal dialysis. Antihypertensive treatment was necessary in 19 (73.1%) patients, monotherapy in 6 (23.1%) and two medications in 13 (50%) patients
Patients’ body composition data are outlined in Table 1. Distribution of FM, FFM, and FFTI were lower in patients compared to healthy controls (p = 0.04, p = 0.055, and p = 0.069 respectively) (Supplementary Table 1). After stratification of patients according to the stage of CKD, we observed that patients with stage 5 CKD presented significantly lower levels of FM and FFM (p = 0.011 and p = 0.045 respectively) and tended to have lower levels of FTI (p = 0.058) (Table 1). Of note, distribution of BMI and BMI height-age z-score did not significantly differ according to CKD stage. In the present study, BMI and BMI height-age z-score were positively correlated to both FTI and FFTI and negatively correlated to FFTI/FTI ratio (R2 = 0.603, p < 0.001, R2 = 0.378, p = 0.001, R2 = 0.276, p = 0.006 and R2 = 0.574, p < 0.001, R2 = 0.388, p = 0.001, R2 = 0.263, p = 0.007 respectively). Underweight and overweight were present in 3 (11.7%) and 3 (11.7%) patients respectively based on BMI z-score and 2 (7.7%) and 4 (15.4%) patients respectively based on BMI height-age z-score. There was a positive association of both FTI and FFTI with BMI status, whereas overweight patients presented a lower FFTI/FTI when compared to normal weight and underweight patients (Table 2).
As expected, serum albumin was significantly lower in patients with stage 5 CKD (Table 1). In our study, serum albumin was positively correlated only with FFM, among the body composition data (R2 = 0.169, p = 0.037) (data non-shown). As illustrated in Table 1, serum albumin was ≥ 3.8 in 18 (69.2%) patients. These patients presented significantly higher levels of FFM when compared to those with serum albumin < 3.8 mg/dl (p = 0.043) (Fig. 1).
In the present study, PWV was high in 16 (61.5%) patients. We observed a positive significant linear correlation between FTI and PWV z-score (R2 = 0.421, p = 0.006) and a tendency toward the higher the FFTI/FTI ratio, the lower the PWV z-score (R2 = 0.294, p = 0.072), after adjustment for age and sex. In total, FFTI/FTI ≥ 2.5, adjusted to age and sex, was significantly inversely correlated to PWV z-score (R2 = 0.317, p = 0.003). Moreover, patients with FFTI/FTI ≥ 2.5 presented lower levels of PWV regardless the need for antihypertensive treatment (Fig. 2). In multiple logistic regression, serum albumin ≥ 3.8 mg/dl and a FFTI/FTI ratio ≥ 2.5 were significantly associated with a lower risk for high PWV, regardless the age, sex, and eGFR (OR 0.009, 95% CI 0.000–0.729, p = 0.036, and OR 0.039, 95% CI 0.002–0.680, p = 0.026, respectively). Of note, when serum phosphorus levels were added in the multiple regression model, FFTI/FTI ratio ≥ 2.5 remained negatively associated with high PWV (OR 0.029, 95% CI 0.001–0.686, p = 0.028), whereas serum albumin ≥ 3.8 mg/dl lost its statistical significance as a protective factor (OR 0.004, 95% CI 0.000–1.457, p = 0.066) (Table 3). We further examined the effect of nutritional status on PWV z-score after Bonferroni corrections for multiple comparisons using multivariate general linear model analysis. The differences between patient groups with FFTI/FTI ≥ 2.5 and < 2.5 remained significant after adjustment for Bonferroni’s multiple comparisons (estimated marginal means of PWV z-score for age, eGFR, and phosphorus weighted by sex were 2.524, 95% CI 1.612–3.436 in those with FFTI/FTI ≥ 2.5 versus 1.067, 95% CI 0.244–1.889 in those with FFTI/FTI < 2.5, p = 0.020). Differences between patient groups of albumin < 3.8 and ≥ 3.8 were not significant (estimated marginal means of PWV z-score for age, eGFR, and phosphorus weighted by sex were 2.399, 95% CI 1.162–3.636 versus 1.191, 95% CI 0.504–1.879, respectively, p = 0.122).
