Abstract
Background
Rituximab (RTX) is effective in maintaining remission in patients with nephrotic syndrome (NS), but a standard protocol of RTX administration has not been established.
Methods
This study was a 2-year multicenter observational study, in which consistent treatments and evaluations were performed. We enrolled pediatric patients with refractory NS between January 2015 and December 2015. RTX infusion was performed four times at 6-month intervals, followed by mizoribine pulse therapy with early discontinuation of calcineurin inhibitor (CNI). Primary endpoints were the relapse-free survival rate and the number of relapses after RTX administration. Secondary endpoints were changes in side effects associated with long-term steroid administration.
Results
Twenty-two patients were analyzed. The relapse-free survival rate at 1 year and 2 years was 50 and 46%, respectively. Twenty-one patients accomplished our protocol and the frequency of relapse was reduced under the discontinuation of CNI. Although two patients were diagnosed with frequent relapse and/or steroid dependency during the observation period, the frequency of relapse decreased with each rituximab dose. Statistically significant improvements in all steroid complications were observed in the final examination, but no significant improvements were observed from 1 to 2 years after RTX administration. One patient had agranulocytosis, and three patients showed electrocardiographic abnormalities.
Conclusions
Our protocol was useful and safe for refractory NS. However, RTX administration four times might have been excessive in patients who had no relapse by 1 year after the initial RTX administration. Further investigation of the most appropriate method of RTX administration is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rituximab (RTX) is an effective treatment for children with refractory nephrotic syndrome (NS) such as frequently relapsing nephrotic syndrome (FRNS) or steroid-dependent nephrotic syndrome (SDNS) [1, 2]. However, most patients experience relapses with recovery of peripheral B cell counts [3,4,5]. Therefore, it was suggested that repeated RTX administration and post-RTX therapy with immunosuppressive agents are reasonable treatment options to maintain long-term remission [6,7,8,9].
To date, a number of studies have reported the effectiveness of RTX administration in patients with refractory NS, although the dose, frequency, and interval of RTX administration in these studies varied [3, 5, 10,11,12,13]. In addition, some of these studies were retrospective studies and were conducted in absence of uniform protocols [14,15,16]. The method of optimal RTX administration has not been established. Moreover, the number of circulating B cells is not necessarily helpful in predicting relapse when determining the optimal method of administration, although the recovery of switched memory B cells may predict relapses [17].
Based on these findings, we designed a protocol consisting of periodic single-dose RTX administrations every 6 months for a total of four times followed by mizoribine (MZB) administration. We aimed to evaluate whether this protocol could be used as an alternative to the conventional therapy with prednisolone (PSL) combined with a calcineurin inhibitor (CNI) for patients with refractory NS. We also focused on whether this protocol can improve complications associated with long-term steroid administration. The purpose of the present study was to examine the advantages and disadvantages of our protocol, thereby exploring the optimal method of RTX administration.
Materials and methods
Study design and definitions
This study was a multicenter observational study. We enrolled patients at five hospitals in Hokkaido, Japan, between January 2015 and December 2015. The definitions of complete remission, relapse, frequent relapse, steroid dependency, and steroid resistance were those of the International Study of Kidney Disease in Children [18]. B cell depletion was defined as a CD19+ cell count < 10 cells/mm3 using flow cytometry.
Patients
We enrolled pediatric patients with refractory NS who developed FRNS or SDNS during CNI administration or after discontinuation of CNI, and met two or more of the following four criteria: severe steroid dependency requiring a high dose of PSL (> 0.5 mg/kg/day) to maintain remission, history of receiving two or more immunosuppressive drugs, CNI use for over 3 years, and severe steroid complications such as cataract, short stature [height standard deviation score (SDS) < − 3 SD], obesity (obesity index > 30%), low bone mineral density (BMD) (Z-score < − 2.5 SD), compression fracture or deformity of the spine, thromboembolism, diabetes, and osteonecrosis of the femoral head. Patients who had received RTX before enrollment were excluded from this study.
