Abstract
Background
Rituximab (RTX) is a promising option for treating childhood-onset steroid-dependent (SDNS), frequently relapsing (FRNS), and steroid-resistant (SRNS) nephrotic syndrome.
Methods
We retrospectively surveyed RTX treatment for these conditions to evaluate its indications, efficacy and adverse events. Questionnaires were sent to 141 hospitals in Japan.
Results
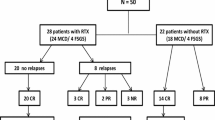
Seventy-four patients (52 SDNS; 3 FRNS; 19 SRNS) were treated with RTX because of resistance to various immunosuppressive agents. Most patients received a single administration of RTX (85%). Forty-one of 53 SDNS/FRNS (77%) and 5 of 17 SRNS (29%) patients successfully discontinued prednisolone (16 SDNS/FRNS and 6 SRNS achieved their first discontinuation since onset), and 17 out of 53 SDNS/FRNS patients (31%) discontinued cyclosporine. However, 28 of the 53 patients (51%) relapsed. Although immunosuppressive agents did not extend B cell depletion, relapses were significantly less if immunosuppressive agents were continued after RTX (P = 0.006; hazard ratio = 0.2). Among the SRNS patients, complete (n = 6) and partial remission (n = 6) were achieved. No life-threatening adverse events were experienced.
Conclusions
Although this was a multi-center survey where treatment of nephrotic syndrome varied between centers, the steroid-sparing effect of RTX in SDNS/FRNS was excellent. If single administration of RTX is chosen, continuation of immunosuppressive agents is recommended for prevention of relapse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment of refractory childhood nephrotic syndrome, such as frequently relapsing nephrotic syndrome (FRNS), steroid-dependent nephrotic syndrome (SDNS), and steroid-resistant nephrotic syndrome (SRNS), is still challenging. Although various immunosuppressive agents are effective for these conditions, in substantial numbers of children it remains intractable.
Most such patients suffer serious adverse effects of steroid and immunosuppressive agents. Recently, rituximab (RTX), a monoclonal antibody targeting the B cell-specific antigen CD20, has been proven to be effective for FRNS/SDNS and SRNS in children [1–8].
The incidental discovery of the effects of this drug has improved the prognosis of childhood SDNS and SRNS [9, 10]. Although RTX is still off-label and its safety for use in nephrotic syndrome has not yet been proven, pediatric nephrologists have started to use RTX in many countries. We previously reported a prospective study of single-dose therapy with RTX for 12 children with intractable SDNS [3]. All patients were able to discontinue steroids after RTX administration. We also found a significant decrease in the frequency of relapses, period of steroid use and mean steroid dosages after RTX treatment. However, the efficacy of the single-dose treatment was not always long-lasting, and the subsequent recovery of B cells in the peripheral blood sometimes resulted in disease recurrence. Therefore, maintenance therapy with some immunosuppressive agents after a single dose of RTX may be a good option to lengthen remission [11, 12]. Although RTX is relatively well tolerated, some severe or life-threatening adverse events, including progressive multifocal leukoencephalopathy [13], interstitial pneumonia [14], and ulcerative colitis [15], have been anecdotally reported. Such severe life-threatening adverse events are rarely experienced with existing immunosuppressive agents, including cyclosporine (CsA), cyclophosphamide, mycophenolate mofetil, tacrolimus, and mizoribine. We conducted a national survey of RTX use for refractory nephrotic syndrome to investigate its indications, efficacy, and adverse events.
Patients and methods
Patients
Questionnaires regarding the use of RTX against childhood-onset SDNS, FRNS, and SRNS were sent to 141 university, children’s, and main regional hospitals in Japan in March 2010. Sixty-eight hospitals returned answers. Among them, 14 hospitals reported the actual use of RTX in 74 children by June 2010.
