Abstract
Introduction
This cross-sectional study investigates the association between insulin resistance (IR) and serum uric acid (sUA) and relative fat (RFM) and lean mass (RLM) profiles in children with chronic kidney disease (CKD).
Material and methods
RLM and RFM were assessed by bioimpedance spectroscopy in 41 children and adolescents. Normal weight obesity (NWO) was defined as normal height-age body mass index and RFM >85th percentile, according to age and sex. Homeostatic model assessment of insulin resistance (HOMA-IR) level >95th percentile, according to sex and pubertal stage, and sUA >7 mg/dl were used to define IR and hyperuricemia, respectively.
Results
High RFM (15 patients) and NWO (7 patients) were associated with higher HOMA-IR in total (p < 0.001) and normal-weight patients (p = 0.004), respectively. RFM was positively and RLM negatively correlated to HOMA-IR (rs = 0.500, p = 0.001 and rs = −0.539, p < 0.001, respectively) and sUA (rs = 0.370, p = 0.017 and rs = −0.325, p = 0.038, respectively), while sUA was positively correlated to HOMA-IR (rs = 0.337, p = 0.031). Hyperuricemia (16 patients) was positively associated with higher RFM and HOMA-IR (p = 0.001 and p = 0.010, respectively). The correlation between sUA and HOMA-IR lost significance after adjustment for RFM. In logistic regression analysis, a 5% increase in RFM was associated with IR (11 patients) independently of the age, sex, sUA, and CKD stage in both total (OR 2.174, 95% CI 1.115–4.225) and normal-weight (OR 3.504, 95% CI 1.110–11.123) patients.
Conclusion
Children with high RFM, including those presenting NWO, are at risk for IR regardless of CKD stage. RFM is probably the mediator of the link between sUA and IR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) has been consistently linked to a disturbed metabolic profile, including dysregulation of glucose homeostasis and lipoprotein metabolism and imbalance in body composition indices, involving a progressive degradation of muscle mass and an enhancement of visceral fat accumulation, eventually leading to metabolic syndrome. Among these conditions, insulin resistance (IR) has been associated with deleterious consequences on CKD progression and cardiovascular morbidity [1]. Therefore, early recognition and timely management of IR is of high benefit for the prognosis of these patients. Obesity is the traditional major risk factor for IR in the general population [2]. Body mass index (BMI) as a sole marker of adiposity has been currently disputed due to the emerging rising incidence of normal weight obesity (NWO), defined as a high body fat percentage with a normal BMI. Although data in children are scarce, this condition has been consistently correlated to increased cardiometabolic risk in the adult population [3, 4]. Patients with CKD are susceptible to exhibit NWO due to the frequently observed adoption of a Western high-fat diet, low physical activity, and muscle wasting. Nevertheless, data on the prevalence and the possibly related impact on the glucose and insulin homeostasis of this condition in children with CKD have not been fully available to date [5].
Even though the pathogenesis of IR in CKD has not been fully elucidated, possible causal factors include vitamin D deficiency, metabolic acidosis, anemia, and progressive systemic accumulation of uremic toxins, which induce dysregulation of glucose metabolic pathways [6]. Among uremic toxins, serum uric acid (sUA), although previously considered just as a sequela of CKD, has recently gained scientific interest regarding its contributing role to CKD progression and related morbidity [7, 8]. Hyperuricemia has been associated with IR in various studies but whether this relationship is direct or not remains unsolved, since sUA has also been associated with obesity [9, 10]. The association between sUA and the presence of IR in children with CKD has not yet been studied. The purpose of this single-center cross-sectional study is to investigate the association between relative fat and lean mass, sUA, and IR in children with CKD.
Methods
We conducted a cross-sectional study from June 2018 to February 2019 on pediatric patients with CKD, already followed up at the Pediatric Nephrology Units of the 1st and 3rd Departments of Pediatrics at the Hippokratio General Hospital of Thessaloniki. Inclusion criteria included (i) age between 6 and 19 years old, (ii) estimated glomerular filtration rate (eGFR) ≤ 80 ml/min/1.73 m2, as calculated by the revised Schwartz formula [11], (iii) BMI height-age z score > − 2 SD, (iv) absence of history of kidney transplantation or history of steroid or immunosuppressive therapy, and (v) absence of diabetes mellitus and purine metabolic disorder. None of the patients included in this study was under urate-lowering therapy. Finally, patients with missing data were excluded.
