Abstract
Background
Chronic haemodialysis (HD) in small children has not been adequately investigated.
Methods
This was a retrospective investigation of the use of chronic HD in 21 children aged <2 years (n = 12 aged <1 year) who were registered in the Italian Pediatric Dialysis Registry. Data collected over a period of >10 years were analysed.
Results
The median age of the 21 children at start of HD was 11.4 [interquartile range (IQR) 6.2–14.6] months, and HD consisted mainly of haemodiafiltration for 3–4 h in ≥4 sessions/week. A total of 51 central venous catheters were placed, and the median survival of tunnelled and temporary lines was 349 and 31 days, respectively (p < 0.001). Eight children (38 %) showed evidence of central vein thrombosis. Although 19 % of patients received growth hormone and 63.6 % received enteral feeding, the weight and height of these patients remained suboptimal. During the HD period the haemoglobin level increased in all patients, but not to normal levels (from 8.5 to 9.6 g/dl) despite erythropoietin administration (503–600 U/kg/week). The hospitalisation rate was 1.94/patient-year. Seventeen patients underwent renal transplantation at a median age of 3.0 years. Four patients, all affected by severe comorbidities, died during follow-up (in 2 cases due to absence of a vascular access). The 5- and 10-year cumulative survival was 82.4 and 68.7 %, respectively.
Conclusions
Extracorporeal dialysis is feasible in children aged <2 years, but comorbidities, vascular access, growth and anaemia remain major concerns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite significant advances in the management of very young paediatric patients with end-stage renal disease (ESRD), haemodialysis (HD) is still only used in 3–14 % of cases [1–9]. Compared to HD, peritoneal dialysis (PD) has experienced increasingly popularity as therapy modality in this age range due to its lack of requirement for a vascular access, relative technical simplicity and increased likelihood of preserving residual renal function [10–12]. It is therefore not surprising that there are only a few published small single-centre reports on the use of chronic HD in young children [13–17] and that there are actually no published studies which specifically describe infants starting HD in the first year of life. Among the published studies involving infants on HD, Shroff et al. described 18 patients aged <2 years, of whom only seven underwent HD for >3 months [15], and Quinlan et al. described nine children weighing <10 kg who underwent HD for >6 months [13]. However, even though HD is sometimes considered the first-choice option even in small children, including those with primary oxalosis or anatomical or psychosocial contraindications to PD [10], there is a scarcity of information on chronic HD in small children.
In this study we retrospectively investigated the use of chronic HD in children with ESRD aged <2 years. Data were obtained from the Italian Pediatric Dialysis Registry over a period of >10 years.
Patients and methods
The files of patients entered in the Italian Pediatric Dialysis Registry (a permanent, nationwide chronic dialysis network of all 12 Italian pediatric dialysis units) between 1 January 2000 and 31 October 2013 who started HD before the age of 2 years were retrospectively reviewed. Chronic HD was defined as a continuous HD period lasting >3 months: patients treated with HD for <3 months were excluded from the analysis. The term “HD cycle” used here refers to each continuous HD period of >3 months from the beginning of HD to either transplantation, switch to PD, death or last follow-up.
A specific ad hoc form was sent to each participating unit in order to acquire additional patient information.
Data on dialysis schedule, medications, enteral feeding, vascular access, anthropometry and hospitalisations were collected at the beginning of the HD cycle and every 6 months thereafter. The parameters studied are given in Table 1. The outcome of the HD cycle, data on renal transplantations and the long-term outcome of each patient were also analysed. Patient survival and technique survival were calculated. Technique survival was calculated from the time of initiation of HD to death or conversion to another dialysis treatment, with both of the latter considered to be modality failures.
Statistical analysis
The data were expressed as median values and interquartile ranges (IQR) and statistically analysed using the Mann–Whitney test for continuous variables and the chi-square test for dichotomous variables. Survival was assessed using Kaplan–Meier analysis. A p value of < 0.05 was considered to be statistically significant.