Therapeutic protocol
All patients were admitted to our hospital for RTX administration and were monitored for at least 24 h after RTX administration for infusion reactions. Patients were in complete remission at the start of each RTX administration. RTX was administered intravenously in a single dose of 375 mg/m2 body surface area (maximum 500 mg). In order to minimize infusion reactions, we administered methylprednisolone (1–1.5 mg/kg) intravenously, and acetaminophen (10 mg/kg, maximum 300 mg) and d-chlorpheniramine maleate (0.4–2.0 mg depending on the patient’s age) orally 30 min before RTX infusion. RTX administration was performed four times at 6-month intervals. As post-RTX therapy, MZB was given orally twice a week in a dose of 500 mg on day 1 and 550 mg on day 2. Sulfamethoxazole-trimethoprim and fluconazole were given to all patients during the period of B cell depletion for prophylaxis against pneumocystosis and cryptosporidiosis. Tapering of CNI began at the time of the first dose of RTX, and the CNI was discontinued 1 week later. Tapering of PSL began at 2 weeks after the first dose of RTX with discontinuation 2 to 3 months later. When patients had a relapse during the study period, they received 60 mg/m2 oral PSL (maximum 60 mg) in three divided doses until proteinuria disappeared for three consecutive days. Thereafter, PSL was switched to alternate days, and the dose was gradually tapered every 2 weeks over a 2-month period.
Follow-up
The observation period was 2 years from the initial RTX administration. During the period of 6 months from the initial administration, biochemical and complete blood count (CBC) tests were performed at 1, 2, and 4 weeks and every month thereafter. The number of CD19-positive cells was measured at 1, 2, and 4 weeks and at 2, 4, and 6 months. After 6 months, the biochemistry and CBC tests and measurement of CD19-positive cells were performed every 2 to 3 months. Urinalysis was performed throughout the observation period using a dipstick test at home to confirm relapses and remissions. X-ray, electrocardiogram, respiratory function test, and BMD measurement of the lumbar spine using dual-energy radiograph absorptiometry were performed during each semiannual hospital stay for RTX administration. In order to detect complications before RTX administration, echocardiography; abdominal echography; head, spine, and hip joint magnetic resonance imaging tests; and oral glucose tolerance test (OGTT) were performed at the time of hospital stay for the initial administration. When testing showed abnormal findings, a re-examination was performed at the completion of the observation period. Virus antibody titers were determined before the initial RTX administration and 2 years later. In addition, the level of human anti-chimeric antibody (HACA) in serum was measured at the completion of the observation period.
Outcome
A patient was considered to have a relapse if the result of proteinuria on dipstick was 3+ or more for more than three consecutive days, or the patient developed FRNS or SDNS. Primary endpoints were the relapse-free survival rate, survival rate without FRNS or SDNS, and the number of relapses after the start of RTX administration. Secondary endpoints included changes in adverse reactions associated with long-term steroid administration such as short stature, obesity, low BMD, and diabetes. Height was evaluated using the SDS obtained from the Japanese pediatric standards by age group, respectively, and obesity was evaluated using the obesity index which was calculated as 100 × (body weight − standard body weight) / standard body weight. BMD was measured for the lumbar spine (L1–L4) by dual-energy X-ray absorptiometry densitometers (Discovery A; Hologic, Inc., USA). The BMD Z-score provided an estimate of the SDS away from “height age” and sex for Japanese children [19]. We substituted “height age,” the age at which a child’s height is the median height for age on the growth chart, for chronologic age as a means of adjusting for short stature. Measurements were compared among the time points of before RTX administration, 1 year, and 2 years of administration. Adverse events associated with RTX administration were also evaluated.
Statistical analysis
The Kaplan-Meier method was used for analysis of relapse-free survival and the time taken to develop either FRNS or SDNS. The Wilcoxon signed rank test was performed for evaluation of changes in the number of relapses during the 2-year period before and the 2-year period after the start of RTX administration, evaluation of the changes over time in the side effects of steroid and IgG antibody titer, and comparison of vaccination antibody titers before and after the start of RTX administration. Significance was set at P < 0.05. As for the changes over time in the side effects of steroid and IgG antibody titer, Bonferroni’s correction was applied to compensate for multiple testing. All analyses were performed with JMP version 11.0 (SAS Institute Japan, Tokyo, Japan).
