Abstract
Background
Children with steroid-dependent nephrotic syndrome (SDNS) often suffer from serious adverse events, including growth retardation and obesity. Rituximab (RTX) is a promising therapeutic option to overcome steroid dependency. We have examined the impact of RTX on growth and obesity in children with SDNS.
Methods
Thirteen pediatric patients with SDNS who were refractory despite treatment with multiple immunosuppressive agents received RTX infusions. Mean follow-up was 2.3 years from the first administration of RTX. Improvement in the height and obesity indexes from prior to the initial RTX infusion through to the last visit was assessed.
Results
After RTX, the number of relapses [2.8 (before RTX) vs. 0.8/year (after RTX); p = 0.0008] and the prednisolone dose (287.9 vs. 70.7 mg/kg/year, respectively; p = 0.0002) were significantly decreased. Marked improvement in the height standard deviation score (SDS) was achieved by ten of the 13 patients (77 %) [n = 13; −1.6 (before RTX) vs. −0.8 SDS (after RTX); p = 0.03]. Notably, the height SDS of seven of the eight patients whose height was less than average at the first RTX improved from −2.6 to −1.4 SDS with RTX therapy. At the same time, the obesity index of 12 of the 13 patients (92 %) significantly improved (n = 13; 16.9 vs. 3.1 %; p = 0.004).
Conclusion
Therapy with RTX may contribute to an improvement in the growth and obesity indexes in some patients suffering from severe side effects of steroids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While most patients (90 %) with idiopathic nephrotic syndrome respond to steroid therapy, up to 60 % of patients experience frequently relapsing nephrotic syndrome (FRNS) or steroid-dependent nephrotic syndrome (SDNS) [1–3]. Patients with FRNS/SDNS may require prolonged steroid therapy which can lead to serious adverse events, including growth retardation, obesity, osteoporosis, osteonecrosis, glaucoma, cataracts and hypertension. Alternative therapeutic options, including cyclophosphamide (CPA), chlorambucil, cyclosporine A (CsA), tacrolimus, mizoribine (MZR) and mycophenolate mofetil (MMF), have been developed to avoid or minimize such adverse events. However, despite the various immunosuppressive agents currently available, some patients remain refractory.
Growth retardation and obesity are likely to be related to the duration and intensity of the steroid treatment. Alteration of the secretory pattern of growth hormone, inhibition of insulin-like growth factor-1 bioactivity and a direct effect on the skeletal tissue matrix are actions that have been attributed to steroids [4]. Catch-up growth after cessation of steroid treatment can be expected in patients who outgrow SDNS or are treated with alternate-day prednisolone (PSL). However, the effectiveness of PSL may be insufficient in patients with refractory SDNS [5–8].
Growth retardation and obesity negatively influence a patient’s self-esteem and quality of life. Additionally, cosmetic issues, such as hypertrichosis, acne and cutaneous striae, especially in adolescence, often disrupt a patient’s adherence to steroids, resulting in poor control of the disease. Furthermore, steroids can cause psychological problems in patients, creating mental stress for parents and caregivers [9]. Therefore, the therapeutic goal in SDNS/FRNS has always been to accomplish a sustained longer remission with a minimal dose of steroids. However, the appropriate treatment strategy for patients with refractory SDNS remains challenging as severe growth retardation and obesity are likely to develop despite the use of alternative immunosuppressive agents. For such patients, rituximab (RTX), an anti-CD20 monoclonal antibody, is an emerging option [10–14]. We have previously reported that RTX significantly reduces the number of relapses and required doses of steroids in patients with refractory SDNS [12]. However, to our knowledge, there are no reports focusing on the efficacy of RTX to improve height and weight in pediatric patients with SDNS/FRNS. Consequently, in this study, we attempted to determine the clinical benefits of RTX on these two aspects.
Patients and methods
Treatment strategy
We retrospectively analyzed 13 patients with SDNS who were refractory—that is, unresponsive to conventional immunosuppressants such as CsA, CPA, MZR and MMF. These patients were treated with RTX from January 2007 until December 2010 in the National Center for Child Health and Development, Tokyo, Japan. Patients aged >18 years and those who had reached adult bone age were excluded from the study. RTX was introduced as a therapeutic option for patients who required high doses of steroids to maintain remission or who were suffering from severe steroid-related side effects, such as growth retardation or obesity.