Weight, height, and BMI z-scores were calculated according to the World Health Organization (WHO) reference values for healthy children matched for age and sex. Patients were classified as normal weight, overweight, and obese based on BMI adjusted to height age (BMI height-age) z-score. Patients were considered as normal weight, overweight and obese with BMI height-age z-score between – 2 SD (3rd percentile) and +1 SD (85th percentile), between +1 SD and +2 SD (97th percentile), and above +2 SD, respectively. Body composition was assessed using a portable multifrequency bioimpedance spectroscopy (BIS) device (Body composition monitor, BCM; Fresenius). The participants were instructed to refrain from eating and drinking 8 h before measurement. For patients on peritoneal dialysis, body composition was assessed 3 h post dialysis session. None of the patients had a daytime dwell that could have affected the accuracy of the readings. For patients on hemodialysis, BIS measurement was effectuated 1 h after the end of a hemodialysis session. Electrodes were placed on the left hand and foot of the body with the patient in the supine position. Fat tissue mass (FM) and lean tissue mass (LM), as well as relative fat tissue mass (RFM) and relative lean tissue mass (RLM), which corresponded to the percentage of fat and lean tissue mass, respectively, were derived from the impedance data. The cutoff point for high RFM was set at the 85th percentile, as for BMI, based on the fat mass reference curves assessed by bioelectrical impedance method in healthy Turkish children and adolescents, according to age and sex [12]. Normal weight obesity (NWO) was defined as the presence of RFM >85th percentile with normal BMI height-age z-score.
Patients’ serum glucose and serum insulin were measured after a fasting duration of at least 8 h. All blood samples were analyzed in the same laboratory. Plasma insulin levels were measured using a solid-phase, enzyme-labeled chemiluminescent immunometric assay (IMMULITE 2000 XPi, Siemens Healthineers Global). The cutoff point for high plasma glucose was set at 100 mg/dl. The homeostasis model assessment of insulin resistance (HOMA-IR) was used as an indicator of IR. We calculated HOMA-IR based on the following equation: [serum insulin (mUI/L) × serum glucose (mg/dl)]/405 [13]. Insulin resistance was defined as HOMA-IR >95th percentile based on reference values in healthy Italian children and adolescents according to sex and pubertal stage based on the Tanner staging criteria [13]. Tanner stage was assessed for each patient after a thorough examination by an experienced pediatric endocrinologist. Finally, we collected data on the sUA (mg/dl) level during the last 6-month period for all patients. The mean value of the last 6-month sUA was calculated for each patient. Hyperuricemia was identified for sUA >7 mg/dl.
Data are expressed as median values and ranges. All statistical tests were realized with SPSS Statistics software for Windows. Fisher’s exact test and Mann–Whitney test were used to define associations between the on-study categorical and continuous variables, respectively. Spearman correlation analysis was used to assess the univariate correlations between the on-study variables, whereas Spearman partial correlation analysis was used to assess the correlations between the on-study variables after adjustment for possible confounders. ROC curve analysis was applied to compare the associations between the on-study variables and high HOMA-IR. Multiple logistic regression was used to define risk factors for IR after adjustment for age, sex, and CKD stage. All selected variables were added to a single regression model. We further proceeded with a backward stepwise elimination process to find the best fit model. A two-tailed p value of <0.05 was considered statistically significant.
Results
Forty-one patients (27 boys, 14 girls) with a median age of 12.8 years (range 6.1–18.8) were included in this study. Primary kidney disease was a congenital abnormality of the kidney and urinary tract in 29 (70.7%), hemolytic uremic syndrome in 3 (7.3%), focal segmental glomerulosclerosis in 3 (7.3%), ciliopathy in 5 (12.2%), and unknown in 1 (2.4%) patient(s), respectively. Among them, 16 (39%) patients presented stage 5 CKD (CKD 5): 14 under chronic peritoneal dialysis and 2 under chronic hemodialysis, with a median duration of extrarenal epuration of 3.7 years (0.3–9). The distribution of the stages of CKD in the rest of the patients was as follows: stage 2 in 4 (9.75%), stage 3 in 17 (41.5%), and stage 4 in 4 (9.75%) patients, respectively. Of note, no patient was under enteral feeding via gastrostomy or nasogastric tube or under nutritional supplements.