Results
Study patients
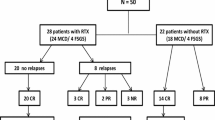
Twenty-five patients with NS were enrolled. Among them, three patients had SDNS under CNI administration but did not meet two or more of the criteria. After excluding these 3 patients, 22 patients were included in the analyses. Table 1 shows their background characteristics. In one patient who had severe steroid dependency, relapse occurred 3 days after the initial RTX administration during treatment with 50 mg oral PSL every other day. The physician decided to resume CNI treatment, which was discontinued after the second RTX administration. We regarded this patient as treatment failure (Fig. 1).
Flow diagram. The patients with refractory nephrotic syndrome who showed frequent relapse or steroid dependency during CNI administration or after CNI discontinuation and who met two or more of the following four conditions were included in the analysis: severe steroid dependency requiring high-dose prednisolone (> 0.5 mg/kg/day) to maintain remission prior to rituximab administration, history of receiving two or more immunosuppressive drugs, use of CNI for over 3 years, and severe steroid complications. CNI calcineurin inhibitors
Primary endpoints
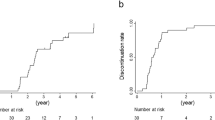
The relapse-free survival rate at 1 year and 2 years after the start of RTX administration was 50 and 46%, respectively. One patient was diagnosed with FRNS/SDNS and one patient with FRNS during the 2-year observation period. The survival rate without FRNS or SDNS at 1 year and 2 years was 91 and 86%, respectively (Fig. 2). The frequency of relapses significantly decreased from 5.8 times/patient/2 years before the initial RTX administration, to 1 time/patient/2 years after the start of RTX treatment (P < 0.001). Although CNI treatment was discontinued in all patients, no increase in the frequency of relapses was observed. Relapses were mostly observed within the first month or beyond 5 months after each RTX administration, and the frequency of relapses decreased with each RTX dose (Fig. 3). There were no relapses with steroid resistance.
Kaplan-Meier curves for relapse-free survival (a) and non-FRNS/SDNS survival (b). a The relapse-free survival rate at 1 year and 2 years was 50 and 46%, respectively. The patients who had no relapse within the first year tended to maintain remission thereafter. Most cases of first relapse occurred before the second rituximab administration. b One patient was diagnosed with FRNS/SDNS 205 days after the initial rituximab administration, and one patient was diagnosed with FRNS 365 days after the initial rituximab administration. In these two patients, relapses during the second year were not FRNS/SDNS. FRNS frequently relapsing nephrotic syndrome, SDNS steroid-dependent nephrotic syndrome. Dotted lines show each rituximab administrations
a Comparison of the number of relapses during the 2-year period before the initial rituximab administration and during the 2-year period after the initial rituximab administration. Although calcineurin inhibitor was discontinued in all patients, no increase in the frequency of relapse was observed. b Total number of relapses after each rituximab administration in the 22 patients. The frequency of relapse during rituximab administration decreased with each rituximab dose. c Timing of relapse in the intervals between consecutive rituximab administrations. Relapses were mostly observed within the first month or beyond 5 months after each rituximab administration. Relapses beyond 5 months from rituximab administration were associated with B cell recovery
Steroid complications
Significant improvements in height, weight, and BMD were observed at 1 year and 2 years compared with the respective parameter before the initial RTX administration. No significant improvements were observed from 1 to 2 years after RTX administration, although the trends for improvement continued (Fig. 4). For the evaluation of growth stage, the bone age was evaluated in all patients, although no patient showed closed epiphysis, either at the start of RTX administration, or at 2 years. Two patients were diagnosed with diabetes and six patients with prediabetes before the initial RTX administration. After 2 years of treatment, the number of patients with diabetes and prediabetes decreased to 0 and 5, respectively.