Steroid dependency was defined as the occurrence of two consecutive relapses during the tapering-off period, or within 2 weeks from the discontinuation of steroid therapy. Steroid treatment was based on the International Study of Kidney Disease in Children protocol. At primary onset, patients were initially treated with PSL at a dose of 60 mg/m2/day for 4 weeks. Thereafter, the PSL dose was reduced to 40 mg/m2 on alternate days for 4 weeks. Relapse was treated with PSL at a dose of 60 mg/m2/day until remission (0 or trace proteinuria for 3 consecutive days) was achieved, following which PSL was reduced to the same dose given on alternate days for 2 weeks. The dosage was subsequently further reduced to 40 mg/m2/2 days for 2 weeks, followed by 20 mg/m2/2 days for 2 weeks. Prior to the initiation of RTX treatment eight patients had suffered from steroid-resistant nephrotic syndrome (SRNS) and were treated with methylprednisolone pulse therapy to induce remission.
Upon receiving approval from the ethics committee in our institution for off-label use of RTX together with parental consent, we administered RTX (375 mg/m2) to the patients. The median follow-up after the first RTX infusion was 2.3 (range 1.0–3.8) years. A total of 26 courses of RTX were administered, with six, three, two and two patients receiving one, two, three and four courses, respectively. All patients continued to receive immunosuppressive agents, including CsA (n = 5), MZR (n = 1) and MMF (n = 10), after RTX therapy to prevent further relapse.
Using the same set of clinical records, we retrospectively assessed improvement of the patients’ height and obesity indexes prior to and after RTX therapy. Height is expressed as a height standard deviation score (SDS). Standard height and weight were based on Japanese children’s growth curves. The obesity index was calculated by the following method: obesity index (%) = 100 × (body weight − standard body weight)/standard weight. The normal range for the obesity index is −20 to 20 %). Standard weight was based on the standard height of Japanese children.
Simultaneously, we assessed the decrement in steroid dose and the number of relapses before and after RTX therapy.
Statistical analysis
Data are expressed as medians. Statistical analysis was performed with GraphPad Prism software (GraphPad Software, Inc., San Diego, CA). All p values are two-sided, and p values of <0.05 were considered to be statistically significant. Comparisons were performed using the Wilcoxon non-parametric test.
Results
Characteristics of patients
Thirteen pediatric patients (12 males, 1 female) were included in this study. Table 1 shows characteristics of the patients prior to the initiation of the RTX infusions. Before the commencement of the RTX infusions, one patient had experienced vertebral fracture and femoral head necrosis, three patients had cataracts and one patient had glaucoma. Eight patients were using alendronate, of whom six were also using alfacalcidol, and one was using a luteinizing hormone-releasing (LHRH) analog in addition to alendronate and alfacalcidol to delay puberty and prolong his growing period. The median age at the last visit in 2010 was 13.6 (range 5.8–19.9) years. The median follow-up period after the discontinuation of RTX therapy was 2.3 (range 1.0–3.8) years. Renal biopsy revealed focal and segmental glomerulosclerosis in two patients, and minimal change nephrotic syndrome in 11 patients. Although all patients had been treated with various immunosuppressive agents, including CsA (n = 13), CPA (n = 10), MZR (n = 13) and MMF (n = 1) in combination with PSL, they suffered from SDNS. Eight patients had a previous history of SRNS (2 with primary SRNS and 6 with late SRNS). All patients had a glomerular filtration rate, estimated from serum creatinine, that fell within the normal range throughout the follow-up period.
Number of relapses and steroid dose before and after RTX
Following RTX therapy, the number of relapses [2.8 (before RTX) vs. 0.8/year (after RTX); p = 0.02, Wilcoxon non-parametric test; Fig. 1a] and the PSL dose [287.9 (before RTX) vs. 70.7 mg/kg/year (after RTX); p = 0.0002, Wilcoxon non-parametric test; Fig. 1b] were significantly decreased.
