Abstract
Background
Steroids, the mainstay of treatment for nephrotic syndrome in children, have multiple adverse effects including growth suppression.
Methods
Anthropometric measurements in children < 18 years enrolled in the Nephrotic Syndrome Study Network (NEPTUNE) were collected. The longitudinal association of medication exposure and nephrotic syndrome characteristics with height z-score and growth velocity was determined using adjusted Generalized Estimating Equation regression and linear regression.
Results
A total of 318 children (57.2% males) with a baseline age of 7.64 ± 5.04 years were analyzed. The cumulative steroid dose was 216.4 (IQR 61.5, 652.7) mg/kg (N = 233). Overall, height z-scores were not significantly different at the last follow-up compared to baseline (− 0.13 ± 1.21 vs. − 0.23 ± 1.71, p = 0.21). In models adjusted for age, sex, and eGFR, greater cumulative steroid exposure (β − 7.5 × 10−6, CI − 1.2 × 10−5, − 3 × 10−6, p = 0.001) and incident cases of NS (vs. prevalent) (β − 1.1, CI − 2.22, − 0.11, p = 0.03) were significantly associated with lower height z-scores over time. Rituximab exposure was associated with higher height z-scores (β 0.16, CI 0.04, 0.29, p = 0.01) over time.
Conclusion
Steroid dose was associated with lower height z-score, while rituximab use was associated with higher height z-score.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nephrotic syndrome is a group of chronic kidney diseases diagnosed in 2–7 of every 100,000 children that is characterized by nephrotic-range proteinuria and edema or hypoalbuminemia [1]. Children with nephrotic syndrome have several risk factors for growth abnormalities including but not limited to proteinuria, progressive chronic kidney disease, and medication exposures. The mainstay of treatment of nephrotic syndrome is prednisone, a glucocorticoid that has adverse effects, including growth suppression [2, 3]. In many cases, patients require prednisone treatment for long periods of time, due to persistent nephrosis and/or relapses which compounds steroid toxicity and side effects [4]. In those with frequently relapsing, steroid-dependent, and steroid-resistant nephrotic syndrome (FRNS/SDNS/SRNS), additional immunosuppressive treatments such as calcineurin inhibitors (tacrolimus and cyclosporine), mycophenolate mofetil (MMF), and rituximab are often used [5].
While steroids remain the primary treatment in children with nephrotic syndrome, the literature regarding growth while on steroid treatment in children with nephrotic syndrome is conflicting. Although there is evidence that steroids correlate with growth retardation in a dose-dependent manner, there are varying results in studies of nephrotic syndrome [2, 3]. For example, a recent study in the UK showed that despite steroid use in children with nephrotic syndrome, there was a statistically significant decrease in body mass index (BMI) and increase in height from baseline to the last recorded visit [6]. Some studies suggest improved growth with calcineurin inhibitor or rituximab treatment, though the evidence is neither conclusive nor specific to nephrotic syndrome [7, 8]. Close to normal growth in children with nephrotic syndrome treated with cyclophosphamide and steroids has been described, but no comparison was made with monotherapy [9]. A better understanding of the risk factors associated with growth abnormalities in children with nephrotic syndrome will guide clinicians to improve care.
The purpose of this study was to examine growth over time in children enrolled in the Nephrotic Syndrome Study Network (NEPTUNE), a prospective, multicenter observational cohort study of children and adults with proteinuric glomerulopathies [10]. The objectives were to describe longitudinal growth (height z-score, growth velocity) patterns of children with nephrotic syndrome and determine the associations between proteinuric glomerulopathy characteristics, including medications, and growth in this population. We also sought to characterize the progression of growth in nephrotic syndrome, comparing longitudinal growth indices in newly identified incident cases to individuals with an existing diagnosis. We hypothesized that greater steroid exposure would be associated with lower height z-score and slower growth velocity over time.