We analyzed the results of the questionnaires. Approval for this study protocol was obtained from the institutional review boards of the National Center for Child Health and Development. No patients who had been enrolled in a placebo-controlled double-blind clinical trial of RTX for SDNS and FRNS to gain Japanese government approval were included in this survey. Patients with two consecutive relapses of NS while receiving prednisolone on alternate days or within 15 days of its discontinuation were defined as having SDNS. Patients with two or more relapses within 6 months after initial therapy or four relapses in any 12-month period were defined as having FRNS. Patients with the inability to induce remission with 4 weeks of daily steroid therapy (60 mg/m2 or 2 mg/kg, maximum 80 mg/day) were defined as having SRNS [16]. Relapse was defined as proteinuria 3+ by dipstick for more than 3 consecutive days. Complete remission (CR) was defined as the disappearance of proteinuria. Partial remission (PR) of SRNS was defined as more than a 50% reduction in proteinuria and recovery from hypoalbuminemia of less than 2.5 g/dl.
Statistical analysis
The Kaplan–Meier method and log-rank test were used for analyses of relapse-free survival. Statistical significance was established at P < 0.05.
Results
Characteristics of the patients
The characteristics of the patients are summarized in Table 1. Seventy-four patients (44 male and 30 female) were administered RTX for FRNS (n = 3), SDNS (n = 52), and SRNS (n = 19). All of the patients with SDNS/FRNS were treated with RTX under remission. The follow-up duration after the first RTX administration was 24.2 ± 19.8 (standard deviation, SD) months (range: 8–51 months; median: 24 months). All of the patients had already been treated with various types of immunosuppressive agents. However, levamisole has not been approved in Japan and was not administered to any patients.
Overall, 59.5% of patients (n = 44) had a previous (n = 25) or present (n = 19) history of SRNS. Of these 44 patients, 20 developed SRNS at onset (primary SRNS) and 24 had changed from steroid-sensitive nephrotic syndrome to SRNS (late SRNS). Twenty-five patients among those 44 patients changed their clinical course from SRNS to steroid-sensitive nephrotic syndrome mainly by immunosuppressive agents and/or methyl prednisolone pulse therapy. However, 19 patients had been continuously resistant to not only steroids, but also to various immunosuppressive agents, and therefore, were treated with RTX. These findings suggest that many patients with SRNS overcome steroid resistance, but still suffer from SDNS/FRNS.
The mean number of RTX administrations was 1.9 ± 1.4 (SD) (SDNS/FRNS: 1.8 ± 1.4; SRNS: 2.3 ± 1.4; range: 1–7). A total of 106 courses of RTX were administered to the 74 patients, comprising 90 courses of single administration, 4 courses of 2-weekly administration, 1 course of 3-weekly administration, and 11 courses of 4-weekly administration. Of the 74 patients, 2, 3, 4, and 5 courses of RTX were given to 18, 2, 2, and 1 patient respectively. Therefore, 51 patients were treated by a single injection of RTX.
There was no standardized indication for RTX for SDNS/FRNS between the centers, but most of the patients had suffered many adverse effects of steroids and CsA, and had failed to control disease activity in spite of trials of various types of immunosuppressive agents. RTX was given during remission in all patients with SDNS and FRNS.
Steroid and CsA-sparing effect of RTX
Steroid and CsA discontinuation after RTX administration in patients with SDNS/FRNS is shown in Fig. 1. In the SDNS/FRNS patients, 41 of the 53 patients under steroid treatment (77%) successfully discontinued steroids, after suffering various long-term adverse effects of steroid administration. In addition, 18 of the 30 patients with SDNS/FRNS under CsA treatment (60%) discontinued CsA after RTX (Fig. 1), because they suffered from various adverse effects of CsA.
In patients with SRNS, 7 of the 17 patients under steroid treatment (41%) discontinued steroids after RTX because of CR (n = 3), PR (n = 2), or no efficacy (n = 2). However, almost all of the patients with SRNS could not discontinue CsA even after RTX (Fig. 2). For 2 patients with SDNS and 2 patients with SRNS, we could not obtain information on the discontinuation of steroids because they changed hospital and were lost to follow-up.
In addition, among 37 patients (25 SDNS/FRNS and 12 SRNS) who had never discontinued steroids before RTX, 22 (16 SDNS/FRNS and 6 SRNS) had their first experience of discontinuation of steroids since onset (Fig. 3). The mean duration of steroid use was 49 ± 36 (SD) months in these 37 patients before RTX.
Amelioration of the adverse effects of steroids and CsA
Short stature and obesity were the two main adverse effects of steroids, followed by hypertension, cataracts, and glaucoma. Hypertrichosis and CsA nephropathy were the two main adverse effects of CsA. RTX improved the various adverse effects of steroids and CsA (Fig. 4).