According to the BMI height-age z-score, 32 (78%) patients were normal weight, 6 (14.6%) were overweight, and 3 (7.3%) were obese. The patients were then divided according to both the RFM and BMI height-age z-score in normal RFM with normal weight (BMI z-score ≤ +1 SD) or overweight/obese (BMI z-score > +1 SD) and high RFM with normal weight (BMI z-score ≤ +1 SD) or overweight/obese (BMI z-score > +1 SD) (Fig. 1). More than one third of the patients presented high RFM and approximately 50% of whom were not overweight. Among the 26 patients with normal RFM, only 1 was overweight. In total, 9 (22%) patients were overweight or obese, whereas 7 (17.1%) presented NWO. The distribution of BMI z-score and body composition data according to the stage of CKD are illustrated in Fig. 1 and in Table 1. We observed that although patients with CKD 5 presented lower z-score levels of weight, height, and BMI (p < 0.001, p < 0.001, and p = 0.012, respectively), distribution of RFM and RLM and the prevalence of high RFM did not significantly differ from those in CKD stages 2–4 (Table 1). In detail, NWO corresponded to 4 (16%) patients with CKD stages 2–4 and 3 (18.8%) patients with CKD 5, whereas the prevalence of overweight with high RFM was present in 6 (24%) and in 2 (12.5%) patients with CKD stages 2–4 and CKD 5, respectively (Fig. 1).
In this study, fasting serum glucose levels were high in only 2 (4.9%) patients, while HOMA-IR level was increased in 11 (26.8%) patients. The distribution of the serum glucose, serum insulin and HOMA-IR profile did not statistically differ between the patients with CKD 5 and those with CKD stages 2–4 (Table 1). As expected, patients with high RFM presented higher levels of serum insulin and HOMA-IR and higher rate of increased HOMA-IR (p < 0.001, p < 0.001, and p = 0.008, respectively). Nevertheless, the abovementioned associations remained significant in the normal-weight group of patients (p = 0.013, p = 0.004, and p = 0.026, respectively) (Table 2). In the correlation analysis, RLM was negatively correlated to serum insulin (rs = − 0.440, p = 0.004) and HOMA-IR (rs = − 0.479, p = 0.002) levels, whereas RFM was positively correlated to serum insulin (rs = 0.500, p = 0.001) and HOMA-IR (rs = 0.539, p < 0.001) levels. These correlations remained significant in the group of normal-weight patients (Table 3). Of note, the correlations between BMI height-age z-score and insulin or HOMA-IR were weaker than those observed between RFM and insulin or HOMA-IR in both groups of patients (Table 3).
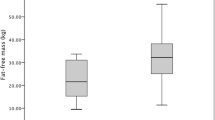
In this study, sUA was high in 16 (39%) patients and did not significantly differ between patients with CKD stage 2–4 and CKD 5. Levels of RLM were lower (p = 0.004) and levels of RFM were higher (p = 0.001) in patients with hyperuricemia (Fig. 2). Furthermore, hyperuricemia was significantly associated with HOMA-IR levels (p = 0.010). Patients with high RFM and NWO presented higher sUA levels (p = 0.020 and p = 0.048, respectively) and higher rate of hyperuricemia (p = 0.009 and p = 0.032, respectively) (Table 2). In the correlation analysis, sUA was negatively correlated to RLM (rs = − 0.325, p = 0.038) and positively correlated to RFM (rs = 0.370, p = 0.017), serum insulin (rs = 0.354, p = 0.023), and HOMA-IR (rs = 0.337, p = 0.031) (Table 3). All these correlations did not reach significance in the normal-weight group.