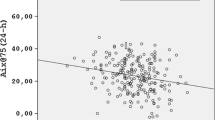
The correlation of BMI z-score with PWV z-score was statistically more significant as a U-shaped curve (R2 = 0.233, p = 0.047) than as a linear curve (R2 = 0.092, p = 0.132) (Fig. 3). All underweight and overweight patients, based on either BMI or BMI height-age z-score, presented high PWV. Among normal weight patients (based on BMI height-age), 10 (50%) presented high PWV. In this group, patients with FFTI/FTI ratio ≥ 2.5 presented lower levels of PWV z-score (p = 0.013) (Fig. 4).
Discussion
Pediatric CKD has been associated with divergent nutritional disorders [19]. On the one hand, poor dietary intake, systemic inflammation, metabolic acidosis, increased oxidative stress, and endocrine imbalance contribute to fat and muscle wasting [16, 20,21,22,23]. On the other hand, lack of physical activity and universal adoption of a diet high in saturated fat products gives rise to overweight and obesity rate [19, 24]. In concordance with these facts, both underweight and overweight patients, according to either BMI or BMI height-age, were present in our study. We also observed significantly lower levels of FFM and FFM according to bio-impedance analysis data, in patients with advanced stages of CKD, based on modified Schwartz equation for eGFR. Of note, given the fact that serum creatinine is influenced by the body composition indices, an eGFR formula based on serum cystatin C could have been more reliable in the determination of the impact of the CKD stage on the nutritional status of patients [25].
Both muscle wasting and obesity have been related to adverse clinical outcomes in pediatric CKD, such as poor growth and life quality, increased mortality rate, and limited access to kidney transplantation [19, 21, 26,27,28]. Therefore, precise assessment of fat and muscle mass is important for the optimal management of these patients. Measurement of triceps skinfold thickness and mid-arm muscle circumference for estimation of fat and muscle mass have been nowadays abandoned, mainly due to the lack of precision given the high level of inter-observer variability and the failure to estimate total muscle mass [29]. Dual-energy X-ray absorptiometry (DXA) has been used to evaluate muscle mass in children with CKD in various studies, indicating mainly leg skeletal mass loss in patients with late CKD [22, 30]. Nevertheless, the benefits of application of this expensive technique for monitoring nutritional status in patients with chronic disease have not yet been proven. In our study, we used bio-impedance analysis for estimation of FM and FFM adjusted to patient’s height. This technique is simple and rapid and has been widely used for measurement of body composition indices in general pediatric population. We observed that BMI and BMI height-age z-score were strongly correlated to FTI, FFTI, and to FFTI/FTI. Moreover, all body composition indices were significantly different in overweight and underweight when compared to normal weight patients. Of note, adjustment of BMI z-score to height-age led to fall in underweight prevalence from 11.7 to 7.7% and rise in overweight from 11.7 to 15.4%, implying that the application of BMI z-score in children with CKD is probably limited, due to the high incidence of growth retardation [31]. These findings highlight that BMI adjusted to height-age status embodies the finest anthropometric marker of nutritional status in children with CKD, representative of both FM and FFM. Nevertheless, the large variation of body composition indices in normal weight patients observed in this study indicates that bio-impedance analysis may be useful for precise nutritional evaluation in this group of patients.
In our study, we used arterial stiffness as an intermediate cardiovascular endpoint. FTI was significantly correlated to PWV and a ratio of FFTI/FTI ≥ 2.5 was associated with a lower risk of PWV regardless the need for antihypertensive treatment. These results show the adverse effects of relatively increased adiposity on the arterial stiffness in children with CKD. Nevertheless, we noticed that all overweight children presented high PWV, suggesting that body composition assessment does not improve upon BMI in the assessment of arterial stiffness risk of overweight children with CKD. Our results are supported by recent studies held in general pediatric population, where body FM did not seriously affect the strong association between BMI and metabolic syndrome or cardiovascular risk [32, 33]. However, we demonstrated that a higher FFTI/FTI had a protective effect against arterial stiffness not only in the total of patients but also in normal weight patients. The adverse clinical outcomes of normal weight obesity, defined as increased FM with normal BMI, have been also reported in adult patients with CKD [34]. Toward the same direction, Sgambat et al. highlighted that a combination of BMI with waist to height ratio, as a marker of fat distribution, better predicted cardiovascular risk, assessed by BP, left ventricular mass, and lipid profile, when compared to sole BMI in children with CKD [35]. The results of the present study may suggest that body composition assessment by bio-impedance analysis may be valuable for assessment of the nutritional impact on the cardiovascular disease in normal weight children with CKD, but the small size patient group does not allow safe conclusions.