Results
Patient characteristics
A total of 133 children aged <2 years started chronic dialysis during the study period. Of these, 21 (15.8 %) underwent a total of 22 HD cycles in six centres for >3 months. Twelve patients started chronic HD during the first year of life, the youngest of whom was aged 2.3 months at start of HD. Of the 133 patients started on chronic dialysis, eight (6 %) started HD as their first dialysis modality due to abdominal surgery (4 patients), enlarged kidneys (1) and unknown reasons (3), and 13 (9.8 %) were transferred from PD to HD due to peritonitis (7 patients), a diagnosis of primary oxalosis (3), ultrafiltration failure (2) and unknown reason (1).
Table 2 shows the characteristics of the patients at the beginning of HD treatment. The most frequent primary kidney diseases were congenital nephrotic syndrome and kidney dysplasia (6 cases each, 28.6 %). Four patients had primary oxalosis. Nine children (42.8 %) were affected by at least one non-renal comorbidity, of which the most frequent were central nervous system abnormalities and anorectal/enteric malformations.
HD schedule and medications
Data on the HD schedules are presented in Fig. 1. Haemodiafiltration (HDF) was the preferred form of dialysis in 58.8 % of patients at the start of treatment, in 50 % after 6 months and in 63.6 % after 12 months. The percentage of children undergoing ≥4 sessions/week at these three time points was 64.7, 71.4 and 63.6 %, respectively (2 of the 4 patients with oxalosis underwent 6 sessions/week, and the other 2 underwent 3 and 4 sessions/week, respectively), with a session duration of 3–4 h in 64.7 % of children at start of the treatment, 78.6 % after 6 months of treatment and 81.8 % after 12 months of treatment. Median blood flow was 8.0 (IQR 6.7–12.0) ml/kg/min at the start of HD, 9.0 (8.2–10.1) ml/kg/min after 6 months and 8.7 (7.6–11.2) ml/kg/min after 12 months. There were no significant differences between patients aged <1 year and those aged >1 year in any of these dialysis parameters.
All of the patients were treated with recombinant human erythropoietin (rhEPO); the median dose was 600 (IQR 395–661) U/kg/week at the start of HD, 577 (469–900) U/kg/week after 6 months and 503 (303–545) U/kg/week after 12 months. The median dose at these same time points in the subgroup of patients starting HD during the first year of life was 453, 634 and 401 U/kg/week, respectively. Of the 21 patients who started on chronic HD during the study period, four (19 %) received recombinant human growth hormone (rhGH), including three of the 12 (25 %) treated in the first year of life.
Nutritional data were available for only 11 patients, seven of whom (63.6 %) were enterally fed by means of a gastrostomy (1 patient) or nasogastric tube (6).
Vascular access
A total of 51 central venous catheters (CVCs) were placed in children aged ≤ 2 years, and data were available on the placement of 45 of these (27 temporary and 18 tunnelled CVCs). The insertion site of the temporary CVCs was a jugular vein in 13 patients, a femoral vein in 12 patients and a subclavian vein in two patients. The cuffed CVCs [type and manufacturer: 6.5Fr Tesio® (8 patients), Quinton Permcath (4), 8Fr Medcomp (3) and Bard (3)] were placed in a jugular vein in 13 patients and in a subclavian vein in three patients (the site was not reported in 2 cases). The CVC exchange rate was 5.9/1000 days, and the median survival of the tunnelled CVCs was significantly longer than that of the temporary CVCs (349 vs. 31 days, respectively; p < 0.001). Specifically, the survival of cuffed and uncuffed CVCs was 83.3 vs. 52 % at 30 days, 83.3 vs. 18.9 % at 90 days and 77.8 vs. 14.2 % at 180 days (p < 0.001), respectively (Fig. 2).