Changes in side effects of steroid. Changes in the a obesity index, b Height SDS, and c BMD Z-score before the initial rituximab administration and 1 year and 2 years after the initial rituximab administration. The five horizontal bars represent the maximum value, 75th percentile, median, 25th percentile and minimum value in range from 75th percentile + 1.5 × IQR to 25th percentile − 1.5 × IQR. Dots represent outliers. The median height SDS improved to − 1.3 SD, the median obesity index improved to − 3%, the median BMD Z-score improved to − 1.4 SD at 2 years after the initial RTX administration. The obesity index was calculated by the following method: obesity index (%) = 100 × (body weight − standard body weight) / standard weight. The normal range for the obesity index is − 20 to 20%. RTX rituximab, BMD bone mineral density, SDS standard deviation score
Adverse events
Infusion reactions were observed after 47% (41/88) of the RTX administrations. All of these reactions were mild with Common Terminology Criteria for Adverse Event Grade1 (Table 2) and did not require therapeutic interventions including medication or reduction of administration rate. Adverse events are shown in Table 3. The patient with agranulocytosis was detected by follow-up examination 2 months after the second RTX administration. He had no symptoms during decreasing in granulocyte count and granulocyte count increased spontaneously. One patient with decrease in granulocytes was detected after the second RTX administration accompanied by tonsillitis, which was treated by antibiotics. The other two patients had no symptoms and were detected by follow-up examination 3 and 5 months after the first RTX administration. No recurrence of neutropenia was detected in all these patients. Respiratory function tests showed no change in all patients during the observation period. HACA was not detected in any of the patients 2 years after the start of RTX administration. Serum levels of immunoglobulin did not show significant decreases at 1 year, but significantly decreased over the 2-year period. The titers of specific anti-viral antibodies against various vaccinations did not show significant decreases.
Transition of B cells
Three patients at the fourth RTX administration had not recovered from B cell depletion. In other words, B cells had been out of depletion at all the other RTX administrations. The average number of B cells decreased at each RTX administration.
Discussion
Switching from the conventional treatment to our protocol, consisting of four periodic single-dose RTX administrations followed by MZB for refractory NS, showed excellent outcomes. Patients who received this protocol did not require resumption of CNI treatment during the study period, although in one patient regarded as treatment failure, discontinuation of CNI had been delayed because of early relapse with severe steroid dependency. In addition, this protocol reduced the frequency of relapse and improved steroid complications. Our protocol achieved a 1-year relapse-free survival of 50%, whereas the previous clinical trial by Iijima et al. reported a 1-year relapse-free survival of 29% as a result of RTX administration every week for a total of four times [5]. In addition, all patients in that study experienced relapse within 19 months, whereas our protocol achieved better outcomes with a 2-year relapse-free survival of 46%. In our study, the patients who had no relapse within 1 year tended to maintain remission thereafter. A similar trend was shown in a previous report, in which repeated RTX administrations led to B cell depletion for at least 15 months [3]; in patients who undergo repeated administration of RTX, the presence or absence of relapse within 1 year after starting RTX administration may be used to evaluate the response to RTX.
In patients who received our protocol, CNI could be tapered immediately and CNI administration did not need to be resumed. In most studies, the immunosuppressive agents were continued for 3 to 6 months or longer after RTX administration [3, 5, 11, 12], while some studies did not mention the method of tapering. Ravani et al. reported that once or twice RTX administration led to remission for 6 to 12 months in 50% of patients with early discontinuation of CNI. On the other hand, in patients who had a second relapse within 5 months, RTX was additionally administered and CNI treatment was resumed [4]. In the present study, RTX administration reduced the frequency of relapse without resumption of CNI. Even in the two patients who developed FRNS/SDNS during the first year, relapses during the second year were not FRNS/SDNS. Although this could be the result of natural disease amelioration over time, it might be the long-term effects of repeated RTX administrations [16].