In the Spearman partial correlation analysis, the correlation between sUA and HOMA-IR lost significance after adjustment for RFM (rs = 0.238, p = 0.140) whereas the correlation between RFM and HOMA-IR remained significant after adjustment for sUA (rs = 0.474, p = 0.002). In the ROC curve analysis, RFM was a stronger predictor of high HOMA-IR compared to BMI height-age z-score in the total patients (AUC = 0.785 and AUC = 0.709, respectively) and in the normal-weight group (AUC = 0.783 and AUC = 0.686, respectively) (Fig. 3). In the multiple logistic regression analysis, RFM was significantly associated with the risk for high HOMA-IR independently of sUA, age, sex, and CKD stage in the total and in the normal-weight group of patients (p = 0.022, OR 1.168, 95% CI 1.022–1.334 and p = 0.033, OR 1.285, 95% CI 1.021–1.619). In other words, an RFM increase of 5% was a risk factor for high HOMA-IR with an OR of 2.174 (95% CI 1.115–4.225) in the total patients and with an OR of 3.504 (95% CI 1.110–11.123) in normal-weight patients. Serum UA was not significantly associated with the risk for high HOMA-IR in both groups of patients (p = 0.636, OR 0.844, 95% CI 0.419–1.701 for the total patients and p = 0.772, OR 0.869, 95% CI 0.335–2.253 for normal-weight patients). Of note, after a 4-step backward elimination process, the best fit logistic regression model included RFM (p = 0.014, OR 1.114, 95% CI 1.022–1.215) and CKD stage (p = 0.086, OR 2.008, 95% CI 0.906–4.449) as covariates of high HOMA-IR.
Discussion
Insulin resistance is defined as an abnormal response of peripheral body tissues, including adipose and mass tissue, to blood insulin levels concerning glucose uptake, metabolism, or storage. While abnormally high fasting serum glucose levels is an uncommon finding in non-diabetic patients with CKD, hyperinsulinemia, and glucose intolerance have been widely described in both adult and pediatric patients [7]. Our results highlight increased HOMA-IR in 26.8% and high fasting serum glucose in only 4.9% of patients, respectively, which is in accordance with previous epidemiologic studies, where the prevalence of IR, expressed as either abnormal oral glucose test or increased HOMA-IR levels concerned 21–51.5% and 15.2–47.1% of children with CKD, respectively [14,15,16]. Although the risk for IR is higher when glomerular filtration rate greatly decreases [6, 17], it may be present even in the early stages of CKD [18,19,20]. In our study, increased HOMA-IR level was detected in 20% and 37.5% of patients with CKD stages 2–4 and CKD 5, respectively. Insulin resistance has been incriminated in both cardiovascular (CV) and kidney damage. In detail, it has been implicated in the pathogenesis of atherosclerosis and hypertension through upregulation of the renin–angiotensin aldosterone system, contribution to the development of dyslipidemia, and promotion of endothelial dysfunction [6]. Although the CV impact of this condition in children with CKD has not been clearly evidenced yet [17, 20], possibly because of the short course of the disease, IR has been solidly associated with higher risk of CV-related mortality and events, as well as with left ventricular hypertrophy and arterial stiffness in several studies including adult patients [1]. Moreover, IR is one of the components of metabolic syndrome significantly correlated to CKD progression in both adult and pediatric patients [1, 19], implying that this condition may directly induce both glomerular and tubular structural injury [21]. Given this data, understanding the mechanisms responsible for the pathogenesis of IR is essential for the optimal screening of these patients and identification of novel therapeutic targets.
Obesity is considered as a major contributing condition to IR in the general population. Increased adipose tissue induces alteration in the release of free fatty acids and the secretion of adipokines and proinflammatory cytokines, which are involved in the modulation of insulin sensitivity [2]. As expected, BMI has been significantly associated with a higher risk of hyperinsulinemia in both the general pediatric population and in children with CKD [15, 22]. In our study, we noted a discrepancy between the prevalence of high BMI and high RFM, with the first accounting for 22% and the second for 36.6% of patients, respectively. Results from healthy children are contradictory regarding the benefits of using adiposity level, compared to BMI, for the assessment of cardiometabolic risk [23, 24]. Nevertheless, in children with CKD, it has been recently observed that the combination of BMI and waist-to-height ratio, as an index of abdominal adiposity, favors predicting the risk of hyperglycemia compared to each of these parameters individually [5]. The association between NWO and IR, which is a prominent and preceding condition of hyperglycemia, although already mentioned in adult patients with CKD, has not yet been studied in a pediatric population [25]. In our study, we have observed that high RFM was significantly associated with increased HOMA-IR levels and that RFM levels were significantly correlated to serum insulin and HOMA-IR levels in both total and normal-weight patients. Moreover, RFM was significantly associated with the risk for high HOMA-IR after adjustment for possible confounders in both groups of patients and was a stronger predictor of high HOMA-IR compared to BMI height-age z-score. These results indicate that RFM level is a risk factor for IR even in normal-weight children with CKD and that patients with NWO are prone to a disturbed glucose and insulin metabolism.