A total of 27 CVCs were placed in the first year of life, including 11 temporary and 12 tunnelled CVCs [type and manufacturer: 6.5Fr Tesio (3 patients), Permcath (3), Bard (3) and unknown (4)]. All of the lines were placed in the internal jugular vein. The median survival of the tunnelled and non-tunnelled CVCs was 347 and 17 days, respectively (p < 0.005) (Fig. 2).
Eight episodes of catheter-related bloodstream infection were observed in the overall population, resulting in an incidence of 0.6/1000 CVC days. The bacteria isolated were Staphylococcus aureus (4 episodes), S. epidermidis (2), Pseudomonas aeruginosa (1) and unknown (1).
Of the 21 children on HD, eight (38 %) showed ultrasound or computed tomographic evidence of central vein thrombosis, which was either absent or not investigated in the remaining 13 patients.
One arteriovenous fistula was successfully placed in a child aged 1.23 years.
Anthopometric, blood pressure and laboratory data
The anthropometric data and blood pressure values of the 21 children aged <2 years started on HD are presented in Table 3. Both body weight and height-SDS remained suboptimal during the HD period, with a non-significant improvement in body weight observed after renal transplantation (rTx).
There were no significant changes in most of the biochemical values during the first year of HD (Table 4). Median haemoglobin levels increased, but not to the target values (from 8.5 to 9.6 g/dl; p < 0.05);t the levels of the other parameters varied widely (particularly phosphate and parathyroid hormone levels), but their median values were close to the recommended targets. No correlations were found between the erythropoietin dose and haemoglobin or parathyroid hormone levels.
The comparison of anthropometric, blood pressure and laboratory data of patients treated with HDF and those treated with bicarbonate HD showed that children on HDF had higher creatinine (5.7 vs. 4.2 mg/dl; p < 0.05) and albumin (3.6 vs. 3.0 g/dl; p < 0.05) levels and lower phosphate levels (4.9 vs. 6.1 mg/dl; p < 0.05) than those on bicarbonate HD. The only significant difference between patients treated with 2–4 sessions/week and those treated with 5–7 sessions/week was lower urea levels in those undergoing more frequent dialysis (84 vs. 123.5 mg/dl; p < 0.05).
Hospitalisations and outcomes
Data on 39 hospital admissions were available for 14 children aged <2 years (25 hospitalisations occurred in the first year of life), for a median cumulative hospital stay of 23 (IQR 11–38) days per patient, and an incidence of 1.94 hospitalisations per patient-year. Table 5 shows the causes of the hospitalisations, with most due to infections and CVC complications, followed by cardiovascular complications and surgery.
In terms of the outcome of the first HD cycle, after a median period of 16.6 (IQR 6.9–33.6) months, 11 patients underwent rTx, six were switched to PD, three had died and one was still undergoing HD. The reasons for switching to PD were family choice (3 patients), the lack of a vascular access (1) and unknown (2). Technique survival was 71 and 65 % after 12 and 24 months, respectively.
Seventeen patients underwent a total of 19 rTx. Median age and median body weight at the time of the first rTx was 3.0 (IQR 2.0–3.5) years and 11.1 (7.9–12.6) kg, respectively. Postoperative graft recovery was immediate in 15 of these 17 patients; the remaining two patients developed renal artery graft thrombosis, resulting in the perioperative death of one patient and loss of the graft after 9 months in the other. The median post-rTx follow-up was 1.54 (IQR 0.56–5.3) years, and the overall 5-year graft survival was 72 %. Four of the 19 rTx involved patients aged <2 years, of which three renal grafts were still functioning after 5.33, 0.66 and 0.16 years, respectively.
