Abstract
The present study was designed to evaluate the risk of permanent linear growth impairment in a selected group of 42 children with steroid-dependent nephrotic syndrome (SDNS) and 14 children with frequently relapsing nephrotic syndrome (FRNS). Longitudinal height measurements were available in all patients from the onset of the disease for a mean follow-up of 11.7±3.5 years. During the prepubertal period, patients lost 0.49±0.6 height SD score (HtSDS) (P<0.001). Twenty-three patients have reached their final height with an average loss of 0.92±0.8 HtSDS from the onset of their disease (P<0.001) and 0.68±0.7 from their target HtSDS (P<0.001). The pubertal growth spurt was mildly delayed in male but not female patients. Steroid therapy, calculated as the mean duration of prednisone (PDN) treatment or as the average cumulative PDN dose, was the only predictor of poor growth evolution. Partial catch-up growth occurred after PDN withdrawal. Children with early onset NS and adolescent patients, who were still receiving PDN after the age of 9 years in girls and 11 years in boys, were at higher risk for HtSDS loss. In conclusion, children with severe steroid-responsive NS are at risk of permanent growth retardation secondary to prolonged courses of steroid treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children with steroid-responsive nephrotic syndrome (NS) receiving repeated treatments with prednisone (PDN) are known to be at risk for growth failure. Although PDN has been used as the first line of treatment for these patients for nearly half a century [1], only a limited number of studies have investigated the effects of steroid treatment on linear growth [1, 2, 3, 4, 5, 6, 7, 8, 9]. The available data are often conflicting and difficult to compare because of differences in the length of follow-up and severity of NS. Moreover, most available data are limited to prepubertal children. Final height has been well documented only in one report that included a large number of children with only mild NS [6].
The aim of the present study was to analyze in a selected group of children with severe steroid-responsive NS the long-term impact of PDN treatment on linear growth and on the final postpubertal height.
Patients and methods
Patient selection criteria
Medical records of patients with steroid-responsive NS followed in our renal division since 1982 were reviewed. Fifty-six patients met the following criteria and were enrolled in the study.
-
1.
Steroid-dependent NS (SDNS) or frequently relapsing NS (FRNS) [10].
-
2.
Minimum follow-up of 6 years.
-
3.
Accurate height measurements obtained with a stadiometer available at the onset of NS and at least every year thereafter.
-
4.
Measured parental height available.
-
5.
Numbers of relapses and treatment modality carefully recorded during the entire follow-up.
We have estimated that approximately 70% of patients with SDNS or FRNS that were followed in our division for more than 6 years during the study period have been enrolled. For the remaining 30% of patients, follow-up data did not meet the selection criteria, preventing reliable longitudinal analysis. No patient meeting the inclusion criteria has withdrawn from the study for any other reason.
Growth evaluation
Height measurements were expressed as height standard deviation score (HtSDS) based on the Tanner-Whitehouse reference growth curves [11] and synchronized for each patient at 1-year intervals as described by others [12]. A recent cross-sectional study on children aged 6–20 years in Italy has shown that the British curves match the data of southern Italian children well [13]. A total number of 744 height data were collected (range 7–23 for individual patients). Prepubertal growth was considered until the age of 9 years in girls and the age of 11 years in boys. Analysis of growth after the prepubertal period was performed by synchronizing pubertal growth spurts as described by Haffner et al. [14]. Briefly, from each individual height velocity growth curve, the age of minimal prepubertal velocity, of peak height velocity and of termination of growth was measured. The time scale of each individual curve was then transformed to align these three points to their respective means [14]. Peak height velocity was expressed as Z-score according to Tanner and Whitehouse [11].
Final height was assessed by the absence of height growth over 1 year. Target HtSDS was calculated from measured parental height according to Tanner [15].
Treatment regimens
Patients were initially treated with 2 mg/kg per day of PDN with a maximum of 70 mg/day over 4 weeks, followed by 4 weeks of the same daily steroid dose given on alternate days. After the first one or two relapses, treatment was individualized based on the clinical evolution, previous response to steroids, and side effects. Specifically, PDN induction therapy was prescribed for 2–3 weeks after remission of NS and tapered more gradually over 8–12 weeks. Maintenance therapy was prescribed to most steroid-dependent children. When clinical signs of steroid toxicity were observed (e.g., hypertension, severe hirsutism, severe obesity, diabetes, marked growth stunting) or if patients continued to experience frequent relapses on low-dose alternate-day PDN treatment, steroid-sparing treatments were sequentially used in a stepwise approach, including levamisole, cyclophosphamide, chlorambucil, azathioprine, or cyclosporin A.