Materials and methods
NEPTUNE
The NEPTUNE cohort study has been previously described in detail [10]. In brief, NEPTUNE is a multicenter observational cohort study of patients with glomerular diseases causing nephrotic syndrome across North America. Pediatric participants were enrolled from two different NEPTUNE cohorts: Incident cohort (Cohort B) and Prevalent cohort (Cohort A). For incident cases of NS, participants had less than 30 days of immunosuppression treatment for the new onset of nephrotic syndrome at the time of enrollment. Nephrotic syndrome was defined as proteinuria on dipstick with ≥ 2 + protein or UPC ≥ 2 g/g, edema, and serum albumin < 3 g/dL. For prevalent cases of NS, participants with proteinuria ≥ 500 mg/day on a 24-h urine sample or urine protein/creatinine ratio (UPC) ≥ 0.5 g/g on a spot urine specimen were enrolled at the time of kidney biopsy. Individuals with systemic disease, solid organ transplant, or life expectancy < 6 months were not eligible for enrollment in NEPTUNE. Participants were followed longitudinally every 4 months in the first year and then every 6 months thereafter for up to 3 years for incident cases and up to 5 years for prevalent cases. For the current analysis, children < 18 years enrolled from NEPTUNE with at least 1 year of follow-up and at least three documented anthropometric measurements were eligible for inclusion. The study period included children enrolled between January 2012 and January 2021. Following IRB approval, informed consent/assent was obtained from all participants enrolled in NEPTUNE. This study was deemed exempt human subjects research by the Institutional Review Board of Northwell Health. See Fig. 1 for a distribution of the cohort.
Growth measurements
At each visit, height was measured to the nearest 0.1 cm with a stadiometer and weight to the nearest 0.1 kg with a digital scale. Age- and sex-specific z-scores for height, weight, and BMI were calculated [11]. Growth velocity was calculated by subtracting the first listed height in centimeters from the final listed height in centimeters and dividing by the change in time in years to establish a growth velocity in cm/year. Weight velocity (kg/year) and BMI velocity (kg/m2/year) were calculated by subtracting the first recorded measure from the final recorded measure and dividing over the change in time in years.
There were a considerable number of missing values for Tanner staging, therefore age was used as a proxy for pubertal status at baseline. Sensitivity analyses of primary outcomes were conducted for various cut-off ages for puberty (i.e., 8/9 years, 9/10 years, 10/11 years for girls and boys, respectively). Results were similar across the different age cut-offs; therefore, the most conservative age cut-offs were used (see Supplementary Tables). For the primary analysis, age < 8 years was considered pre-pubertal and age ≥ 8 years was considered pubertal for girls. For boys, pre-pubertal status was considered age < 9 years and age ≥ 9 years was considered pubertal [12, 13].
Treatment medications and nephrotic syndrome characteristics
The primary exposures of interest were treatment medications. Steroid exposure was quantified at each study visit. The total exposure between two consecutive visits was calculated in mg/kg/visit as the total dose given at each interval prior to a study visit divided by the weight at the study visit. Additionally, the cumulative exposure of steroids during the study period was calculated as a sum of the steroid exposure at each study visit in mg/kg. Steroid use was further classified as low dose (< 2 mg/kg) or high dose (≥ 2 mg/kg). Tacrolimus, MMF, and rituximab use were documented as dichotomous variables (yes/no) at each study visit.
Baseline demographics and clinical variables were collected on the following: age; sex; self-identified race/ethnicity; glomerular disease diagnosis (minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), other); annual household income; insurance status; follow-up time (months); presence of edema (including location); medications; urine protein/creatinine ratio (UPC); and serum creatinine. The CKiD U25 formula was used to calculate eGFR.
Statistical analysis
Descriptive statistics included means with standard deviation and median with interquartile range for continuous variables and frequencies with percentages for dichotomous variables. T-tests and chi-square were used to compare baseline demographic and clinical characteristics of the incident NS group vs. prevalent NS group, as appropriate. Paired t-tests were used to compare z-scores for height, weight, and BMI at the first measurement and at the last measurement in the overall group, as well as stratified by enrollment cohort and pubertal status. Growth velocities were also compared by pubertal status using t-tests.
Generalized estimating equation (GEE) linear regression was used to examine the association of medication exposure and nephrotic syndrome characteristics with height z-scores over time. Each variable of interest was entered in separate models adjusted for age, sex, and eGFR. Steroid use was included in the models for tacrolimus, MMF, and rituximab. Separate linear regression models adjusted for age, sex, and eGFR were used to examine the association of medication exposure and nephrotic syndrome variables with growth velocity. Variables forced in the model were chosen a priori based on a literature review. Two-sided p values < 0.05 were statistically significant, and all analyses were done using SPSS version 27 (IBM).