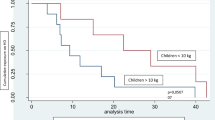
With respect to the long-term outcome, four of the 21 patients on HD died during follow-up (19 %): three during the first HD cycle after 3–91.9 months on HD, and the fourth during a second HD cycle after a period on PD. The causes of death were the lack of a vascular access (2 patients), surgical complications (1) and unknown (1 case). All of the deaths occurred in patients with comorbidities: cerebropathies (2 patients), short bowel (1) and polymalformative syndrome (1). The 2-, 5- and 10-year cumulative survival were 95.2, 82.4 and 68.7 %, respectively (Fig. 3). After a median follow-up of 4.8 (IQR 3.1–8.8) years, 15 patients had a functioning transplant, four had died and two were on chronic HD.
2 of the children treated in the first year of life died and 10 had a functioning transplant after a median follow-up of 4.2 years (1.7–12.2). There was no significant difference in the survival of the patients starting HD in the first and second year of life (p = 0.98) (Fig. 3).
Discussion
Although preemptive or early rTx, if feasible, is the best option for small children with ESRD, and PD remains the preferred form of renal replacement therapy (RRT) in this population, under some circumstances HD represents the only possible RRT option. However, given the small number of cases treated in each centre, there is still a scarcity of published data on the use of HD in infants and toddlers [13–17]. The four major studies published to date included a total of only 28 patients aged <2 years or weighing <10 kg who underwent HD for >3 months [13–16], and there are no published case series specifically describing patients who started HD during the first year of life. To address this gap in our knowledge, we retrospectively reviewed the cases of 21 children starting chronic HD at an age of <2 years entered in the Italian Registry of Pediatric Dialysis, including 12 children treated during the first year of life.
Our series of small children treated with chronic extracorporeal therapy is the largest published to date which involves a homogeneous population, i.e. children aged <2 years treated with HD for >3 months, including a relatively high number of infants treated during the first year of life. The points of strength of this study include the homogeneity of the population and a comprehensive set of clinical data not previously reported for this particular population.
Our findings confirm that HD is rarely used in infants and toddlers: the percentage of small children treated with extracorporeal dialysis in different studies varies from 3 to 14 % [1–9], and only 15.8 % of our registered ESRD patients aged <2 years underwent chronic HD, which represents the first-choice RRT option in only 6 % of the cases.
It has been clearly demonstrated that daily dialysis has beneficial effects on many outcome measures in children, including growth. Most of our patients underwent HD sessions of 3–4 h for four or more times per week (most of these were treated before the publication of the results on intensified HD schedules in children). It is interesting to note that the percentage of patients undergoing more than three sessions per week (63.6–71.4 %) was similar to that reported by Leonard et al. in the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) database [9].
Almost half of the children in our series were treated with HDF. We found that these patients on HDF had higher creatinine and albumin levels, which can be interpreted as markers of a better nutritional status, and lower phosphate levels than children on bicarbonate HD. Although a comparison of different HD modalities is beyond the scope of the study, our data, which can be viewed as only preliminary, confirm that despite the results of various adult studies and the potential advantages of convective therapies in pediatric patients, there is still an urgent need for conclusive proof on the benefits of HDF over bicarbonate HD in children, with all of the technical aspects of HDF in small children also taken into account.
Notwithstanding the technical difficulties of HD in small children, our data show that it can lead to acceptable metabolic equilibrium and blood pressure control, although growth remains a major concern. In our pediatric patient population undergoing chronic HD both weight and height were relatively stable during the period of HD treatment, but the children’s growth rate remained unsatisfactory. These data are in line with those reported in previous small case series of small children on HD [13–15], but they do not confirm the improvement of growth reported after the beginning of PD or after rTx [18]. The most important strategy to optimise growth in small children with ESRD is to guarantee adequate protein and calorie intake: 63.6 % of our children were enterally fed, a percentage similar to that reported by Quinlan et al. (6/9 patients) but higher than that reported by Kovalski et al. (3 gastrostomised children among 11 patients) [13, 14]. Almost 20 % of the children in our series were treated with GH (25 % in the first year of life), which is surprising given that there are very little published data on the use of rhGH in infants, and none of the studies involved infants on HD [19–21]. We did not observed post-rTx catch-up growth, probably due to the short follow-up after rTx (median 1.5 years). Data on parental target height, which could help in the diagnosis of a true height deficit in children, were not available in the Registry database.