In general, high levels of adipose tissue boost circulation of free fatty acids and secretion of cytokines and adipokines, promoting low-grade inflammation, endothelial dysfunction, and ultimately atherosclerosis [36]. Moreover, lipotoxicity due to lipid overload is incriminated for impairment in insulin sensitivity of muscle and liver [37]. On the other hand, skeletal mass represents a regulator of glucose homeostasis, since it is the main site of glucose uptake and disposal, playing a protective role against insulin resistance and metabolic syndrome [38, 39]. Besides, loss of muscle mass has been associated with increased mortality and cardiovascular events in adult patients with CKD, suggesting that enhanced muscle mass is also indicative of a fine cardiorespiratory fitness [12, 13, 40]. Hence, targeting a higher proportion of FFTI to FTI seems of critical importance for assuring an optimal cardiometabolic profile in patients with CKD, who present a predetermined high risk of cardiovascular disease.
Apart from the cardiovascular impact of obesity, there is growing evidence that protein energy wasting (or muscle wasting) has potential adverse cardiovascular effects, mainly through the stimulation of systemic inflammatory cytokines release [16]. The presence of malnutrition-inflammation-atherosclerosis syndrome, although observed in adult CKD, has not been confirmed yet in pediatric population [41, 42]. Inflammatory biomarkers such as CRP and IL-6 levels would provide significant insights, but were also not available in the present study. We used BMI and serum albumin as surrogate markers of protein energy wasting as proposed by previous studies [16]. All underweight patients presented high PWV and serum albumin ≥ 3.8 mg/dl was significantly associated with lower risk of high PWV. These results could support the hypothesis that enhanced muscle mass may be protective against arterial stiffness in children with CKD. However, the fact that the association between high PWV and serum albumin ≥ 3.8 mg/dl lost significance after inclusion of serum phosphorus levels in the multivariate models implies that poorer metabolic control (particularly CKD-MBD) and other confounding factors may mediate higher PWV particularly in underweight patients. Of note, serum phosphorus levels were not associated with high PWV possibly due to the fact that all patients, even those with stage 5 CKD, did not present very high levels of serum phosphorus, because of the wide use of phosphate binders. In the present study, we also pointed out that serum albumin was correlated to FFM, and patients with serum albumin ≥ 3.8 mg/dl presented significantly higher levels of FFM. Both these results indicate the good correspondence of serum albumin with the FFM, assessed by bio-impedance analysis. Therefore, bio-impedance analysis could be a valuable tool for monitoring both FM and FFM in children with CKD. Prospective cohort studies are necessary to confirm our results.
Maintenance of muscle reserve and inhibition of fat accumulation in patients with CKD remains a clinical challenge for the nephrologist. Firstly, correction of metabolic acidosis is valuable for impeding skeletal muscle catabolism [43]. Adherence to Mediterranean diet, low in saturated fat products, has been associated with prevention of obesity and fat overload [44]. Application of this diet, with a careful monitoring of serum potassium in patients with ESRD, seems to have beneficial effects on lipid profile in patients with CKD [45]. Exercise has been demonstrated as a positive impact on the loss of skeletal muscle mass and strength in adults with CKD [46]. Finally, for patients on hemodialysis, online hemodiafiltration instead of conventional hemodialysis has been associated with prevention of protein energy wasting [47]. The potential favorable effects of these measures in the adipose-dependent cardiovascular risk in children with CKD need to be answered in the future.
Our study has several limitations. While bio-impedance analysis has been recently validated as a reliable method for assessing total body water in children with CKD, its use for body composition assessment has been highly controversial [48]. It seems that this technique is useful for precise assessment of FM, but not lean mass, mainly due to its overestimation in overhydrated patients [49]. To overlap this problem, we performed this test at the estimated dry weight in patients on peritoneal dialysis. Nevertheless, the presence of subclinical overhydration, giving potential bias to our results cannot be excluded. Moreover, the fact that this is a cross-sectional study does not allow us to confirm the long-term impact of FFTI/FTI on the cardiovascular risk in pediatric CKD. Finally, the limited number of patients included in this study precluded us to make definite conclusions. Therefore, large-scale cohort studies are needed to confirm our results.