Results
Study population
Of the 357 children < 18 years enrolled in NEPTUNE, a total of 318 children who contributed 36.3 ± 17.9 months of follow-up met eligibility criteria and were included in the analysis. Demographic and clinical characteristics at baseline are presented in Table 1. Among the cohort, the mean age was 7.64 ± 5.04 years, with 57.2% males and 29.9% identifying as Black. Overall, 58.2% of children were pre-pubertal (median age 4.9 years) and 41.8% of children were pubertal (median age 15.0 years) at baseline. Diagnoses included 41.2% MCD, 26.7% FSGS, 2.8% other, and 29.2% were not biopsied. The baseline median eGFR was 104 (IQR 87, 130) ml/min/1.73 m2, and the UPC ratio was 1.47 (IQR 0.12, 6.9). Enrollment was at the time of kidney biopsy (prevalent group) for 173 children with a disease duration median of 2.5 IQR 1, 12 months. Enrollment was at the at the time of diagnosis within 30 days of treatment (incident group) for 145 children. Those in the prevalent group were significantly older, had a higher proportion with MCD and FSGS, lower UPC and eGFR, higher exposure to steroids and tacrolimus, and were followed for a longer period compared to those in the incident cases group (Table 1).
Growth parameters
Height z-score, weight z-score, and BMI z-score over time are presented in Fig. 2. Among all participants, the mean height z-score at the last follow-up (− 0.13 ± 1.21) was not significantly different from baseline (− 0.23 ± 1.71), p = 0.21 (Table 2). Over half of the children (55%) had a higher height z-score at the final visit compared to the initial (N = 175). Growth velocity was 5.14 ± 2.87 cm/year. There were no significant differences in weight z-score and BMI z-score from baseline to the last follow-up. The weight velocity was 3.61 kg/year, and the BMI velocity was 0.532 kg/m2/year.
When examined by cohort, the weight z-score was significantly lower at the last follow-up in children in the prevalent cases group (p = 0.003). Conversely, the weight z-score was higher at the last follow-up compared to baseline in cohort B (p = 0.02). BMI z-score was also lower in patients at the last follow-up in the prevalent group compared to baseline. The incident group had a higher BMI z-score at the last follow-up compared to baseline. When comparing weight velocity, the prevalent group vs. incident group had a statistically significant difference (p = 0.06), and the same trend was noted in BMI velocity when comparing the incident group to prevalent group (p > 0.001) (Table 2).
Growth parameters by enrollment cohort and puberty
When stratified by enrollment cohort and puberty, within the prevalent group, the pre-pubertal weight z-score was significantly less at the last follow-up compared to baseline (p = 0.04), and BMI z-score was significantly lower at the last follow-up (p = 0.05). In the incident group, post-pubertal patients had a difference in their height z-score being higher at the last follow-up (p = 0.004) and weight z-score being lower at the last follow-up (p = 0.03). When looking at incident cases, pre-pubertal patients had a higher height z-score over time (p = 0.04), weight z-score was also higher over time (p = 0.03), and BMI z-score was higher over time (p = 0.04). In the post-pubertal group within the incident group, there were no statistically significant changes (Table 3). These trends are presented in Fig. 3. Due to the controversial cut-off points of pubertal age and the paucity of Tanner staging data, analyses were conducted with varying cut-off points all with the same trend when stratifying by pubertal score. Supplementary Tables 1–3 show these additional analyses.
Medication exposure
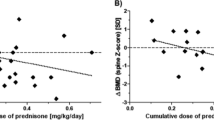
During the observation period, 233 (73.2%) participants were exposed to steroids. The median cumulative medication dose was 216.4 (IQR 61.5, 652.7) mg/kg for those who were exposed to steroids. The mean height z-scores over time comparing high dose (> 2 mg/kg), low dose (< 2 mg/kg), and no steroid exposure are presented in Fig. 4. Eighty-seven (27.4%) participants were exposed to tacrolimus, 77 (24.2%) participants were exposed to MMF, and 25 (7.9%) participants were exposed to rituximab at some point during the observation period.