Relapse after RTX in SDNS/FRNS
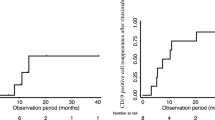
Relapse after RTX occurred in 28 of the 55 patients with SDNS/FRNS. The time to relapse after RTX was 6.6 ± 5.57 (SD) months (range: 1–24 months; median: 5 months). The remaining 27 patients were free from relapse for 17.3 ± 7.8 (SD) months (range: 7–31 months; median 16 months). The mean duration of CD20 cell depletion was 5.2 ± 1.4 (SD) months (n = 48; range: 2–8 months; median: 5 months). The timing of CD20 cell recovery did not significantly differ between the relapsed (n = 24) and remitted (n = 16) patients (4.9 ± 1.0 vs 5.4 ± 1.4 months). We also examined the use of maintenance immunosuppressive agents after RTX. The patients who continued immunosuppressive agents after RTX showed a significantly lower risk of relapse (relapse occurred in 15 of the 40 patients) than the patients who discontinued immunosuppressive agents (relapse occurred in 13 of the 15 patients; Fig. 5; log-rank test, P = 0.006; hazard ratio = 0.201; 95% confidence interval = 0.079–0.514). The mean duration of CD20 cell depletion was 5.0 ± 1.4 (SD) months in patients who continued immunosuppressive agents (n = 33) after RTX, and 4.9 ± 1.0 (SD) months in patients without immunosuppressive agents after RTX (n = 15; not significant). Immunosuppressive agents after RTX did not extend the duration of CD20-positive cell depletion. As maintenance therapy after RTX, mycophenolate mofetil, CsA, tacrolimus and mizoribine were used. However, there were no specific immunosuppressive agents that were preferable for prevention of relapse after RTX.
Effect of RTX against SRNS
All 19 patients with SRNS were resistant to various immunosuppressive agents (Table 1). None of the patients had a family history of SRNS, but 3 underwent genetic analysis, including WT1 and NPHS2. After RTX therapy, CR was achieved in 6 patients (3 primary SRNS; 3 late SRNS) and PR was achieved in 6 patients (2 primary SRNS; 4 late SRNS). Conversely, 7 patients showed no response (NR) to RTX (3 primary SRNS; 4 late SRNS). One non-responder was subsequently found to have a WT1 mutation.
Complete or partial remission were achieved 5.1 ± 3.1 (SD) months (mean 6 months; range 1–12 months) after RTX. Seven out of 12 patients achieved CR or PR under continued CsA (additional mycophenolate mofetil in 2 and additional mizoribine in 1). The urinary protein to creatinine ratio before vs after RTX was 52 ± 113 (SD) vs 1.01 ± 1.3 in the CR group, 24.6 ± 36.6 vs 7.4 ± 9.8 in the PR group, and 73.6 ± 160.4 vs 30.8 ± 41.6 in the NR group.
The latest renal biopsy findings of these 19 patients were as follows: FSGS (n = 11; 4 CR; 2 PR; 5 NR), minimal change nephrotic syndrome (n = 8; 2 CR; 4 PR; 2 NR). Two FSGS patients had post-transplant relapse (1 CR; 1 NR). Therefore, the type of histology did not influence the response to RTX.
Adverse events of RTX
The adverse events of RTX are summarized in Table 2. Infusion reactions were frequently experienced, but were generally mild. Two patients developed granulocytopenia as a late severe adverse event. One of these patients had the complication of sepsis. However, both patients recovered well after treatment with granulocyte-stimulating factor and antibiotics.
Satisfaction with RTX
We also asked the parents and/or patients about their satisfaction with RTX (n = 69). Overall, 29 of them (42%) felt very satisfied and 27 (39.1%) felt relatively satisfied. “Neither” and “unsatisfactory” were the responses in 15.9% and 2.9%, respectively. Overall, 81.2% had a good impression of RTX treatment.
Discussion
Rituximab treatment for nephrotic syndrome is still off-label in Japan. The results of this survey revealed that RTX has been broadly used for patients with childhood-onset refractory nephrotic syndrome. In our study, dramatic steroid- and calcineurin inhibitor-sparing effects of RTX against SDNS and FRNS are consistent with previous reports in France, India, Italy, Germany, and the United States [1–8]. Compared with these previous reports, the unique finding in our study was that immunosuppressive agents followed by a single dose of RTX could extend the relapse-free period after RTX administration.