In conclusion, in this cross-sectional study, both underweight and overweight were related to arterial stiffness in children with CKD. A higher FFTI/FTI was associated to a lower risk of arterial stiffness not only in the total of patients but also in normal weight patients. Therefore, normal BMI coupled with enhanced lean-to-adipose tissue mass ratio may be protective against vascular damage in these patients. We may suggest that bio-impedance analysis is a useful tool for assessment of body composition indices. Further studies are needed to confirm our results and to provide strategies in order to improve the nutritional status of children with CKD.
References
Mitsnefes MM (2012) Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 23:578–585
Shroff R, Weaver DJ, Mitsnefes MM (2011) Cardiovascular complications in children with chronic kidney disease. Nat Rev Nephrol 7:642–649
Savant JD, Furth SL, Meyers KE (2014) Arterial stiffness in children: pediatric measurement and considerations. Pulse (Basel) 2:69–80
Stabouli S, Papakatsika S, Kotronis G, Papadopoulou-Legbelou K, Rizos Z, Kotsi (2015) Arterial stiffness and SBP variability in children and adolescents. J Hypertens 33:88–95
Kotsis V, Antza C, Doundoulakis I, Stabouli S (2017) Markers of early vascular ageing. Curr Pharm Des 23:3200–3204
Patange AR, Valentini RP, Du W, Pettersen MD (2012) Vitamin D deficiency and arterial wall stiffness in children with chronic kidney disease. Pediatr Cardiol 33:122–128
Kis E, Cseprekál O, Bíró E, Kelen K, Ferenczi D, Kerti A, Szabó AJ, Szabó A, Reusz GS (2009) Effects of bone and mineral metabolism on arterial elasticity in chronic renal failure. Pediatr Nephrol 24:2413–2420
Shroff RC, Donald AE, Hiorns MP, Watson A, Feather S, Milford D, Ellins EA, Storry C, Ridout D, Deanfield J, Rees L (2007) Mineral metabolism and vascular damage in children on dialysis. J Am Soc Nephrol 18:2996–3003
Schmidt BMW, Sugianto RI, Thurn D, Azukaitis K, Bayazit AK, Canpolat N, Eroglu AG, Caliskan S, Doyon A, Duzova A, Karagoz T, Anarat A, Deveci M, Mir S, Ranchin B, Shroff R, Baskin E, Litwin M, Özcakar ZB, Büscher R, Soylemezoglu O, Dusek J, Kemper MJ, Matteucci MC, Habbig S, Laube G, Wühl E, Querfeld U, Sander A, Schaefer F, Melk A, 4C Study Consortium (2018) Early effects of renal replacement therapy on cardiovascular comorbidity in children with end-stage kidney disease. Transplantation 102:484–492
Schaefer F, Doyon A, Azukaitis K, Bayazit A, Canpolat N, Duzova A, Niemirska A, Sözeri B, Thurn D, Anarat A, Ranchin B, Litwin M, Caliskan S, Candan C, Baskin E, Yilmaz E, Mir S, Kirchner M, Sander A, Haffner D, Melk A, Wühl E, Shroff R, Querfeld U, 4C Study Consortium (2017) Cardiovascular phenotypes in children with CKD: the 4C study. Clin J Am Soc Nephrol 12:19–28
Marcelli D, Usvyat LA, Kotanko P, Bayh I, Canaud B, Etter M, Gatti E, Grassmann A, Wang Y, Marelli C, Scatizzi L, Stopper A, van der Sande FM, Kooman J, MONitoring Dialysis Outcomes (MONDO) Consortium (2015) Body composition and survival in dialysis patients: results from an international cohort study. Clin J Am Soc Nephrol 10:1192–1200
Lin TY, Peng CH, Hung SC, Tarng DC (2018) Body composition is associated with clinical outcomes in patients with non–dialysis-dependent chronic kidney disease. Kidney Int 93:733–740
Beddhu S, Pappas LM, Ramkumar N, Samore M (2003) Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol 14:2366–2372
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1240–1243
Oliveira EA, Cheung WW, Toma KG, Mak RH (2018) Muscle wasting in chronic kidney disease. Pediatr Nephrol 33:789–798
Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T, Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries (2012) Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 30:445–448