Regression analysis
In GEE models adjusted for age, sex, and eGFR, greater cumulative steroid exposure was significantly associated with lower height z-scores over time in the overall group. Each 10 mg/kg of cumulative steroid exposure was associated with a decrease in height z-score of − 7.5 × 10−6 (CI − 1.2 × 10−5, − 3 × 10−6), p = 0.001. Rituximab exposure ever was associated with a higher height z-score over time (β 0.16, CI 0.04, 0.29, p = 0.01). The incident group vs. the prevalent group was significantly associated with lower height z-score over time (β − 1.1, CI − 2.22, − 0.11, p = 0.03). There were no significant associations between MMF use, tacrolimus use, eGFR, type of NS (FSGS vs. MCD), and height z-score over time. There were no associations with growth velocity over time (Table 4).
Discussion
In this large, multicenter cohort of children with nephrotic syndrome enrolled in NEPTUNE, height z-scores were not significantly different at the last follow-up compared to baseline in the overall group. However, differences in growth patterns emerged when stratified by enrollment cohort and pubertal status. Interestingly, in adjusted regression analysis, higher cumulative steroid dose exposure and enrollment at the time of kidney biopsy were inversely associated with height z-score over time, and rituximab use was associated with a higher height z-score over time. No other nephrotic syndrome characteristics were associated with height z-score or growth velocity.
Proposed mechanisms of steroid-induced growth restriction include steroid effects on trabecular bone growth via suppression of osteoblastogenesis and increased apoptosis of osteoblasts/osteocytes causing osteonecrosis and decreased bone formation [14, 15]. Calcium loss through the kidneys and gut due to steroid treatment can also cause secondary hyperparathyroidism, further affecting bone growth. It is also thought that an increase in somatostatin tone, secondary to steroids, causes a decrease in growth hormone secretion [14,15,16].
In this contemporary cohort, cumulative steroid dose exposure was associated with lower height z-scores over time in children with nephrotic syndrome. This suggests that the effects of steroids may be affected by duration of administration. There is some prior literature consistent with our findings. One study evaluated the effects of steroids on growth in steroid-dependent nephrotic syndrome by assessing height over approximately 11.7 years. Investigators found that children with severe nephrotic syndrome had permanent growth retardation due to prolonged steroid treatment, but this study did not include information on other medications and their effects on growth [17]. An additional study evaluating children with steroid-dependent nephrotic syndrome showed that longer steroid treatment was associated with a higher risk of lower final height standard deviation score [18]. However, the evidence surrounding these findings has historically been inconsistent. A study of Indian and African children found no association between steroid duration and height with no change in mean height standard deviation score [19]. Another study on children with steroid-sensitive nephrotic syndrome showed no difference in height percentile between baseline and final visit [20]. A recent study in the UK showed that despite steroid use, there were statistically significant decreases in BMI and increases in height from baseline to the last recorded visit [6]. However, the cumulative steroid dose in this study was almost one-half of the cumulative steroid dose in our study. A number of studies have also shown contrasting findings of steroid doses affecting height and bone density [21]. While the evidence is contradictory, none of these prior studies have taken place in the context of nephrotic syndrome in recent years or factored in the use of more recent steroid-sparing treatments.
Our results demonstrated that tacrolimus and MMF exposure were not associated with growth in nephrotic syndrome. There was one prospective study that looked at final height in patients post-transplant after withdrawing from steroids while on various medications, including calcineurin inhibitors. In this study, the final height standard deviation was significantly improved with steroid withdrawal in children older than 5 years undergoing kidney transplantation [9].
The relationship between rituximab use and improvement of height z-score is consistent with other literature. While some studies have shown no significant effect of rituximab on height [21], others found a beneficial effect of rituximab on growth parameters. In other studies, rituximab use resulted in an improvement in height standard deviation over time [7, 23], improvement in height slope (velocity) over time [8], and improvement in BMI in children with nephrotic syndrome [7]. In some previous studies, presumably, this rituximab-associated height improvement was related to steroid minimization, but our study controlled for this and still showed the same effect. One possible explanation could have been a lack of compliance with steroids resulting in the necessity of rituximab, especially for steroid-dependent patients. One challenge in interpreting the effect of steroid-sparing agents is that they may be more likely to be utilized in children who are having more adverse effects from corticosteroids, including growth retardation.