Patients were instructed to perform regular urine dipstick tests for proteinuria at home. For each patient the number of relapses, months on daily or alternate-day steroid treatment, dosage of PDN expressed as milligrams per kilogram, and other non-steroid treatments were recorded.
Steroid withdrawal was defined as the discontinuation of steroid treatment for more than 2 years. The date of the last recorded steroid dose was assigned as the date of steroid withdrawal. Patients were considered in permanent remission or "cured" if they remained relapse free without any treatment and without proteinuria for at least 3 years. The date of the last recorded treatment was assigned as the date of achievement of permanent remission.
Statistical analysis
Statistical analysis was performed with NCSS 2000 statistical package software (NCSS, Kaysville, Utah, USA). Comparisons between data were performed with chi-squared test, t-test, or non-parametric Mann-Whitney U test if samples failed normality tests. Logistic regression analysis was used to identify potential positive or negative predictors of height growth using a stepwise forward selection procedure. Simple and multiple regression analyses were performed to analyze the impact of PDN treatment. The probability of persistence of NS was evaluated by the Kaplan-Meier survival curve and comparison between sub-groups was performed by log-rank test. All P values are two-sided and considered statistically significant for P values <0.05. Data are expressed as mean±SE in figures and as mean±SD in the text.
Results
Patients characteristics and evolution of NS
Fifty-six children were enrolled in the study, including 37 males and 19 females. Forty-two patients had SDNS (75%) and 14 patients had FRNS (25%). Detailed patient characteristics are reported in Table 1.
During the entire study period, 733 relapses were recorded, corresponding to a mean relapse rate of 1.8±0.9 relapses/patient per year of disease. Within the first 3 years of follow-up, the average number of relapses/year was significantly higher (2.6±1.2 relapses/year, P<0.05). At the last follow-up, 52% of patients were considered cured and 69% of patients had not received steroid treatment for more than 2 years. No patient became steroid resistant during the entire study period.
No statistically significant difference was found between male and female patients when compared for the age at onset, duration of follow-up, number of relapses, and treatment modalities (Table 1).
Complete prepubertal growth was available for all patients. Final height was reached in 23 patients, among whom 8 still had active disease at their last follow-up. By Kaplan-Meier product analysis, 35% of the cohort was projected to have active disease at the age of 20 years.
Log-rank analyses did not show any differences in the age of achievement of permanent remission between sexes and between children with early or late onset NS.
Linear growth
During prepubertal growth the cohort lost 0.49±0.6 HtSDS (P<0.001). Patients that have reached their final height have lost 0.92±0.8 HtSDS from the onset of their disease (P<0.001) and 0.68±0.7 from their target HtSDS (P<0.001). In 3 patients the final adult height was lower than −2 SD. As illustrated in Fig. 1 however, a large scatter of the data was observed. The final HtSDS was -0.60±1.3 in boys and -0.44±1.2 in girls (P=NS, male versus female comparison). Male patients that have completed growth had received a cumulative PDN dose of 1,560±720 mg/kg over 79±45 months, while female patients received 1,310±750 mg/kg over 75±44 months (P=NS).
Evolution of linear growth. Left panel: height standard deviation score (HtSDS) evolution during the prepubertal period (n=56) and until completion of growth (n=23). Right panel: mean±SE HtSDS and target HtSDS of patients that have achieved their final height (empty circles and square) and of the remaining 33 patients until the beginning of puberty (filled circles)
The peak pubertal growth spurt was significantly delayed in boys compared with the reference population (+0.7±0.8 years, P<0.03), but not in girls. The mean peak velocity Z-scores in both male (-0.32±0.8) and female patients (-0.48±0.9) were lower but not statistically different from the reference population. The final height was reached at a normal age of 16.3±0.4 years in females, but was delayed to 19.0±0.6 years in boys (P<0.01 compared with the reference population).
Children aged less than 3.5 years at the onset of NS had significantly worse linear growth before puberty, as shown in Fig. 2, when compared with children that started their disease later during childhood. On average, they also received steroids for longer periods of time (9.8 versus 6.1 months/year, P<0.005), reaching higher cumulative doses of PDN (228±90 mg/kg per year versus 124±60 mg/kg per year, P<0.001) and experienced more relapses (2.3±1.0 versus 1.4±0.8 relapses/year, P<0.03).