Changes in the number of relapses and in steroid dose after initiation of rituximab (RTX) therapy. After RTX infusions, the number of relapses [2.9 (before RTX) vs. 0.8/year (after RTX); p = 0.0008, Wilcoxon non-parametric test] and the prednisone dose [287.9 (before RTX) vs. 70.7 mg/kg/year (after RTX); p = 0.0002, Wilcoxon non-parametric test] were significantly decreased compared with those before RTX infusions
RTX impact on height
Changes in height before and after RTX use are shown in Fig. 2. At the initial administration of RTX, eight patients had a height SDS of less than zero (growth-retarded patients), and their median duration from disease onset to administration of the first RTX dose was 7.7 (range 1.8–11.1) years. In comparison, the median duration from disease onset to the first RTX dose in the five patients whose SDS were more than the average 0 SDS (non-growth retarded patients) at the initiation of RTX therapy was 3.0 years (range 2.5–3.3 years; p = 0.02). In total, the height SDS of ten of the 13 patients (77 %) improved significantly after RTX therapy. The mean value of −1.6 SDS before RTX improved to −0.8 SDS after RTX therapy (Fig. 2; p = 0.03). Among the eight growth-retarded patients, seven achieved an improvement of height SDS from −2.6 SDS to −1.4 SDS with RTX therapy (not significant). The remaining patient who experienced increased relapses despite RTX therapy (Fig. 1a) had severe progressive growth retardation (−2.5 to −3.8 SDS; Fig. 2). Annual changes in the height SDS after RTX therapy are shown in Fig. 3, while bone age at the first administration of RTX is shown in Table 1.
RTX impact on weight
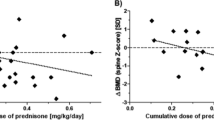
The obesity index of 12 of the 13 patients (92 %) improved significantly with RTX therapy—from 16.9 % before the administration of RTX to 3.1 % after discontinuation of RTX (Fig. 4; p = 0.004).
Adverse effects
Almost half of the patients suffered a mild acute infusion reaction to RTX. Agranulocytopenia was observed in one patient but was successfully treated with granulocyte-colony stimulating factor. No other adverse events were observed.
Discussion
The results of our study add to the increasing body of evidence for the efficacy of RTX against refractory SDNS. While several studies have reported that RTX can achieve a sustained longer remission and maintain a minimal dose of steroids [10–14], they did not focus on the effect of RTX on height and weight. We found that RTX therapy significantly reduced the number of relapses and the doses of steroids (Fig. 1) and that it contributed to an improvement of growth retardation and the obesity index.
According to reports by Foote et al. [15] and Rüth et al. [16], most patients with steroid-sensitive nephrotic syndrome (SSNS) have a decreasing relapse rate as they grow older, with the final height not significantly affected. Leroy et al. reported that 25 % of children with SDNS experience severe growth retardation during the course of their disease but that most can achieve catch-up growth during late puberty [17]. In contrast, Kashtan et al. reported that mean final height SDS in five patients with SDNS was −1.2 SDS [5]. A total of 23 patients with FRNS or SDNS reported by Emma et al. had an average loss of 0.9 SDS in height from the onset of nephrotic syndrome when they reached their final height [6]. Furthermore, Kitamura et al. reported that the final height in 30 % of 17 patients with FRNS was below −2.0 of the height SDS [7]. Another study reported that combined administration of the immunosuppressive agents CPA and CsA significantly reduced growth impairment caused by steroids [18]. In our study, the children had a median height of −1.6 SDS prior to receiving RTX infusions, and after the RTX infusions, their median height SDS improved to −0.8 SDS (Fig. 2). Overall, height improvement was seen in ten of the 13 patients (77 %), of whom seven displayed growth retardation prior to RTX treatment.
The five patients who did not show growth retardation before RTX treatment were resistant to multiple immunosuppressive therapy. Hence these patients were treated with RTX to prevent relapses and reduce the PSL dose. Compared to growth-retarded patients at the first RTX infusion, non-growth retarded patients did not achieve a significant improvement in growth after RTX therapy (Fig. 2). However, the median duration from onset of NS to the first RTX infusion between these two groups was significantly different (growth-retarded patients vs. non-growth retarded patients: 7.7 vs 3.0 years; p = 0.02), suggesting that non-growth-retarded patients might have experienced growth retardation if SDNS had persisted. Based on these results, we hypothesize that RTX would prevent future loss of height SDS in some non-growth retarded patients.