Many studies have shown that, differently from PD, anaemia is still a major problem in infants undergoing HD: all of the patients in our cohort were treated with rhEPO, but haemoglobin levels remained unsatisfactory as the average level was only 8.5–9.6 g/dl. The median EPO dose (600 U/kg/week) was higher than that used by Quinlan et al. (100–550 U/kg/week) and Shroff et al. (100–300 U/kg/week) but in line with that used by Kowalski et al. (330–800 U/kg/week) and Feinstein et al. (340–1252 U/kg/week) [13–15, 17]. Unfortunately, the Registry database does not contain data on patients’ iron status.
The preparation and management of a vascular access is undoubtedly one of the major factors limiting the use of HD in small children [14, 22, 23]. Our study confirms that the use of an arteriovenous fistula was exceptional, with almost all of the children treated by means of a central venous line. Overall CVC survival was clearly better using a tunnelled rather than an uncuffed catheter, but despite the availability of suitable pediatric cuffed CVCs and unacceptably poor outcomes of temporary CVCs, the latter are still frequently employed in small children. The incidence of CRBSI (0.6 episodes/1000 CVC days) was low among our pediatric patients, although it may have been underestimated. However, there was a very high prevalence of catheter-related central vein thrombosis (38 %), which may have been even more frequent as the Registry centres did not systematically investigate its presence. Central vein thrombosis can have a negative impact on the possibility to perform an arteriovenous fistula in the future, with dramatic consequences on quality of life and survival. Even more importantly, the lack of a vascular access was the most common cause of death in our cohort (2 cases). New strategies need to be developed to preserve the integrity of central veins in small children who undergo HD through a CVC. Given that short- and long-term access complications are less common in PD, CVC remains at present the most important drawback of HD in small children.
Our findings confirm that infants and toddlers on chronic HD frequently need to be hospitalised, with CVC-related complications being second only to intercurrent infectious diseases as the reason for hospitalisation (accounting for 20 % of the cases). Furthermore, the retrospective nature of our study may have led to our hospitalisation rate (1.9 admissions/patient-year) being at least partially underestimated given that it is lower than that recorded by Shroff et al. (8.2/patient-year) and Kovalski et al. (0.7/patient-month) [14, 15].
Previously published studies reported successful rTx in 40–73 % of small children treated with HD, with mortality rates ranging from 0 to 30 % [13–16]. Seventeen of our patients underwent rTx at a median age of 3.0 years, with a 5-year graft survival rate of 72 %. The four patients who died during a median follow-up of 4.8 years all had severe comorbidities, thus confirming that chronic renal failure per se rarely leads to death even in small children. Previous studies have clearly demonstrated that extrarenal comorbidities are major factors influencing the prognosis of infants with ESRD [3, 17]. It is worth noting that ten of our 12 patients who started HD in the first year of life had a functioning transplant at the time of the last observation. Registry data clearly showed that the survival of patients starting RRT as infants is lower than that in those aged >1 year at the start of dialysis (75.1 % at 3 years according to NAPRTCS data) [1]. Our survival rates (95.2, 82.4 and 68.7 % at 2, 5 and 10 years, respectively) are not significantly different from the 79 % of the 1-, 2- and 5-year survival rates reported by Wedekin et al. [3] in 29 infants treated with PD (27 patients) or preemptive rTx (2 cases), but they are far from those reported by large studies after rTx (91.4–95.5 % at 5 years) [1, 24].
Based on these data taken together, the search for new strategies to improve the outcome of small children on chronic HD is essential. Technical advances could help overcome the current limitations of HD in this population, and the promising results obtained using the CARPEDIEM machine, which is specifically designed for continuous RRT in neonates, and the Nidus system are the first steps in this direction [25, 26].