Influence of the age at onset on prepubertal growth. Prepubertal cumulative ΔHtSDS in children with early and late-onset nephrotic syndrome (NS) defined as an onset of NS before or after the age of 3.5 years. Numbers indicate number of patients. Asterisks indicate P values <0.05. Children aged less than 3.5 years at the onset of NS had a mean age of 2.3±0.4 years and a HtSD at onset of 0.25±0.81. Children aged more then 3.5 years at the onset had a mean age of 5.1±1.6 years and a HtSD at onset of 0.32±0.73
Impact of steroid treatment on linear growth
During relapses, patients received a mean dose of 1.8±0.2 mg/kg per day of PDN for 3.1±0.2 weeks, followed by 1.1±0.3 mg/kg of PDN every other day over 10.2±3.8 weeks during the tapering schedule; 45 patients received at any time during the follow-up 0.5±0.2 mg/kg of PDN on alternate days as a low-dose maintenance treatment, during a mean of 31±20 months (range 4–72 months).
Logistic regression analysis was used to identify positive or negative predictors of linear growth, defined as the average ΔHtSDS per year for each patient during the study period, including gender, the number of months on PDN per year, the number of relapses per year, age at onset of NS, the HtSDS at the onset of NS, and the use of additional steroid-sparing agents. PDN treatment was the only variable associated with a negative ΔHtSDS (P=0.003, χ 2=8.7). Although sharing similar chi-squared values, the relapse rate did not add significant weight to the model because it was heavily associated with steroid treatment, as demonstrated by simple regression analysis (r=0.91, P<0.001). The odds ratio for a loss in HtSDS was 7.2 (confidence limits 2.1–25.6) when children received PDN for more than 6 months per year (P<0.02).
Regression analysis was performed to evaluate which PDN treatment modality and which method to quantify PDN treatment correlated more with negative HtSDS scores. All regression coefficients were statistically significant (P<0.0001) and were similar, whether ΔHtSDS was correlated with steroid treatment expressed as milligrams per kilogram of PDN (r=-0.58), as the total number of months of PDN treatment (r=-0.61), or as the months of PDN therapy during induction (r=-0.58), tapering (r=-0.54), and low-dose maintenance schedules (r=-0.52).
As expected, the relapse rate was highly correlated with the use of PDN during induction and tapering schedules, but also with the use of maintenance PDN treatment (r=0.49, P<0.001), indicating that patients with more relapsing disease were more likely to be left on low-dose steroid therapy after each relapse.
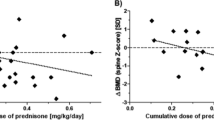
The impact of steroid treatment on linear growth is best illustrated in Fig. 3A, which shows the cumulative ΔHtSDS before puberty in patients that received more than 6 months per year of PDN compared with the rest of the cohort. Significant differences between these two groups were apparent 3 years after the onset of the disease.
Influence of prednisone (PDN) on linear growth. A Cumulative ΔHtSDS during the prepubertal period according to the intensity of PDN treatment, expressed as months of PDN per years. HtSDS at the onset was 0.31±0.76 for children receiving more than 6 months of PDN per year and 0.24±0.80 for children receiving less than 6 months of PDN per year. B Changes in HtSDS during puberty in patients that received PDN after the prepubertal period (filled circles) and in patients that were withdrawn from PDN before the beginning of puberty (open circles). C Catch-up growth after complete withdrawal of PDN. Data are synchronized at the date of the last recorded PDN dose. Numbers indicate number of patients. Asterisks indicate P values <0.05
During puberty, PDN had a similar negative impact. Among the 23 patients that have reached their final height (Fig. 3B), 12 were receiving PDN after the prepubertal period (mean dose 74±39 mg/kg per year) and experienced on average 0.5±0.3 relapses per year. During puberty, these patients have lost 0.66±0.65 HtSDS compared with a loss of 0.08±0.54 HtSDS in patients that were not receiving PDN (P<0.05). Their peak height velocity was also lower (−0.68±0.93 SD versus 0.08±0.92 SD), but differences failed to reach statistical significance. Of 12 patients that received PDN during puberty, 10 (83%) had a final height lower than their target compared with 4 of 11 (36%) of those that were no longer on steroid treatment before entering puberty (P<0.03).
Thirty-three patients were permanently withdrawn from PDN for more than 2 years during the study. As shown in Fig. 3C, partial catch-up growth was observed (P<0.001 after 5 years). Acceleration of growth velocity began generally during the 2nd year after steroid withdrawal and the rate of recovery thereafter was similar to the rate of loss of HtSDS during PDN treatment.
Discussion
Chronic steroid treatment has long been recognized as a major risk for growth retardation in children [1, 16]. In many cases, growth stunting can also be attributed to the severity of the underlying disease. In children with steroid-resistant NS, for example, progressive loss of HtSDS has been related to chronic urinary protein losses [17].