It has been demonstrated that children who continue to receive steroids after the age of 9 years (for girls) and 11 years (for boys) may be at a higher risk of growth retardation due to the loss of the growth spurt in adolescence [5]. On the other hand, a late pubertal growth spurt allows a certain degree of catch-up growth even at an older age [16, 17]. In our study, the five patients who had their bone age measured before RTX treatment showed a marked delayed bone age (median age difference between chronologic age and bone age was 2.5 years, range 0.5–7.4 years). In particular, patients No. 7 and No. 12 showed excellent catch-up growth after RTX treatment, as indicated by their improved height SDS (Fig. 2; Table 1). This result indicates that RTX in older children may contribute to growth if their bone age is delayed but the epiphyseal line is still open. We have shown that RTX improved the height of three patients who were older than 16 years at the first RTX infusion (median improvement of height SDS: 0.9 SDS, range 0.6–1.4 SDS; Table 1; Fig. 3). Among these, the LHRH analog was used for one patient (patient No.12) to delay his puberty so as to prolong his growing period. However, with the records available, we were unable to evaluate how the LHRH analog affected growth in this particular patient.
The body shape resulting from long-term exogenous exposure to glucocorticoids is described as cushingoid, in reference to the body shape observed in Cushing’s disease. Cushing’s disease is classically characterized by obesity, with redistribution of fat from the limbs to the trunk, decreased lean mass and proximal muscle wasting. According to reports by Elzouki et al. [19] and Meritt et al. [20], 35–43 % children with SSNS are obese. Foster et al. reported that obesity is a common complication among children with SSNS; in their study, 41 % of children with SSNS were obese compared with 16 % of the 186 community-based reference subjects [21]. In our study, the obesity index was 16.9 % before RTX therapy, improving to 3.1 % after RTX (Fig. 4).
Several studies have demonstrated behavioral and psychological difficulties in children with nephrotic syndrome which are likely to affect the overall outcome of the disease in an adverse manner. Rüth et al. evaluated the health-related quality of life and psychosocial adjustment in patients with SSNS and showed that the quality of life subscale “social functioning” was significantly impaired [22]. A number of other studies have reported a significant correlation between treatments with high doses of corticosteroids and behavioral problems [23–25]. As growth retardation, obesity, hypertrichosis, acne and cutaneous striae can have a negative effect on the patient’s self-esteem and quality of life, RTX may have great value in preventing these negative outcomes by reducing the patient’s dependency on steroids.
Our study is limited by several factors: the small number of patients, the heterogeneity of the patient population and a relatively short observation period. At the same time, the cumulative dose, duration of continuous versus alternate-day steroid therapy before RTX, variability in pharmacokinetics and/or susceptibility to steroids between individuals, hypoproteinemia and pubertal status could all influence growth. We were unable to evaluate the effect of these factors on growth in our patients due to the retrospective nature of the study.
While RTX is generally well tolerated, it does occasionally cause severe or life-threatening adverse events, including progressive multifocal leukoencephalopathy, interstitial pneumonia and ulcerative colitis [26]. As such, to evaluate the potential side effects of RTX, a larger number of patients together with a longer follow-up are necessary for future studies. Nonetheless, our study has shown that RTX may have a significant steroid-sparing effect that improves growth and reduces obesity in a group of children with SDNS who suffered from severe steroid side effects.