When comparing children who were enrolled at the time of biopsy (prevalent group) vs. those with new diagnosis without any substantial immunosuppression (incident group), we found that those enrolled at the time of biopsy had a decrease in weight and BMI over time, while those with new diagnosis had an increase in weight and BMI over time. The increase in weight parameters in the new diagnosis group was likely due to the use of steroids as the initial treatment in this group. The decrease in weight parameters in the biopsy group could be representative of the fact that they had improvement in their disease process over time and/or the use of steroid-sparing agents. They were also generally older at the start of the study which could also explain the relative lack of weight gain and fluctuation in BMI that is more evident with younger, growing children.
There were some limitations of our study. One limitation was the incomplete data for Tanner staging to characterize pubertal status, which limited our ability to draw conclusions about pubertal status from the data. Data on steroid exposure prior to enrollment in NEPTUNE was also not collected for those enrolled at the time of kidney biopsy. This was also a limitation as it did not consider the cumulative effect of steroids from prior administration. This is a plausible explanation for the association of enrollment at the time of kidney biopsy with lower height z-score, as this cohort likely had steroid exposure prior to biopsy. In addition, the administration of steroids (low dose alternate day vs. short high dose) may affect growth parameters. As this was not considered, this is an additional limitation. The use of growth hormone was also not available, which would be important to obtain in future studies. The lack of this data is a major limitation in that this would directly affect outcomes. In future or prospective studies, this would be an important point to address. Additionally, although the median follow-up was close to 3 years, a longer follow-up period would be ideal to determine the long-term effect of medications on growth. As this was an observational study, we can only report on associations and acknowledge that there may have been confounding factors affecting growth that were not captured in this study. While we were able to identify cumulative steroid exposure between visits, we were unable to determine the exact frequency and timing of dose administration (i.e., 1 mg/kg daily vs. 2 mg/kg every other day). Also, data was only available at the time of enrollment and any medications prior to enrollment were not available. Furthermore, the data deficiency of specific types of nephrotic syndrome (frequent relapsing, steroid dependent, etc.) serves as a limitation when evaluating the effects of steroids on growth. Lastly, the study included children enrolled in a prospective observational study from academic centers, which may introduce bias and reduce the generalizability.
The study presented here is novel for many reasons. This study has the most extensive cohort to date. The multicenter design strengthens our conclusions, transcending the limitations typically associated with single-site studies. Additionally, the study encompasses North America, which makes results more generalizable due to the diverse patient population across the countries and multiple centers. A further distinguishing feature of this study is the longitudinal aspect, including analysis of a population with newly diagnosed nephrotic syndrome and a prevalent cohort with 3.5 years of follow-up, thereby providing a temporal advantage that offers insights into disease progression and treatment efficacy. The contemporary nature of this research is underscored by its inclusion of modern therapeutic agents such as rituximab, mycophenolate mofetil, and tacrolimus.
Overall, we conclude that height z-score was affected negatively by steroid use in children with nephrotic syndrome, positively affected by rituximab use, and did not have a significant association with other steroid-sparing agents. Other medications did not have a significant impact on growth velocity overall or when sub-dividing based on pubertal status. Our findings support that steroids are detrimental to the growth of children with nephrotic syndrome, and thus, steroid-sparing agents should be considered to minimize the exposure to steroids.
Data availability
The datasets generated during and/or analyzed during the current study are available from NEPTUNE, https://www.neptune-study.org/.