Data on the prevalence of NWO are limited in children with CKD. Sgambat et al. have observed that 15.2% of patients with GFR > 30 ml/min/1.73 m2 presented high waist-to-height ratio with normal BMI [5], which is comparable to our results, where NWO, based on BMI and BIS results, was present in 17.1% of the patients, accounting for 16% of patients with CKD stages 2–4 and 18.8% of patients with CKD 5. It is worth mentioning that this condition may be representative not only of increased fat mass but also of muscle wasting, which is particularly observed in children with CKD. Taking into account the fact that muscle mass is the primary site of glucose uptake, some researchers suggest that muscle wasting may contribute substantially to IR in patients with CKD [6, 16]. In clinical studies, IR has been inversely associated with RLM in the general adult population [26] and with muscle breakdown parameters in adults with CKD [27]. To the best of our knowledge, this is the first pediatric study to show an inverse correlation between RLM and levels of HOMA-IR and insulin in both total and normal-weight children with CKD. The link between IR and RLM is probably bidirectional, with IR contributing to the inhibition of glucose uptake by the skeletal muscle, further promoting muscle atrophy [28]. Therefore, therapeutic strategies aimed at the enhancement of muscle mass and reduction of fat mass may be the key for limiting the risk of IR in children with CKD.
Uric acid is the end metabolic product of ingested or endogenous synthesized purines and is generated by xanthine oxidase, an enzyme which catalyzes the oxidation of xanthine to UA. The reduced UA kidney clearance and the increased xanthine oxidase activity observed in CKD [29, 30] may ultimately lead to hyperuricemia, which may be additionally boosted by a high-purine diet (Fig. 4). Uric acid has been associated with IR in various studies in obese children, raising the question of whether the link between UA and IR is direct or dependent on the extent of adiposity [31, 32]. In this study, sUA level was significantly correlated to HOMA-IR, RFM, and RLM levels. The lack of significance in the association between sUA and IR in logistic regression analysis and the results of the partial Spearman correlation analysis suggest that the connection between HOMA-IR and sUA is probably indirect and mediated through fat mass accumulation in children with CKD (Fig. 4). A negative correlation between sUA levels and relative skeletal mass has been already observed in adult studies [33]. In addition, sUA has been suggested as a determinant factor for weight gain and for the development of non-alcoholic fatty liver disease in adult studies [34, 35]. In fact, biochemical studies have shown that UA acts as a pro-oxidant factor in the intracellular space, promoting the stimulation of proinflammatory cytokine synthesis, while increased xanthine oxidase activity induces oxidative stress through the production of reactive oxygen species [36, 37]. Both these pathways may be at the origin of muscle wasting through activation of the ubiquitin–proteasome system [38]. Increased intracellular and mitochondrial oxidative stress may also explain the generating of obesity in case of high UA milieu [9]. Furthermore, there is probably a vicious circle between UA and fat mass accumulation since some researchers suggest that secretion of UA and xanthine oxidase activity from the adipose tissue may be enhanced in the case of obesity [37, 39]. Conclusively, our results suggest that sUA may reflect higher adiposity and lower muscle mass levels in children with CKD.
Both muscle wasting and obesity have been related to long-term adverse clinical outcomes in pediatric CKD, such as high risk of CV complications and rapid progression of CKD [5, 19, 40]. Apart from the recommended physical exercise and eviction of high-fat diet, new therapeutic strategies need to be implemented. Evidence on the protective effect of urate-lowering therapy in the prevention of muscle loss is limited and based on animal models or older individuals [34, 41, 42]. A few clinical studies also highlight the benefits of reducing sUA levels on the reduction of body weight and adiposity in obese patients [43, 44]. The impact of urate-lowering therapy in children with CKD presenting high RFM needs to be illuminated in the future.