Almost all the patients in our survey had been treated with steroid and calcineurin inhibitors for long periods, and they suffered critical adverse effects from steroid and calcineurin inhibitor administration (Fig. 4). The frequency of present or previous use of calcineurin inhibitors reached 98.6%. In addition, various types of immunosuppressive agents, methylprednisolone pulse therapy, and plasma exchange also failed to control their disease activity and allow tapering of steroid and calcineurin inhibitors. Therefore, the use of RTX was anticipated for these patients. In our survey, 41 of the 53 SDNS/FRNS patients (77%) and 5 of the 17 SRNS patients (29%) successfully discontinued steroids, which is similar to previous reports [2, 3, 6, 11]. In addition, we focused on how many patients who had never discontinued steroids since disease onset could become free from steroids after RTX administration. Remarkably, 16 the of 25 SDNS/FRNS patients and 6 of the 12 SRNS patients discontinued steroids for the first time after disease onset. This was a great benefit to the patients. Concurrently, RTX showed a considerable calcineurin inhibitor-sparing effect, because 18 of the 30 SDNS/FRNS patients were able to discontinue CsA. A reduction in the dosage of steroids and calcineurin inhibitors allowed amelioration of the serious adverse effects of both drugs (Fig. 4). Hypertension, glaucoma, and spinal fracture are urgent complications of steroid administration. Half of the patients suffering from these severe adverse events successfully recovered after RTX administration. RTX also resolved the severe adverse events of CsA, such as CsA nephropathy and hypertension, in many patients (Fig. 4). Interestingly, RTX overcame the acquired resistance to CsA. During long-term use of CsA, some patients show acquired resistance to CsA [17, 18]. Since frequent relapse under CsA increases the risk of CsA nephropathy and the dosage of steroids [19, 20], acquired resistance to CsA is problematic. In this survey, 4 of the 8 patients overcame acquired resistance after RTX administration (Fig. 4). Fujinaga et al. [12] reported the value and efficacy of maintenance therapy with CsA after a single dose of RTX, even for patients with previously CsA-refractory SDNS [10]. They also emphasized that RTX improved the drug sensitivity to CsA. CsA is one of the essential drugs used to treat SDNS/FRNS and SRNS [21, 22]. Therefore, it is a beneficial finding that RTX may recover the efficacy of CsA and allow longer use of CsA in patients with long-term intractable nephrotic syndrome. Conversely, Sinha et al. reported that two or three doses of 375 mg/m2 of RTX is as effective as tacrolimus against intractable SDNS. Therefore, RTX could be an alternative option to calcineurin inhibitors for SDNS [23].
Although previous reports have suggested that a single dose of RTX is generally insufficient to achieve long-term remission [3, 11, 12], most of the courses in our survey involved a single administration of RTX (85%). The reason for a single dose being given may be because of the expensive price of RTX. Therefore, half of the patients with SDNS/FRNS had relapses, which occurred at 6.6 ± 5.5 months after RTX administration following recovery of CD20-positive cells in the peripheral blood (4.9 ± 1.0 months). The other half of the patients remained free from relapse for 17.3 ± 7.8 months, even after the recovery of CD20-positive cells (5.2 ± 1.4 months). We concluded that this difference originated from the use of immunosuppressive agents after RTX as maintenance therapy, which allowed a significant reduction in relapses after RTX (Fig. 5). However, no specific agents following RTX therapy have been proven to be beneficial for the prevention of relapses. Although use of immunosuppressive agents after RTX did not extend the duration of CD20-positive cell depletion in our study, RTX may improve drug susceptibility and recover the efficacy of immunosuppressive agents in addition to decreasing disease activity. In contrast, Kemper et al. reported that RTX allowed both steroid and maintenance immunosuppressive agents to be stopped in many patients, but in our study, RTX followed by maintenance immunosuppressive agents was significantly better for prevention of relapse [6]. This difference may be due to the ethnic background, heterogeneity of nephrotic syndrome, and the higher number of single doses of RTX in our study.