In the present study, at least five cases suffered relapse during B cell depletion. Even in these cases, our protocol was fully effective in achieving reductions in the frequency of relapse and relieving steroid complications. However, two of these five cases presented with FRNS/SDNS. RTX is considered to be relatively ineffective in patients experiencing relapse during B cell depletion [20]. On the other hand, two of the five cases experienced early relapse within 2 weeks of RTX administration. It was reported that PSL could be discontinued in such cases and RTX was still effective [7]. In our study, the two patients who experienced early relapse did not experience any subsequent relapse. Moreover, the one patient whose treatment had deviated from our protocol because of early relapse with severe steroid dependency and who was regarded as treatment failure had only two other episodes of relapse in the 2-year period and did not show SDNS anymore. With regard to early relapse, it was discussed that the effects of RTX may partially be due to indirect action mediated by T cells and/or a humoral factor that had been released before RTX administration [7, 12]. RTX is effective even in patients with relapses during B cell depletion. The efficacy of RTX should probably not be assessed based on the episodes of early relapse.
We did a post-hoc analysis of the risk factors for the first relapse after the initial RTX administration. All of the following conditions were not shown to be a significant risk factor in univariate analysis using Log rank test: sex, onset age, treatment age, disease duration, number of relapses during 2-year period before RTX administration, interval between relapse before the first RTX administration and the first RTX administration < 180 days, history of SRNS, and duration of CNI treatment.
One characteristic of our protocol is pulsatile administration of MZB which was used as post-RTX therapy. In Japan, mycophenolate mofetil is not covered by national health insurance for NS, and MZB is often used as a purine metabolic inhibitor in renal disease treatment and renal transplantation. It has been reported that the administration of high-dose MZB is effective in suppressing relapses of NS, and more importantly, it has few side effects [21,22,23,24]. Although MZB may have contributed in part to the favorable outcomes obtained in the present study, further examination is required.
Regarding steroid complications, statistically significant improvements in height, weight, and BMD were observed in the present study. However, in terms of obesity and BMD, some cases showed little improvement or exacerbation despite discontinuation of PSL. In these patients, it was considered that multiple factors including diet and exercise were involved. There are no safe and effective agents for pediatric osteoporosis; therefore, reduction in the dose of PSL is key to prevention of osteoporosis [25].
There has not been a comprehensive report on the results of OGTT in pediatric patients with refractory NS. In the present study, all of the patients with prediabetes had normal fasting blood glucose levels but abnormal OGTT results. In addition, among the five cases with prediabetes at the completion of the observation period, one did not experience relapse during the 2-year observation period, and three experienced relapse only once during the first year of RTX administration. In spite of the very short period of steroid use, they still had impaired glucose tolerance at the end of the study. Moreover, two other cases had impaired insulin secretion based on the insulinogenic index, indicating a risk for developing diabetes. Prediabetes is a steroid complication that can be easily missed, and a long amount of time may be required for improvement after discontinuation of PSL, or the prediabetes may be irreversible [26, 27]. As in the case of low BMD, prevention through reduction in the dose of PSL is considered to be important.
Regarding adverse events, the observed infusion reactions were all mild and severe infection was not obvious. However, attention should be paid to agranulocytosis as reported previously [28]. In addition, three patients showed an abnormal electrocardiogram. The electrocardiographic abnormalities were observed on the day after RTX administration and disappeared during the 6-month period prior to the next administration and the echocardiography showed no findings of abnormality. As a result, we concluded that the electrocardiographic findings were transient changes of little clinical significance. Nevertheless, such changes require careful monitoring as cardiomyopathy, arrhythmia, and ischemic heart disease following RTX administration have been reported previously [29, 30]. Although there was a concern about increased risk for HACA production [14, 31], HACA was not detected in any of the patients in the present study. Previous studies reported the detection rate of HACA to be 9.2 to 40% among cases of rheumatoid arthritis, systemic lupus erythematous, and membranous nephritis, and 12% among NS cases [5, 31,32,33,34]. The discrepancy might be due to the use of MZB as post-RTX therapy. Although RTX did not seem to directly induce decreased IgG levels in other studies [7, 35], our patients showed a significant decrease in IgG level over the 2-year period. Our protocol might inhibit production of de novo antibody. However, various specific anti-viral antibodies were maintained during the 2-year observation period.