Our study has several limitations. Firstly, the gold standard method to investigate insulin sensitivity is the euglycemic hyperinsulinemic clamp [6]. Nevertheless, this technique is time consuming and difficult to perform in a pediatric population compared to HOMA-IR applied in this study, which is a less expensive and quicker method and has already been used as an indicator of IR in various pediatric studies [13]. Moreover, in the general pediatric population, BIS technique is considered as a practical, bedside, inexpensive, and non-irradiant method to estimate RFM in children and adolescents, but its validity and measurement error are not satisfactory when compared to the reference techniques, including dual-energy X-ray absorptiometry, isotope dilution, and underwater weighing [45]. Although BIS has already been used for the evaluation of nutritional status in adult and pediatric patients with CKD 5 [46, 47], it is not as precise as deuterium dilution in the assessment of lean mass at an individual level [48]. The large variance of agreement between RFM assessed by BIS and reference techniques may have affected the strength of our results. In our study, it is worth mentioning that all BIS measurements in children with CKD 5 were performed at their dry weight, in order to minimize possible overestimation of lean mass. Furthermore, although toddlers and preschool children were excluded from the study, age-related variation of sUA may have affected our results. Besides, as already mentioned, the results in this cross-sectional study do not prove cause-and-effect relationships. Finally, the limited number of patients recruited does not allow us to make solid conclusions. Further large-scale longitudinal studies, including patients from different nations and probably different nutritional lifestyles and physical activity levels, are needed to confirm our results.
In conclusion, despite the limitations of our study, it is the first to demonstrate the negative impact of increased adiposity on IR in children with CKD, even in those with normal weight, suggesting the need for monitoring not only BMI but also body composition indices in these patients. Moreover, we highlighted that RFM probably mediates the association between UA and IR, suggesting that hyperuricemia may reflect the relatively higher adiposity levels in CKD. This novel finding supplements the already identified aspects of hyperuricemia in the contribution of CKD-related morbidity.
References
Spoto B, Pisano A, Zoccali C (2016) Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol 311:F1087–F1108
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846
Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, Jensen MD, Parati G, Lopez-Jimenez F (2010) Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 31:737–746
Wiklund P, Törmäkangas T, Shi Y, Wu N, Vainionpää A, Alen M, Cheng S (2017) Normal-weight obesity and cardiometabolic risk: a 7-year longitudinal study in girls from prepuberty to early adulthood. Obesity (Silver Spring) 25:1077–1082
Sgambat K, Roem J, Mitsnefes M, Portale AA, Furth S, Warady B, Moudgil A (2018) Waist-to-height ratio, body mass index, and cardiovascular risk profile in children with chronic kidney disease. Pediatr Nephrol 33:1577–1583
Mak RH (2008) Insulin and its role in chronic kidney disease. Pediatr Nephrol 23:355–362
Weaver DJ Jr (2019) Uric acid and progression of chronic kidney disease. Pediatr Nephrol 34:801–809
Johnson RJ, Nakagawa T, Jalal D, Sánchez-Lozada LG, Kang DH, Ritz E (2013) Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant 28:2221–2228
Kanbay M, Jensen T, Solak Y, Le M, Roncal-Jimenez C, Rivard C, Lanaspa MA, Nakagawa T, Johnson RJ (2016) Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur J Intern Med 29:3–8
Seyed-Sadjadi N, Berg J, Bilgin AA, Grant R (2017) Visceral fat mass: Is it the link between uric acid and diabetes risk? Lipids Health Dis 16:142
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Kurtoglu S, Mazicioglu MM, Ozturk A, Hatipoglu N, Cicek B, Ustunbas HB (2010) Body fat reference curves for healthy Turkish children and adolescents. Eur J Pediatr 169:1329–1335