In children with steroid-sensitive NS, given their limited exposure to heavy proteinuria, it is more likely that decreased growth velocity is related to the long-term effects of corticosteroid treatment.
Previous growth studies in steroid-responsive NS are difficult to compare due to the variability in the duration of follow-up, lack of longitudinal measurements in some series, and differences in the severity of NS [1, 2, 3, 4, 5, 6, 7, 8, 9]. In several studies, inclusion of children with a benign course of NS may severely underestimate the side effects of prolonged steroid treatment and may explain the variability of the results that have been reported thus far.
In clinical practice, patients with more severe disease are more likely to experience toxicity related to prolonged and repeated courses of PDN. For this reason, we have selected a homogeneous group of children with severe disease, classified as SDNS or FRNS [10]. The severity of their disease is demonstrated by the fact that 35% of patients still had active disease when they entered adulthood compared with 6.6% at an average age of 21 years in the series by Trompeter et al. [18] and 1% after a follow-up of 5–14 years in the series by Koskimies et al. [19].
Our data indicate that children with severe NS are at risk of permanent loss in their linear growth potential. Generally, the final growth deficit was moderate but exceeded 1 SD in some patients, particularly if treated with prolonged courses of PDN.
The male-to-female ratio was similar to that previously reported [4, 18]. No differences were observed in the general characteristics, duration, severity, and evolution of the disease between sexes.
Foote et al. [6] reported a mean final HtSDS of –0.22 in 28 postpubertal patients. Target height and longitudinal data were not provided. In addition, a significant number of patients experienced a mild course of NS, as indicated by their relapse rate and cumulative steroid dose that were approximately half of that observed in our selected cohort. Therefore, differences in outcome between these two studies are most likely related to differences in the degree of severity of NS and magnitude of steroid treatment.
As in previous reports we found a moderate but significant delay in the peak of pubertal growth spurt in male patients [2]. Our data also show that adolescent patients treated with PDN during puberty experienced significant growth stunting despite being treated with considerably less PDN than prepubertal children. These results suggest that pubertal growth may be particularly sensitive to steroid treatment or that a cumulative effect resulting from protracted steroid therapy is more apparent during adolescence.
Prednisone treatment was the primary risk factor for growth retardation. None of the steroid-sparing treatment regimens retained statistical significance by logistic regression analysis, indicating that their primary beneficial effect on linear growth was related to their steroid-sparing effect, as previously reported by others [3].
Longer steroid treatment with higher cumulative doses of PDN was also the main cause for higher HtSDS losses in children with early onset NS. Moreover, the probability of achieving permanent remission was related to the chronological age but not to the age at onset of the disease, as previously documented by Trompeter et al. [18]. Thus, children with early onset NS also appear to be at higher risk of growth retardation because they experience longer disease courses.
It should be emphasized that the impact of PDN on linear growth is highly variable among different patients. The r values of simple regression analysis, although statistically significant, were relatively small, as reported by other authors [2, 6]. This indicates that a considerable amount of the variance remains unexplained and could be secondary to other factors such as individual pharmacokinetics and susceptibility to steroids. Identifying these factors will certainly be extremely valuable in clinical practice, but until then, side effects of PDN will always need to be monitored on an individual basis. As changes in HtSDS are often very mild when examined on a yearly basis but can become very significant when cumulated over many years, these clinical judgments are often difficult to make.
Our results help to identify those patients that are at increased risk of impaired linear growth. Specifically, we found that young children receiving PDN for more than 6 months per year were at considerably higher risk of experiencing severe growth stunting. This finding is even more relevant because a gain in HtSD generally occurred upon withdrawal of steroids [1, 6]. Catch-up growth, however, was often partial and slow, requiring a number of years nearly equal to the duration of PDN treatment. Therefore, patients who are withdrawn from steroids late during childhood may have insufficient time to recover lost height.
Several authors have argued that prolonged alternate-day steroid treatment does not impair growth in nephrotic patients [7]. Such regimens have been shown to allow normal or near-normal growth in pediatric renal transplant patients [20, 21]. Our data do not substantiate any conclusions on this matter because maintenance treatments in our patients were generally prescribed to children with more severe and relapsing courses, as shown by regression analysis.
By the same analysis, we also tried to identify the best way to measure the magnitude of steroid therapy. We found no substantial differences whether steroid treatment was expressed as the cumulative dose of PDN or as the duration of steroid treatment. Ultimately, these variables are heavily correlated and appear to be equivalent indicators of the intensity of steroid treatment and of the severity of the disease.