Reusz GS, Cseprekal O, Temmar M, Kis E, Cherif AB, Thaleb A, Fekete A, Szabó AJ, Benetos A, Salvi P (2010) Reference values of pulse wave velocity in healthy children and teenagers. Hypertension 56:217–224
Rees L, Jones H (2013) Nutritional management and growth in children with chronic kidney disease. Pediatr Nephrol 28:527–536
Mak RH, Cheung WW, Zhan JY, Shen Q, Foster BJ (2012) Cachexia and protein-energy wasting in children with chronic kidney disease. Pediatr Nephrol 27:173–181
Abraham AG, Mak RH, Mitsnefes M, White C, Moxey-Mims M, Warady B, Furth SL (2014) Protein energy wasting in children with chronic kidney disease. Pediatr Nephrol 29:1231–1238
Foster BJ, Kalkwarf HJ, Shults J, Zemel BS, Wetzsteon RJ, Thayu M, Foerster DL, Leonard MB (2011) Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol 22:377–386
Apostolou A, Printza N, Karagiozoglou-Lampoudi T, Dotis J, Papachristou F (2014) Nutrition assessment of children with advanced stages of chronic kidney disease-a single center study. Hippokratia 18:212–216
Bonthuis M, van Stralen KJ, Verrina E, Groothoff JW, Alonso Melgar Á, Edefonti A, Fischbach M, Mendes P, Molchanova EA, Paripović D, Peco-Antic A, Printza N, Rees L, Rubik J, Stefanidis CJ, Sinha MD, Zagożdżon I, Jager KJ, Schaefer F (2013) Underweight, overweight and obesity in paediatric dialysis and renal transplant patients. Nephrol Dial Transplant 28:iv195–iv204
Miliku K, Bakker H, Dorresteijn EM, Cransberg K, Franco OH, Felix JF, Jaddoe VW (2017) Childhood estimates of glomerular filtration rate based on creatinine and cystatin C: importance of body composition. Am J Nephrol 45:320–326
Ayestaran FW, Schneider MF, Kaskel FJ, Srivaths PR, Seo-Mayer PW, Moxey-Mims M, Furth SL, Warady BA, Greenbaum LA (2016) Perceived appetite and clinical outcomes in children with chronic kidney disease. Pediatr Nephrol 31:1121–1127
Ku E, Glidden DV, Hsu CY, Portale AA, Grimes B, Johansen KL (2016) Association of body mass index with patient-centered outcomes in children with ESRD. J Am Soc Nephrol 27:551–558
Wong CS, Gipson DS, Gillen DL, Emerson S, Koepsell T, Sherrard DJ, Watkins SL, Stehman-Breen C (2000) Anthropometric measures and risk of death in children with end-stage renal disease. Am J Kidney Dis 36:811–819
Mastrangelo A, Paglialonga F, Edefonti A (2014) Assessment of nutritional status in children with chronic kidney disease and on dialysis. Pediatr Nephrol 29:1349–1358
Cochat P, Braillon P, Feber J, Hadj-Aïssa A, Dubourg L, Liponski I, Saïd MH, Glastre C, Meunier PJ, David L (1996) Body composition in children with renal disease: use of dual energy X-ray absorptiometry. Pediatr Nephrol 10:264–268
Gao T, Leonard MB, Zemel B, Kalkwarf HJ, Foster BJ (2012) Interpretation of body mass index in children with CKD. Clin J Am Soc Nephrol 7:558–564
Weber DR, Leonard MB, Shults J, Zemel BS (2014) A comparison of fat and lean body mass index to BMI for the identification of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab 99:3208–3216
Bohn B, Müller MJ, Simic-Schleicher G, Kiess W, Siegfried W, Oelert M, Tuschy S, Berghem S, Holl RW, APV Initiative and the German BMBF Competence Network Obesity (2015) BMI or BIA: is body mass index or body fat mass a better predictor of cardiovascular risk in overweight or obese children and adolescents? A German/Austrian/Swiss multicenter APV analysis of 3,327 children and adolescents. Obes Facts 8:156–165
Lin TY, Lim PS, Hung SC (2018) Normal-weight obesity and clinical outcomes in nondiabetic chronic kidney disease patients: a cohort study. Am J Clin Nutr 107:664–672
Sgambat K, Roem J, Mitsnefes M, Portale AA, Furth S, Warady B, Moudgil A (2018) Waist-to-height ratio, body mass index, and cardiovascular risk profile in children with chronic kidney disease. Pediatr Nephrol. https://doi.org/10.1007/s00467-018-3987-2