One limitation of the present study is that it was an observational study with no control group. In order to minimize bias, a standardized protocol was used for treatment in patients who met specific inclusion criteria, and the therapeutic effects and adverse events were evaluated in a prospective manner. One challenge of a clinical study on NS is that it is not easy to objectively evaluate the cause of NS and disease progression, resulting in patient heterogeneity. Nevertheless, our subjects were refractory cases in which it had been difficult to discontinue PSL and CNI prior to starting RTX therapy, and are comparable to subjects in existing reports in terms of background characteristics.
A potential problem in our protocol is that the RTX dosing of four times might have been excessive in some patients. Although it is not clear whether the disease amelioration was the result of the natural course of the disease or the therapeutic effect of RTX, long-term remission was maintained for many years with once or twice of RTX administration in 10 to 20% of cases [4, 7]. In the present study, the patients with no relapse by 1 year after the initial RTX administration may not have needed additional RTX administrations. The trend of the improvement in side effects of steroid might justify this. There is also the concern of a possible impact on immune function in children during their development, although B cells recovered following each RTX administration in most cases treated with our protocol. The safety of repeated dosing of five times or more of RTX has yet to be evaluated, and thus, prolonged repeated dosing is not recommended.
Although the primary goal is suitable RTX administration based on the etiology, further investigation is required in terms of the safe and optimal methods of administration according to the patient’s clinical course such as patient characteristics and response to therapy.
References
Iijima K, Sako M, Nozu K (2017) Rituximab for nephrotic syndrome in children. Clin Exp Nephrol 21:193–202. https://doi.org/10.1007/s10157-016-1313-5
Ravani P, Bonanni A, Rossi R, Caridi G, Ghiggeri GM (2016) Anti-CD20 antibodies for idiopathic nephrotic syndrome in children. Clin J Am Soc Nephrol 11:710–720. https://doi.org/10.2215/cjn.08500815
Sellier-Leclerc AL, Baudouin V, Kwon T, Macher MA, Guerin V, Lapillonne H, Deschenes G, Ulinski T (2012) Rituximab in steroid-dependent idiopathic nephrotic syndrome in childhood—follow-up after CD19 recovery. Nephrol Dial Transplant 27:1083–1089. https://doi.org/10.1093/ndt/gfr405
Ravani P, Ponticelli A, Siciliano C, Fornoni A, Magnasco A, Sica F, Bodria M, Caridi G, Wei C, Belingheri M, Ghio L, Merscher-Gomez S, Edefonti A, Pasini A, Montini G, Murtas C, Wang X, Muruve D, Vaglio A, Martorana D, Pani A, Scolari F, Reiser J, Ghiggeri GM (2013) Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int 84:1025–1033. https://doi.org/10.1038/ki.2013.211
Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y (2014) Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384:1273–1281. https://doi.org/10.1016/s0140-6736(14)60541-9
Ito S, Kamei K, Ogura M, Udagawa T, Fujinaga S, Saito M, Sako M, Iijima K (2013) Survey of rituximab treatment for childhood-onset refractory nephrotic syndrome. Pediatr Nephrol 28:257–264. https://doi.org/10.1007/s00467-012-2319-1
Kamei K, Ishikura K, Sako M, Aya K, Tanaka R, Nozu K, Kaito H, Nakanishi K, Ohtomo Y, Miura K, Takahashi S, Morimoto T, Kubota W, Ito S, Nakamura H, Iijima K, Rituximab for Childhood-Onset Refractory Nephrotic Syndrome Study Group (2017) Long-term outcome of childhood-onset complicated nephrotic syndrome after a multicenter, double-blind, randomized, placebo-controlled trial of rituximab. Pediatr Nephrol 32:2071–2078. https://doi.org/10.1007/s00467-017-3718-0