In conclusion, our results show that children with SDNS/FRNS are at risk of permanent growth retardation secondary to steroid treatment. Recovery of the initial channel growth is possible if steroid treatment is discontinued before puberty allowing time for catch-up growth. Children who are younger at onset of NS are at higher risk due to severity of their disease during the early years of life, while adolescent patients appear to be more sensitive to PDN treatment during puberty. These findings should guide the management of children with severe steroid-responsive NS. The use of steroid-sparing agents should be considered, in particular during periods of higher risk for growth retardation.
References
Lam CN, Arneil GC (1968) Long-term dwarfing effects of corticosteroid treatment for childhood nephrosis. Arch Dis Child 43:589–594
Rees L, Greene SA, Adlard P, Jones J, Haycock GB, Rigden SP, Preece M, Chantler C (1988) Growth and endocrine function in steroid sensitive nephrotic syndrome. Arch Dis Child 63:484–490
Padilla R, Brem AS (1989) Linear growth of children with nephrotic syndrome: effect of alkylating agents. Pediatrics 84:495–499
Berns JS, Gaudio KM, Krassner LS, Anderson FP, Durante D, McDonald BM, Siegel NJ (1987) Steroid-responsive nephrotic syndrome of childhood: a long-term study of clinical course, histopathology, efficacy of cyclophosphamide therapy, and effects on growth. Am J Kidney Dis 9:108–114
Chesney RW, Mazess RB, Rose P, Jax DK (1978) Effect of prednisone on growth and bone mineral content in childhood glomerular disease. Am J Dis Child 132:768–772
Foote KD, Brocklebank JT, Meadow SR (1985) Height attainment in children with steroid-responsive nephrotic syndrome. Lancet 2:917–919
Elzouki AY, Jaiswal OP (1988) Long-term, small dose prednisone therapy in frequently relapsing nephrotic syndrome of childhood. Effect on remission, statural growth, obesity, and infection rate. Clin Pediatr (Phila) 27:387–392
Polito C, Oporto MR, Totino SF, La Manna A, Di Toro R (1986) Normal growth of nephrotic children during long-term alternate-day prednisone therapy. Acta Paediatr Scand 75:245–250
Saha MT, Laippala P, Lenko HL (1998) Normal growth of prepubertal nephrotic children during long-term treatment with repeated courses of prednisone. Acta Paediatr 87:545–548
Clark AG, Barratt TM (1999) Steroid-responsive nephrotic syndrome. In: Barrat TM, Avner ED, Harmon WE (eds) Pediatric nephrology, 4th edn. Lippincott Williams and Wilkins, Baltimore, pp 731–747
Tanner JM, Whitehouse RH (1976) Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 51:170–179
Gasser T (1991) Analysing curves using kernel estimators. Pediatr Nephrol 5:447–450
Cacciari E, Milani S, Balsamo A, Dammacco F, De Luca F, Chiarelli F, Pasquino AM, Tonini G, Vanelli M (2002) Italian cross-sectional growth charts for height, weight and BMI (6–20 y). Eur J Clin Nutr 56:171–180
Haffner D, Schaefer F, Nissel R, Wuhl E, Tönshoff B, Mehls O (2000) Effect of growth hormone treatment on the adult height of children with chronic renal failure. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. N Engl J Med 343:923–930
Tanner JM (1986) Normal growth and techniques of growth assessment. Clin Endocrinol Metab 15:411–451
Friedman M, Strang LB (1966) Effect of long-term corticosteroids and corticotrophin on the growth of children. Lancet 2:569–572
Scharer K, Essigmann HC, Schaefer F (1999) Body growth of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 13:828–834
Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS (1985) Long-term outcome for children with minimal-change nephrotic syndrome. Lancet I:368–370
Koskimies O, Vilska J, Rapola J, Hallman N (1982) Long-term outcome of primary nephrotic syndrome. Arch Dis Child 57:544–548
Jabs K, Sullivan EK, Avner ED, Harmon WE (1996) Alternate-day steroid dosing improves growth without adversely affecting graft survival or long-term graft function. A report of the North American Pediatric Renal Transplant Cooperative Study. Transplantation 61:31–36
Broyer M, Guest G, Gagnadoux MF (1992) Growth rate in children receiving alternate-day corticosteroid treatment after kidney transplantation. J Pediatr 120:721–725
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Emma, F., Sesto, A. & Rizzoni, G. Long-term linear growth of children with severe steroid-responsive nephrotic syndrome. Pediatr Nephrol 18, 783–788 (2003). https://doi.org/10.1007/s00467-003-1176-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-003-1176-3