The efficacy against SRNS observed in our study is similar to the results of previous studies [1, 4, 24, 25]. In contrast, a recent randomized control trial showed that RTX did not reduce proteinuria at 3 months compared with standard therapy composed of steroids and calcineurin inhibitors [26]. The difference in the results between this randomized control trial and our study may partly be due to a longer treatment period and ethnicity. CR took 5.7 ± 3.7 months (1–12 months, n = 6) in our study.
All of the patients showed resistance to various existing therapies, except for RTX. However, CR was achieved in 6 and PR in 6 of the 19 patients. The type of histology and mode of onset (primary or late) did not influence the response to RTX. Notably, 4 patients with CR and 3 patients with PR had continued CsA after RTX administration. These findings further suggest that RTX may improve the susceptibility to CsA and decrease disease activity, which synergistically induces remission. However, RTX, especially a single injection of RTX monotherapy without immunosuppressive agents, may be insufficient to induce remission in multidrug-resistant SRNS.
Although RTX has already been used in clinical settings for over 10 years, for hematological malignancies, it can lead to rare but lethal adverse events, such as progressive multiple leukoencephalopathy, interstitial pneumonia, ulcerative colitis, and Pneumocystis jirovecii pneumonia [27–29]. For childhood nephrotic syndrome, there have been no reports of progressive multiple leukoencephalopathy caused by RTX, although 1 patient with SRNS died from interstitial pneumonia after RTX therapy [14]. In our survey, mild respiratory infusion reactions were the most common adverse events, as we reported previously [30]. Two patients developed granulocytopenia. In general, however, the results of this survey indicate that RTX is relatively well-tolerated.
Although RTX has considerable efficacy against childhood SDNS/FRNS and SRNS, many patients are likely to relapse, consistent with the recovery of B cells [3, 12]. Feasible solutions to solve these issues are:
-
1.
Administration of a single dose at regular intervals
-
2.
Consecutive multiple administrations within a short period
-
3.
Maintenance therapy with some immunosuppressive agents after RTX
Efficacy of additional RTX administration just after the re-emergence of B cells has been reported in children with SDNS [6], but the effects of persistent B cell depletion on the developing immune system in children are unknown. Recently, Sellier-Leclerc et al. [8] reported that a 15-month CD19 depletion period induced long-term remission, even after definitive CD19 recovery in many SDNS patients, which showed a new potential for RTX. However, during the B cell deleted period, even inactivated vaccines, such as influenza virus vaccines, are impossible to use because of the lack of ability to produce antibodies [31].
Consecutive multiple administrations of RTX within a short period, such as 2 or 4 consecutive injections every week, have also been reported [3, 6, 12]. Some patients are likely to relapse after the recovery of B cells in the same manner as patients receiving a single dose of RTX [3, 6, 12]. Maintenance therapy with some immunosuppressive agents after RTX is one solution to preventing relapse after the re-emergence of B cells in the peripheral blood, consistent with previous reports [9, 10, 32]. In addition, this strategy may help to avoid repeated use of RTX and contribute to safer use and less serious adverse events of RTX.
There are some limitations to this study. It was a retrospective multi-center survey where treatment of nephrotic syndrome was heterogeneous and varied between the centers. In addition, there was no standardized indication for RTX in the treatment of SDNS/FRNS. However, most of the patients showed resistance to multiple immunosuppressive agents prior to RTX, which suggests that they suffered from intractable nephrotic syndrome.
In our study, RTX was well tolerated and reduced the burden of the severe adverse effects of steroids and CsA. In the future, more effective and harmless modes of RTX treatment are required.