Fantuzzi G, Mazzone T (2007) Adipose tissue and atherosclerosis exploring the connection. Arterioscler Thromb Vasc Biol 27:996–1003
Hardy OT, Czech MP, Corvera S (2012) What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes 19:81–87
Srikanthan P, Karlamangla AS (2011) Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 96:2898–2903
Kim K, Park SM (2018) Association of muscle mass and fat mass with insulin resistance and the prevalence of metabolic syndrome in Korean adults: a cross-sectional study. Sci Rep 8:2703
Harada K, Suzuki S, Ishii H, Aoki T, Hirayama K, Shibata Y, Negishi Y, Sumi T, Kawashima K, Kunimura A, Shimbo Y, Tatami Y, Kawamiya T, Yamamoto D, Morimoto R, Yasuda Y, Murohara T (2017) Impact of skeletal muscle mass on long-term adverse cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol 119:1275–1280
Kahraman S, Yilmaz R, Akinci D, Arici M, Altun B, Erdem Y, Yasavul U, Turgan C (2005) U-shaped association of body mass index with inflammation and atherosclerosis in hemodialysis patients. J Ren Nutr 15:377–386
Canpolat N, Caliskan S, Sever L, Tasdemir M, Ekmekci OB, Pehlivan G, Shroff R (2013) Malnutrition and its association with inflammation and vascular disease in children on maintenance dialysis. Pediatr Nephrol 28:2149–2156
Amodu A, Abramowitz MK (2016) Effects of metabolic acidosis on skeletal muscle. In: Wesson D (ed) Metabolic acidosis: a guide to clinical assessment and management, 1st edn. Springer, New York, pp 101–110
Mistretta A, Marventano S, Antoci M, Cagnetti A, Giogianni G, Nolfo F, Rametta S, Pecora G, Marranzano M (2017) Mediterranean diet adherence and body composition among Southern Italian adolescents. Obes Res Clin Pract 11:215–226
Chauveau P, Aparicio M, Bellizzi V, Campbell K, Hong X, Johansson L, Kolko A, Molina P, Sezer S, Wanner C, Ter Wee PM, Teta D, Fouque D, Carrero JJ, European Renal Nutrition (ERN) Working Group of the European Renal Association–European Dialysis Transplant Association (ERA-EDTA) (2018) Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol Dial Transplant 33:725–735
Roshanravan B, Gamboa J, Wilund K (2017) Exercise and CKD: skeletal muscle dysfunction and practical application of exercise to prevent and treat physical impairments in CKD. Am J Kidney Dis 69:837–852
Molina P, Vizcaíno B, Molina MD, Beltrán S, González-Moya M, Mora A, Castro-Alonso C, Kanter J, Ávila AI, Górriz JL, Estañ N, Pallardó LM, Fouque D, Carrero JJ (2018) The effect of high-volume online haemodiafiltration on nutritional status and body composition: the ProtEin Stores prEservaTion (PESET) study. Nephrol Dial Transplant 33:1223–1235
Dasgupta I, Keane D, Lindley E, Shaheen I, Tyerman K, Schaefer F, Wühl E, Müller MJ, Bosy-Westphal A, Fors H, Dahlgren J, Chamney P, Wabel P, Moissl U (2018) Validating the use of bioimpedance spectroscopy for assessment of fluid status in children. Pediatr Nephrol 33:1601–1607
Milani GP, Groothoff JW, Vianello FA, Fossali EF, Paglialonga F, Consolo S, Edefonti A, Consonni D, van Harskamp D, van Goudoever JB, Schierbeek H, Agostoni C, Oosterveld MJS (2018) Bioimpedance spectroscopy imprecisely assesses lean body mass in pediatric dialysis patients. J Pediatr Gastroenterol Nutr 67:533–537
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the local Ethics Committee and was conducted in accordance with the 1964 Helsinki Declaration or comparable standards. Informed consent was obtained from the parents of all the participants.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 32 kb)
Rights and permissions
About this article
Cite this article
Karava, V., Printza, N., Dotis, J. et al. Body composition and arterial stiffness in pediatric patients with chronic kidney disease. Pediatr Nephrol 34, 1253–1260 (2019). https://doi.org/10.1007/s00467-019-04224-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04224-8