d'Annunzio G, Vanelli M, Pistorio A, Minuto N, Bergamino L, Iafusco D, Lorini R, Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes (2009) Insulin resistance and secretion indexes in healthy Italian children and adolescents: a multicentre study. Acta Biomed 80:21–28
Buyan N, Bideci A, Ozkaya O, Ortac E, Bakkaloglu S, Gonen S, Peru H, Soylemezoglu O, Cinaz P (2006) Leptin and resistin levels and their relationships with glucose metabolism in children with chronic renal insufficiency and undergoing dialysis. Nephrology (Carlton) 11:192–196
Lai HL, Kartal J, Mitsnefes M (2007) Hyperinsulinemia in pediatric patients with chronic kidney disease: the role of tumor necrosis factor-α. Pediatr Nephrol 22:1751–1756
Canpolat N, Caliskan S, Sever L, Guzeltas A, Kantarci F, Candan C, Civilibal M, Kasapcopur O, Arisoy N (2012) Glucose intolerance: Is it a risk factor for cardiovascular disease in children with chronic kidney disease? Pediatr Nephrol 27:627–635
Lindblad YT, Axelsson J, Bárány P, Celsi G, Lindholm B, Qureshi AR, Carrea A, Canepa A (2008) Hyperinsulinemia and insulin resistance, early cardiovascular risk factors in children with chronic kidney disease. Blood Purif 26:518–525
Patel HP, Saland JM, Ng DK, Jiang S, Warady BA, Furth SL, Flynn JT (2017) Waist circumference and body mass index in children with chronic kidney disease and metabolic, cardiovascular, and renal outcomes. J Pediatr 191:133–139
Lalan S, Jiang S, Ng DK, Kupferman F, Warady BA, Furth S, Mitsnefes MM (2018) Cardiometabolic risk factors, metabolic syndrome, and chronic kidney disease progression in children. J Pediatr 202:163–170
Becker B, Kronenberg F, Kielstein JT, Haller H, Morath C, Ritz E, Fliser D, MMKD Study Group (2005) Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol 16:1091–1098
Marlais M, Coward RJ (2015) Paediatrics, insulin resistance and the kidney. Pediatr Nephrol 30:1217–1224
Manios Y, Moschonis G, Kourlaba G, Bouloubasi Z, Grammatikaki E, Spyridaki A, Hatzis C, Kafatos A, Fragiadakis GA (2008) Prevalence and independent predictors of insulin resistance in children from Crete, Greece: the children study. Diabet Med 25:65–72
Savva SC, Tornaritis M, Savva ME, Kourides Y, Panagi A, Silikiotou N, Georgiou C, Kafatos A (2000) Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Relat Metab Disord 24:1453–1458
Blüher S, Molz E, Wiegand S, Otto KP, Sergeyev E, Tuschy S, L'Allemand-Jander D, Kiess W, Holl RW, Adiposity Patients Registry Initiative and German Competence Net Obesity (2013) Body mass index, waist circumference, and waist-to-height ratio as predictors of cardiometabolic risk in childhood obesity depending on pubertal development. J Clin Endocrinol Metab 98:3384–3393
Lin TY, Lim PS, Hung SC (2018) Normal-weight obesity and clinical outcomes in nondiabetic chronic kidney disease patients: a cohort study. Am J Clin Nutr 107:664–672
Srikanthan P, Karlamangla AS (2011) Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the Third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 96:2898–28903
Siew ED, Pupim LB, Majchrzak KM, Shintani A, Flakoll PJ, Ikizler TA (2007) Insulin resistance is associated with skeletal muscle protein breakdown in non-diabetic chronic hemodialysis patients. Kidney Int 71:146–152
Price SR, Gooch JL, Donaldson SK, Roberts-Wilson TK (2010) Muscle atrophy in chronic kidney disease results from abnormalities in insulin signaling. J Ren Nutr 20:S24–S28
Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B (2019) Oxidative stress in chronic kidney disease. Pediatr Nephrol 34:975–991
Gondouin B, Jourde-Chiche N, Sallee M, Dou L, Cerini C, Loundou A, Morange S, Berland Y, Burtey S, Brunet P, Guieu R, Dussol B (2015) Plasma xanthine oxidase activity is predictive of cardiovascular disease in patients with chronic kidney disease, independently of uric acid levels. Nephron 131:167–174
Kubota M (2019) Hyperuricemia in children and adolescents: present knowledge and future directions. J Nutr Metab 2019:3480718
Sakou II, Psaltopoulou T, Sergentanis TN, Karavanaki K, Karachaliou F, Ntanasis-Stathopoulos I, Tzanninis S, Sdogou T, Greydanus D, Tsitsika A (2015) Insulin resistance and cardiometabolic risk factors in obese children and adolescents: a hierarchical approach. J Pediatr Endocrinol Metab 28:589–596