References
Bagga A, Sinha A, Moudgil A (2007) Rituximab in patients with the steroid-resistant nephrotic syndrome. N Engl J Med 356:2751–2752
Guigonis V, Dallocchio A, Baudouin V, Dehennault M, Hachon-Le Camus C, Afanetti M, Groothoff J, Llanas B, Niaudet P, Nivet H, Raynaud N, Taque S, Ronco P, Bouissou F (2008) Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol 23:1269–1279
Kamei K, Ito S, Nozu K, Fujinaga S, Nakayama M, Sako M, Saito M, Yoneko M, Iijima K (2009) Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol 24:1321–1328
Prytuła A, Iijima K, Kamei K, Geary D, Gottlich E, Majeed A, Taylor M, Marks SD, Tuchman S, Camilla R, Ognjanovic M, Filler G, Smith G, Tullus K (2010) Rituximab in refractory nephrotic syndrome. Pediatr Nephrol 25:461–468
Gulati A, Sinha A, Jordan SC, Hari P, Dinda AK, Sharma S, Srivastava RN, Moudgil A, Bagga A (2010) Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol 5:2207–2212
Kemper MJ, Gellermann J, Habbig S, Krmar RT, Dittrich K, Jungraithmayr T, Pape L, Patzer L, Billing H, Weber L, Pohl M, Rosenthal K, Rosahl A, Mueller-Wiefel DE, Dötsch J (2012) Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant 27:1910–1915
Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, Benetti E, Scozzola F, Pasini A, Dallera N, Sica F, Belingheri M, Scolari F, Ghiggeri GM (2011) Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 6:1308–1315
Sellier-Leclerc AL, Baudouin V, Kwon T, Macher MA, Guérin V, Lapillonne H, Deschênes G, Ulinski T (2012) Rituximab in steroid-dependent idiopathic nephrotic syndrome in childhood–follow-up after CD19 recovery. Nephrol Dial Transplant 27:1083–1089
Benz K, Dötsch J, Rascher W, Stachel D (2001) Change of the course of steroid-dependent nephrotic syndrome after rituximab therapy. Pediatr Nephrol 19:794–797
Nozu K, Iijima K, Fujisawa M, Nakagawa A, Yoshikawa N, Matsuo M (2005) Rituximab treatment for posttransplant lymphoproliferative disorder (PTLD) induces complete remission of recurrent nephrotic syndrome. Pediatr Nephrol 20:1660–1663
Ito S, Kamei K, Ogura M, Sato M, Fujimaru T, Ishikawa T, Udagawa T, Iijima K (2011) Maintenance therapy with MMF after rituximab in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 26:1823–1828
Fujinaga S, Hirano D, Nishizaki N, Kamei K, Ito S, Ohtomo Y, Shimizu T, Kaneko K (2010) Single infusion of rituximab for persistent steroid-dependent minimal-charge nephritic syndrome after long-term cyclosporine. Pediatr Nephrol 25:539–544
Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, Laubach J, Bawn SD, Gordon LI, Furmann RR, Winter JN, Vose JM, Zelenets AD, Mamtani R, Raisch DW, Dorshimer GW, Rosen ST, Muro K, Gottardi-Litell NR, Talley RL, Sartot O, Green D, Major EO, lBennett CL (2009) Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 113:4834–4840
Chaumais MC, Garnier A, Chalard F, Peuchmaur M, Dauger S, Jacqz-Agrain E, Deschênes G (2009) Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol 24:1753–1755
Ardelean DS, Gonska T, Wires S, Cutz E, Griffiths A, Harvey E, Tse SM, Benseler SM (2010) Severe ulcerative colitis after rituximab therapy. Pediatrics 126:e243–e246
Gipson DS, Massengill SF, Yao L, Nagaraj S, Smoyer WE, Mahan JD, Wigfall D, Miles P, Powell L, Lin JJ, Trachtman H, Greenbaum LA (2009) Management of childhood onset nephrotic syndrome. Pediatrics 124:747–757
Sairam VK, Kalia A, Rajaraman S, Travis LB (2002) Secondary resistance to cyclosporin A in children with nephrotic syndrome. Pediatr Nephrol 17:842–846
Kemper MJ, Kuwertz-Broeking E, Bulla M, Mueller-Wiefel DE, Neuhaus TJ (2004) Recurrence of severe steroid dependency in cyclosporin A-treated childhood idiopathic nephrotic syndrome. Nephrol Dial Transplant 19:1136–1141
Fujinaga S, Kaneko K, Muto T, Ohtomo Y, Murakami H, Yamashiro Y (2006) Independent risk factors for chronic cyclosporine induced nephropathy in children with nephrotic syndrome. Arch Dis Child 91:666–670