Ito S, Kamei K, Ogura M, Sato M, Fujimaru T, Ishikawa T, Udagawa T, Iijima K (2011) Maintenance therapy with mycophenolate mofetil after rituximab in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 26:1823–1828. https://doi.org/10.1007/s00467-011-1886-x
Fujinaga S, Someya T, Watanabe T, Ito A, Ohtomo Y, Shimizu T, Kaneko K (2013) Cyclosporine versus mycophenolate mofetil for maintenance of remission of steroid-dependent nephrotic syndrome after a single infusion of rituximab. Eur J Pediatr 172:513–518. https://doi.org/10.1007/s00431-012-1913-3
Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, Benetti E, Scozzola F, Pasini A, Dallera N, Sica F, Belingheri M, Scolari F, Ghiggeri GM (2011) Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 6:1308–1315. https://doi.org/10.2215/CJN.09421010
Gulati A, Sinha A, Jordan SC, Hari P, Dinda AK, Sharma S, Srivastava RN, Moudgil A, Bagga A (2010) Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol 5:2207–2212. https://doi.org/10.2215/CJN.03470410
Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasa M, Chianca A, Rubis N, Ene-Iordache B, Rudnicki M, Pollastro RM, Capasso G, Pisani A, Pennesi M, Emma F, Remuzzi G, Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis Study Group (2014) Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 25:850–863. https://doi.org/10.1681/ASN.2013030251
Kimata T, Hasui M, Kino J, Kitao T, Yamanouchi S, Tsuji S, Kaneko K (2013) Novel use of rituximab for steroid-dependent nephrotic syndrome in children. Am J Nephrol 38:483–488. https://doi.org/10.1159/000356439
Sinha A, Bhatia D, Gulati A, Rawat M, Dinda AK, Hari P, Bagga A (2015) Efficacy and safety of rituximab in children with difficult-to-treat nephrotic syndrome. Nephrol Dial Transplant 30:96–106. https://doi.org/10.1093/ndt/gfu267
Tellier S, Brochard K, Garnier A, Bandin F, Llanas B, Guigonis V, Cailliez M, Pietrement C, Dunand O, Nathanson S, Bertholet-Thomas A, Ichay L, Decramer S (2013) Long-term outcome of children treated with rituximab for idiopathic nephrotic syndrome. Pediatr Nephrol 28:911–918. https://doi.org/10.1007/s00467-012-2406-3
Kemper MJ, Gellermann J, Habbig S, Krmar RT, Dittrich K, Jungraithmayr T, Pape L, Patzer L, Billing H, Weber L, Pohl M, Rosenthal K, Rosahl A, Mueller-Wiefel DE, Dotsch J (2012) Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant 27:1910–1915. https://doi.org/10.1093/ndt/gfr548
Colucci M, Carsetti R, Cascioli S, Casiraghi F, Perna A, Rava L, Ruggiero B, Emma F, Vivarelli M (2016) B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol 27:1811–1822. https://doi.org/10.1681/asn.2015050523
(1978) Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. Kidney Int 13:159–165. https://doi.org/10.1038/ki.1978.23
Nishiyama S, Kiwaki K, Inomoto T, Seino Y (1999) Bone mineral density of the lumber spine and total body mass in Japanese children and adolescent. Nihon Shonika Gakkai Zasshi 103:69–76
Sato M, Kamei K, Ogura M, Ishikura K, Ito S (2018) Relapse of nephrotic syndrome during post-rituximab peripheral blood B-lymphocyte depletion. Clin Exp Nephrol 22:110–116. https://doi.org/10.1007/s10157-017-1415-8
Honda M (2002) Nephrotic syndrome and mizoribine in children. Pediatr Int 44:210–216
Kawasaki Y, Hosoya M, Kobayashi S, Ohara S, Onishi N, Takahashi A, Isome M, Suzuki H (2005) Oral mizoribine pulse therapy for patients with steroid-resistant and frequently relapsing steroid-dependent nephrotic syndrome. Nephrol Dial Transplant 20:2243–2247. https://doi.org/10.1093/ndt/gfh996
Ohtomo Y, Fujinaga S, Takada M, Murakami H, Akashi S, Shimizu T, Kaneko K, Yamashiro Y (2005) High-dose mizoribine therapy for childhood-onset frequently relapsing steroid-dependent nephrotic syndrome with cyclosporin nephrotoxicity. Pediatr Nephrol 20:1744–1749. https://doi.org/10.1007/s00467-005-2025-3