Beavers KM, Beavers DP, Serra MC, Bowden RG, Wilson RL (2009) Low relative skeletal muscle mass indicative of sarcopenia is associated with elevations in serum uric acid levels: findings from NHANES III. J Nutr Health Aging 13:177–182
Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML (2003) Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 42:474–480
Sirota JC, McFann K, Targher G, Johnson RJ, Chonchol M, Jalal DI (2013) Elevated serum uric acid levels are associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in the United States: liver ultrasound data from the National Health and Nutrition Examination Survey. Metabolism 62:392–399
Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA (2005) Uric acid and oxidative stress. Curr Pharm Des 11:4145–4151
De Oliveira EP, Burini RC (2012) High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr 4:12
Ferrando B, Gomez-Cabrera MC, Salvador-Pascual A, Puchades C, Derbré F, Gratas-Delamarche A, Laparre L, Olaso-Gonzalez G, Cerda M, Viosca E, Alabajos A, Sebastiá V, Alberich-Bayarri A, García-Castro F, Viña J (2018) Allopurinol partially prevents disuse muscle atrophy in mice and humans. Sci Rep 8:3549
Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, Shirakura T, Kato K, Imaizumi K, Takahashi H, Tamura M, Maeda N, Funahashi T, Shimomura I (2013) Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem 288:27138–27149
Karava V, Printza N, Dotis J, Demertzi D, Antza C, Kotsis V, Papachristou F, Stabouli S (2019) Body composition and arterial stiffness in children with chronic kidney disease. Pediatr Nephrol 34:1253–1260
Derbre F, Ferrando B, Gomez-Cabrera MC, Sanchis-Gomar F, Martinez-Bello VE, Olaso-Gonzalez G, Diaz A, Gratas-Delamarche A, Cerda M, Viña J (2012) Inhibition of xanthine oxidase by allopurinol prevents skeletal muscle atrophy: role of p38 MAPKinase and E3 ubiquitin ligases. PLoS One 7:e46668
Sanchis-Gomar F, Pareja-Galeano H, Perez-Quilis C, Santos-Lozano A, Fiuza-Luces C, Garatachea N, Lippi G, Lucia A (2015) Effects of allopurinol on exercise-induced muscle damage: new therapeutic approaches? Cell Stress Chaperones 20:3–13
Madero M, Rodríguez Castellanos FE, Jalal D, Villalobos-Martín M, Salazar J, Vazquez-Rangel A, Johnson RJ, Sanchez-Lozada LG (2015) A pilot study on the impact of a low fructose diet and allopurinol on clinic blood pressure among overweight and prehypertensive subjects: a randomized placebo controlled trial. J Am Soc Hypertens 9:837–844
Soletsky B, Feig DI (2012) Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 60:1148–1156
Talma H, Chinapaw MJ, Bakker B, HiraSing RA, Terwee CB, Altenburg TM (2013) Bioelectrical impedance analysis to estimate body composition in children and adolescents: a systematic review and evidence appraisal of validity, responsiveness, reliability and measurement error. Obes Rev 14:895–905
Canpolat N, Caliskan S, Sever L, Tasdemir M, Ekmekci OB, Pehlivan G, Shroff R (2013) Malnutrition and its association with inflammation and vascular disease in children on maintenance dialysis. Pediatr Nephrol 28:2149–2156
Broers NJH, Canaud B, Dekker MJE, van der Sande FM, Stuard S, Wabel P, Kooman JP (2020) Three compartment bioimpedance spectroscopy in the nutritional assessment and the outcome of patients with advanced or end stage kidney disease: What have we learned so far? Hemodial Int 4:148–161
Milani GP, Groothoff JW, Vianello FA, Fossali EF, Paglialonga F, Consolo S, Edefonti A, Consonni D, van Harskamp D, van Goudoever JB, Schierbeek H, Agostoni C, Oosterveld MJS (2018) Bioimpedance spectroscopy imprecisely assesses lean body mass in pediatric dialysis patients. J Pediatr Gastroenterol Nutr 67:533–537
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karava, V., Dotis, J., Kondou, A. et al. Association between relative fat mass, uric acid, and insulin resistance in children with chronic kidney disease. Pediatr Nephrol 36, 425–434 (2021). https://doi.org/10.1007/s00467-020-04716-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04716-y