References
Bagga A, Mantan M (1995) Nephrotic syndrome in children. Indian J Med Res 122:13–28
Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr (1997) Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8:769–776
Sinha A, Hari P, Sharma PK, Gulati A, Kalaivani M, Mantan M, Dinda AK, Srivastava RN, Bagga A (2012) Disease course in steroid sensitive nephrotic syndrome. Indian Pediatr 49:881–887
Rees L, Greene SA, Adlard P, Jones J, Haycock GB, Rigden SP, Preece M, Chantler C (1988) Growth and endocrine function in steroid sensitive nephrotic syndrome. Arch Dis Child 63:484–490
Kashtan C, Melvin T, Kim Y (1988) Long-term follow-up of patients with steroid-dependent, minimal change nephrotic syndrome. Clin Nephrol 29:79–85
Emma F, Sesto A, Rizzoni G (2003) Long-term linear growth of children with severe steroid-responsive nephrotic syndrome. Pediatr Nephrol 18:783–788
Kitamura M (1992) Growth retardation in children with frequent relapsing nephrotic syndrome on steroid–improvement of height velocity after administration of immunosuppressive agent Improvement of height velocity after administration of immunosuppressive agent. Nihon Jinzo Gakkai Shi 34:117–124
Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA (2004) Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med 351:868–875
Mitra S, Banerjee S (2011) The impact of pediatric nephrotic syndrome on families. Pediatr Nephrol 26:1235–1240
Bagga A, Sinha A, Moudgil A (2007) Rituximab in patients with the steroid resistant nephrotic syndrome. N Engl J Med 356:2751–2752
Guigonis V, Dallocchio A, Baudouin V, Dehennault M, Hachon-Le Camus C, Afanetti M, Groothoff J, Llanas E, Nivet H, Raynaud N, Taque S, Ronco P, Bouissou F (2008) Rituximab treatment for severe steroid-or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol 23:1269–1279
Kamei K, Ito S, Nozu K, Fujinaga S, Nakayama M, Sako M, Saito M, Yoneko M, Iijima K (2009) Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol 24:1321–1328
Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, Benetti E, Scozzola F, Pasini A, Dallera N, Sica F, Belingheri M, Scolari F, Ghiggeri GM (2011) Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 6:1308–1315
Sellier-Leclerc AL, Baudouin V, Kwon T, Macher MA, Guérin V, Lapillonne H, Deschênes G, Ulinski T (2012) Rituximab in steroid-dependent idiopathic nephrotic syndrome in childhood-follow-up after CD19 recovery. Nephrol Dial Transplant 27:1083–1089
Foote KD, Brocklebank JT, Meadow SR (1985) Height attainment in children with steroid-responsive nephrotic syndrome. Lancet 2:917–919
Rüth EM, Kemper MJ, Leumann EP, Laube GF, Neuhaus TJ (2005) Children with steroid-sensitive nephrotic syndrome. Come of age: long-term outcome. J Pediatr 147:202–207
Leroy V, Baudouin V, Alberti C, Guest G, Niaudet P, Loirat C, Deschenes G, Czernichow P, Simon D (2009) Growth in boys with idiopathic nephrotic syndrome on long-term cyclosporine and steroid treatment. Pediatr Nephrol 24:2393–2400
Hung YT, Yang LY (2006) Follow-up of linear growth of body height in children with nephrotic syndrome. J Microbiol Immunol Infect 39:422–425
Elzouki AY, Jaiswal OP (1988) Long-term, small dose predonisone therapy in frequently relapsing nephritic syndrome of childhood. Effect on remission, statural growth, obesity, and infection rate. Clin Pediatr (Phila) 27:387–392
Merritt RJ, Hack SL, Kalsch M, Olson D (1986) Corticosteroid therapy-induced obesity in children. Clin Pediatr (Phila) 25:149–152
Foster BJ, Shults J, Zemel BS, Leonard MB (2004) Interactions between growth and body composition in children treated with high-dose chronic glucocorticoids. Am J Clin Nutr 80:1334–1341
Rüth EM, Landolt MA, Neuhaus TJ, Kemper MJ (2004) Health-related quality of life and psychosocial adjustment in steroid-sensitive nephrotic syndrome. J Pediatr 145:778–834
Hall AS, Thorley G, Houtman PN (2003) The effects of corticosteroids on behavior in children with nephrotic syndrome. Pediatr Nephrol 18:1220–1223
Mishra OP, Basu B, Upadhyay SK, Prasad R, Schaefer F (2010) Behavioural abnormalities in children with nephrotic syndrome. Nephrol Dial Transplant 25:2537–2541
Neuhaus TJ, Langlois V, Licht C (2010) Behavioural abnormalities in children with nephrotic syndrome–an underappreciated complication of a standard treatment? Nephrol Dial Transplant 25:2397–2399
Chaumais MC, Garnier A, Chalard F, Peuchmaur M, Dauger S, Jacqz-Agrain E, Deschênes G (2009) Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol 24:1753–1755
Acknowledgments
We would like to thank Dr. Julian Tang, Department of Education for Clinical Research, National Center for Child Health and Development, for proofreading and editing this manuscript.
Financial disclosure
The authors have no financial relationships relevant to this article to declare nor any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, M., Ito, S., Ogura, M. et al. Impact of rituximab on height and weight in children with refractory steroid-dependent nephrotic syndrome. Pediatr Nephrol 29, 1373–1379 (2014). https://doi.org/10.1007/s00467-014-2792-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-014-2792-9