Our study has a number of methodological limitations that are typical of a multicentre, registry-based study. These include a lack of detailed information on a number of important clinical aspects, such as dialysis efficacy, dietary intake and medications (except for rhEPO and rhGH). Possible differences in the CVC management protocols and hospitalisation policies of the different participating centres and the absence of standardised criteria for the diagnosis of clinical and CVC-related complications must be acknowledged as limitations of the study. Moreover, data collection every 6 months could be misleading in a population of small children. More importantly, given the absence of a control group, we acknowledge that the study can be considered to be a descriptive analysis only and that care must be taken when interpreting the results.
Conclusions
Technological advances in dialysers and machines over the last years have resulted in HD becoming a feasible treatment option in very small children to obtain fluid status control and metabolic equilibrium, thereby enabling the majority of surviving children to undergo a successful rTx. Despite the use of GH, impaired growth remains a major concern. However, the most important limitation of long-term HD is the use of CVCs, which increases the risks of infection, central vein thrombosis, hospitalisation and even death. Mortality is strictly associated with the presence of non-renal comorbidities. PD and, if possible, preemptive or early rTx remain the first-choice options in small children with ESRD. Investigation of the different dialysis modalities and of the optimal strategies for improving the outcomes of small children undergoing chronic HD remains the unanswered priority.
References
North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) (2012) NAPRTCS 2011 annual dialysis report. The EMMES Corporation, Rockville, MD. Available at: http://www.emmes.com/study/ped/annlrept/annualrept2011.pdf
Hijazi R, Abitbol CL, Chandar J, Seeherunvong W, Freundlich M, Zilleruelo G (2009) Twenty-five years of infant dialysis: a single center experience. J Pediatr 155:111–117
Wedekin M, Ehrich JH, Offner G, Pape L (2008) Aetiology and outcome of acute and chronic renal failure in infants. Nephrol Dial Transplant 23:1575–1580
Alexander RT, Foster BJ, Tonelli MA, Soo A, Nettel-Aguirre A, Hemmelgarn BR, Samuel SM, of the Pediatric Renal Outcomes Group Canada (2012) Survival and transplantation outcomes of children less than 2 years of age with end-stage renal disease. Pediatr Nephrol 27:1975–1983
Shroff R, Rees L, Trompeter R, Hutchinson C, Ledermann S (2006) Long-term outcome of chronic dialysis in children. Pediatr Nephrol 21:257–264
Carey WA, Talley LI, Sehring SA, Jaskula JM, Mathias RS (2007) Outcomes of dialysis initiated during the neonatal period for treatment of end-stage renal disease: a North American pediatric renal trials and collaborative studies special analysis. Pediatrics 119:e468–473
van Stralen KJ, Borzych-Dużalka D, Hataya H, Kennedy SE, Jager KJ, Verrina E, Inward C, Rönnholm K, Vondrak K, Warady BA, Zurowska AM, Schaefer F, Cochat P, ESPN/ERA-EDTA Registry, IPPN Registry, ANZDATA Registry, Japanese RRT Registry (2014) Survival and clinical outcomes of children starting renal replacement therapy in the neonatal period. Kidney Int 86:168–174
Sousa CN, Gama M, Andrade M, Faria MS, Pereira E (2008) Haemodialysis for children under the age of two years. J Ren Care 34:9–13
Leonard MB, Donaldson LA, Ho M, Geary DF (2003) A prospective cohort study of incident maintenance dialysis in children: an NAPRTC study. Kidney Int 63:744–755