Iijima K, Hamahira K, Tanaka R, Kobayashi A, Nozu K, Nakamura H, Yoshikawa N (2002) Risk factors for cyclosporine-induced tubulointerstitial lesions in children with minimal change nephrotic syndrome. Kidney Int 618:1801–1805
Hamasaki Y, Yoshikawa N, Hattori S, Sasaki S, Iijima K, Nakanishi K, Matsuyama T, Ishikura K, Yata N, Kaneko T, Honda M (2009) Cyclosporine and steroid therapy in children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 24:2177–2185
Plank C, Kalb V, Hinkes B, Hildebrandt F, Gefeller O, Rascher W, for Arbeitsgemeinschaft für Pädiatrische Nephrologie (2008) Cyclosporin A is superior to cyclophosphamide in children with steroid-resistant nephrotic syndrome-a randomized controlled multicentre trial by the Arbeitsgemeinschaft für Pädiatrische Nephrologie. Pediatr Nephrol 23:1483–1493
Sinha A, Bagga A, Gulati A, Hari P (2012) Short-term efficacy of rituximab versus tacrolimus in steroid-dependent nephritic syndrome. Pediatr Nephrol 27:235–241
Nakayama M, Kamei K, Nozu K, Matsuoka K, Nakagawa A, Sako M, Iijima K (2008) Rituximab for refractory focal segmental glomerulosclerosis. Pediatr Nephrol 23:481–485
Kari JA, El-Morshedy SM, El-Desoky S, Alshaya HO, Rahim KA, Edrees BM (2011) Rituximab for refractory cases of childhood nephrotic syndrome. Pediatr Nephrol 26:733–737
Magnasco A, Ravani P, Edefonti A, Murer L, Ghio L, Belingheri M, Benetti E, Murtas C, Messina G, Massella L, Porcellini MG, Montagna M, Regazzi M, Scolari F, Ghiggeri GM (2012) Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol 23:1117–1124
Teichmann LL, Woenckhaus M, Vogel C, Salzberger B, Scholmerich J, Fleck M (2008) Fatal Pneumocystis pneumonia following rituximab administration for rheumatoid arthritis. Rheumatology 47:1256–1257
Kumar D, Gourishankar S, Mueller T, Cockfield S, Weinkauf J, Vethanayagam D, Humar A (2009) Pneumocystis jirovecii pneumonia after rituximab therapy for antibody-mediated rejection in a renal transplant recipient. Transpl Infect Dis 11:167–170
Shelton E, Yong M, Cohney S (2009) Late onset Pneumocystis pneumonia in patients receiving rituximab for humoral renal transplant rejection. Nephrology 14:96–99
Kamei K, Ito S, Iijima K (2010) Severe respiratory adverse events associated with rituximab infusion. Pediatr Nephrol 25:1193
Yri OE, Torfoss D, Hungnes O, Tierens A, Waalen K, Nordøy T, Dudman S, Kilander A, Wader KF, Ostenstad B, Ekanger R, Meyer P, Kolstad A (2011) Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood 118:6769–6771
Sharma AP, Filler G (2009) Role of mycophenolate mofetil in remission maintenance after a successful response to rituximab. Pediatr Nephrol 24:423–424
Acknowledgements
This work had no financial support. The authors appreciate the doctors who collaborated on our questionnaire: Daishi Hirano, Saitama Children’s Medical Center, Saitama, Japan; Hiroshi Kaito, Kobe University, Kobe, Japan; Tomonori Harada, Yokohama City University Medical Center, Yokohama, Japan; Hiroshi Tanaka, Hirosaki University, Hirosaki, Japan; Toshio Watanabe, Gunma University, Maebashi, Japan; Masaki Shimizu, Kanazawa University, Kanazawa, Japan; Naohiro Wada, Shizouka Children’s Hospital, Shizuoka, Japan; Osamu Uemura, Aichi Children’s Health and Medical Center, Ohbu, Japan; Masashi Nishida, Kyoto Prefectural University of Medicine, Kyoto, Japan; Kenichi Satomura, Osaka Medical Center and Research Institute for Maternal and Child Health, Osaka, Japan; Rika Fujimaru, Osaka City General Hospital, Osaka Japan; Ryojiro Tanaka, Hyogo Prefectural Kobe Children’s Hospital, Kobe, Japan; and Kohei Maekawa, Hyogo College of Medicine, Nishinomiya, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ito, S., Kamei, K., Ogura, M. et al. Survey of rituximab treatment for childhood-onset refractory nephrotic syndrome. Pediatr Nephrol 28, 257–264 (2013). https://doi.org/10.1007/s00467-012-2319-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-012-2319-1