Fujinaga S, Hirano D, Nishizaki N, Someya T, Ohtomo Y, Ohtsuka Y, Shimizu T, Kaneko K (2011) Single daily high-dose mizoribine therapy for children with steroid-dependent nephrotic syndrome prior to cyclosporine administration. Pediatr Nephrol 26:479–483. https://doi.org/10.1007/s00467-010-1707-7
Ward LM, Konji VN, Ma J (2016) The management of osteoporosis in children. Osteoporos Int 27:2147–2179. https://doi.org/10.1007/s00198-016-3515-9
Burt MG, Willenberg VM, Petersons CJ, Smith MD, Ahern MJ, Stranks SN (2012) Screening for diabetes in patients with inflammatory rheumatological disease administered long-term prednisolone: a cross-sectional study. Rheumatology (Oxford) 51:1112–1119. https://doi.org/10.1093/rheumatology/kes003
Suh S, Park MK (2017) Glucocorticoid-induced diabetes mellitus: an important but overlooked problem. Endocrinol Metab (Seoul, Korea) 32:180–189. https://doi.org/10.3803/EnM.2017.32.2.180
Kamei K, Takahashi M, Fuyama M, Saida K, Machida H, Sato M, Ogura M, Ito S (2015) Rituximab-associated agranulocytosis in children with refractory idiopathic nephrotic syndrome: case series and review of literature. Nephrol Dial Transplant 30:91–96. https://doi.org/10.1093/ndt/gfu258
Cheungpasitporn W, Kopecky SL, Specks U, Bharucha K, Fervenza FC (2017) Non-ischemic cardiomyopathy after rituximab treatment for membranous nephropathy. J Renal Inj Prev 6:18–25. https://doi.org/10.15171/jrip.2017.04
Ng KH, Dearden C, Gruber P (2015) Rituximab-induced Takotsubo syndrome: more cardiotoxic than it appears? BMJ Case Rep. https://doi.org/10.1136/bcr-2014-208203
Ahn YH, Kang HG, Lee JM, Choi HJ, Ha IS, Cheong HI (2014) Development of antirituximab antibodies in children with nephrotic syndrome. Pediatr Nephrol 29:1461–1464. https://doi.org/10.1007/s00467-014-2794-7
Keystone E, Fleischmann R, Emery P, Furst DE, van Vollenhoven R, Bathon J, Dougados M, Baldassare A, Ferraccioli G, Chubick A, Udell J, Cravets MW, Agarwal S, Cooper S, Magrini F (2007) Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum 56:3896–3908. https://doi.org/10.1002/art.23059
Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E, Hladunewich M, Cattran DC (2008) Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 73:117–125. https://doi.org/10.1038/sj.ki.5002628
Albert D, Dunham J, Khan S, Stansberry J, Kolasinski S, Tsai D, Pullman-Mooar S, Barnack F, Striebich C, Looney RJ, Prak ET, Kimberly R, Zhang Y, Eisenberg R (2008) Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Ann Rheum Dis 67:1724–1731. https://doi.org/10.1136/ard.2007.083162
Delbe-Bertin L, Aoun B, Tudorache E, Lapillone H, Ulinski T (2013) Does rituximab induce hypogammaglobulinemia in patients with pediatric idiopathic nephrotic syndrome? Pediatr Nephrol 28:447–451. https://doi.org/10.1007/s00467-012-2361-z
Acknowledgements
We thank all pediatricians at affiliated hospitals for data collection. Part of this work will be presented at the 55th Congress of the ERA-EDTA Congress, May 24-27, 2018, Copenhagen, Denmark. This work was supported by The Naomichi Yamada Memorial Research Foundation for Pediatric Disease (YAMAME) (T.T.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Research Ethics Committee of Hokkaido University Hospital.
Informed consent
Informed consent was obtained from the parents of all patients included in the study.
Rights and permissions
About this article
Cite this article
Takahashi, T., Okamoto, T., Sato, Y. et al. Periodically repeated rituximab administrations in children with refractory nephrotic syndrome: 2-year multicenter observational study. Pediatr Nephrol 34, 87–96 (2019). https://doi.org/10.1007/s00467-018-4063-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-4063-7