Zurowska AM, Fischbach M, Watson AR, Edefonti A, Stefanidis CJ, on behalf of the European Paediatric Dialysis Working Group (2013) Clinical practice recommendations for the care of infants with stage 5 chronic kidney disease (CKD5). Pediatr Nephrol 28:1739–1748
Feber J, Schärer K, Schaefer F, Míková M, Janda J (1994) Residual renal function in children on haemodialysis and peritoneal dialysis therapy. Pediatr Nephrol 8:579–583
Fischbach M, Terzic J, Menouer S, Soulami K, Dangelser C, Helmstetter A, Gehant F (2001) Effects of automated peritoneal dialysis on residual daily urinary volume in children. Adv Perit Dial 17:269–273
Quinlan C, Bates M, Sheils A, Dolan N, Riordan M, Awan A (2013) Chronic hemodialysis in children weighing less than 10 kg. Pediatr Nephrol 28:803–809
Kovalski Y, Cleper R, Krause I, Davidovits M (2007) Hemodialysis in children weighing less than 15 kg: a single-center experience. Pediatr Nephrol 22:2105–2110
Shroff R, Wright E, Ledermann S, Hutchinson C, Rees L (2003) Chronic hemodialysis in infants and children under 2 years of age. Pediatr Nephrol 18:378–383
Al-Hermi BE, Al-Saran K, Secker D, Geary DF (1999) Hemodialysis for end-stage renal disease in children weighing less than 10 kg. Pediatr Nephrol 13:401–403
Feinstein S, Rinat C, Becker-Cohen R, Ben-Shalom E, Schwartz SB, Frishberg Y (2008) The outcome of chronic dialysis in infants and toddlers—advantages and drawbacks of haemodialysis. Nephrol Dial Transplant 23:1336–1345
Ledermann SE, Scanes ME, Fernando ON, Duffy PG, Madden SJ, Trompeter RS (2000) Long-term outcome of peritoneal dialysis in infants. J Pediatr 136:24–29
Haffner D, Fischer DC (2009) Growth hormone treatment of infants with chronic kidney disease: requirement, efficacy, and safety. Pediatr Nephrol 24:1097–1100
Mencarelli F, Kiepe D, Leozappa G, Stringini G, Cappa M, Emma F (2009) Growth hormone treatment started in the first year of life in infants with chronic renal failure. Pediatr Nephrol 24:1039–1046
Santos F, Moreno ML, Neto A, Ariceta G, Vara J, Alonso A, Bueno A, Afonso AC, Correia AJ, Muley R, Barrios V, Gómez C, Argente J (2010) Improvement in growth after 1 year of growth hormone therapy in well-nourished infants with growth retardation secondary to chronic renal failure: results of a multicenter, controlled, randomized, open clinical trial. Clin J Am Soc Nephrol 5:1190–1197
Paul A, Fraser N, Manoharan S, Williams AR, Shenoy MU (2011) The challenge of maintaining dialysis lines in the under twos. J Pediatr Urol 7:48–51
Coulthard MG, Sharp J (2001) Haemodialysing infants: theoretical limitations, and single versus double lumen lines. Pediatr Nephrol 16:332–334
McDonald SP, Craig J (2004) Long-term survival of children with end-stage renal disease. N Engl J Med 350:2654–2662
Ronco C, Garzotto F, Brendolan A, Zanella M, Bellettato M, Vedovato S, Chiarenza F, Ricci Z, Goldstein SL (2014) Continuous renal replacement therapy in neonates and small infants: development and first-in-human use of a miniaturised machine (CARPEDIEM). Lancet 383:1807–1813
Coulthard MG, Crosier J, Griffiths C, Smith J, Drinnan M, Whitaker M, Beckwith R, Matthws JNS, Flecknell P, Lambert HJ (2014) Haemodialysing babies weighing < 8 kg with the Newcastle infant dialysis and ultrafiltration system (nidus): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol 29:1873–1881
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Paglialonga, F., Consolo, S., Pecoraro, C. et al. Chronic haemodialysis in small children: a retrospective study of the Italian Pediatric Dialysis Registry. Pediatr Nephrol 31, 833–841 (2016). https://doi.org/10.1007/s00467-015-3272-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3272-6