References
Kidney disease: improving global outcomes (KDIGO) glomerular diseases work group (2021) KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 100:S1–S276. https://doi.org/10.1016/j.kint.2021.05.021
Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, Zink A, Buttgereit F (2009) Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis 68:1119–1124
Lam CN, Arneil GC (1968) Long-term dwarfing effects of corticosteroid treatment for childhood nephrosis. Arch Dis Child 43:589–594
Mohan KR, Kanitkar M (2009) Growth in children with steroid sensitive nephrotic syndrome. Med J Armed Forces India 65:4–6
Kang HG, Ha IS, Cheong HI (2016) Recurrence and treatment after renal transplantation in children with FSGS. Biomed Res Int 2016:6832971
Göknar N, Webb H, Waters A, Tullus K (2022) Long-term obesity prevalence and linear growth in children with idiopathic nephrotic syndrome: is normal growth and weight control possible with steroid-sparing drugs and low-dose steroids for relapses? Pediatr Nephrol 37:1575–1584
Sinha R, Banerjee S, Mukherjee A, Akhtar S, Pradhan S (2021) Impact of rituximab on anthropometric indices among childhood steroid-dependent nephrotic syndromes. Arch Dis Child 106:283–285
Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasà M, Chianca A, Rubis N, Ene-Iordache B, Rudnicki M, Pollastro RM, Capasso G, Pisani A, Pennesi M, Emma F, Remuzzi G, Rituximab in nephrotic syndrome of steroid-dependent or frequently relapsing minimal change disease or focal segmental glomerulosclerosis (NEMO) study group (2014) rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 25:850–863
Motoyama O, Hasegawa A, Aikawa A, Shishido S, Honda M, Tsuzuki K, Kinukawa T, Hattori M, Ogawa O, Yanagihara T, Saito K, Takahashi K, Ohshima S (2012) Final height in a prospective trial of late steroid withdrawal after pediatric renal transplantation treated with cyclosporine and mizoribine. Pediatr Transplant 16:78–82
Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, Sampson MG, Kopp JB, Lemley KV, Nelson PJ, Lienczewski CC, Adler SG, Appel GB, Cattran DC, Choi MJ, Contreras G, Dell KM, Fervenza FC, Gibson KL, Greenbaum LA, Hernandez JD, Hewitt SM, Hingorani SR, Hladunewich M, Hogan MC, Hogan SL, Kaskel FJ, Lieske JC, Meyers KE, Nachman PH, Nast CC, Neu AM, Reich HN, Sedor JR, Sethna CB, Trachtman H, Tuttle KR, Zhdanova O, Zilleruelo GE, Kretzler M (2013) Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 83:749–756
Centers for Disease Control and Prevention (2023) healthy weight, nutrition, and physical activity. Children’s BMI Tool for Schools. https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/tool_for_schools.html
Wheeler MD (1991) Physical changes of puberty. Endocrinol Metab Clin North Am 20:1–14
Novello L, Speiser PW (2018) Premature adrenarche. Pediatr Ann 47:e7–e11
Strauss AJ, Su JT, Dalton VM, Gelber RD, Sallan SE, Silverman LB (2001) Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol 19:3066–3072
Rickers H, Deding A, Christiansen C, Rødbro P (1984) Mineral loss in cortical and trabecular bone during high-dose prednisone treatment. Calcif Tissue Int 36:269–273
Pantelakis SN, Sinaniotis CA, Sbirakis S, Ikkos D, Doxiadis SA (1972) Night and day growth hormone levels during treatment with corticosteroids and corticotrophin. Arch Dis Child 47:605–608
Emma F, Sesto A, Rizzoni G (2003) Long-term linear growth of children with severe steroid-responsive nephrotic syndrome. Pediatr Nephrol 18:783–788
Ribeiro D, Zawadynski S, Pittet LF, Chevalley T, Girardin E, Parvex P (2015) Effect of glucocorticoids on growth and bone mineral density in children with nephrotic syndrome. Eur J Pediatr 174:911–917
Adhikari M, Manikkam NE, Coovadia HM (1992) Effects of repeated courses of daily steroids and of persistent proteinuria on linear growth in children with nephrotic syndrome. Pediatr Nephrol 6:4–9
Donatti TL, Koch VH, Fujimura MD, Okay Y (2003) Growth in steroid-responsive nephrotic syndrome: a study of 85 pediatric patients. Pediatr Nephrol 18:789–795
Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA (2004) Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med 351:868–875
Topaloğlu R, Gülhan B, Çelegen K, İnözü M, Hayran M, Düzova A, Ozaltin F (2019) Rituximab for children with difficult-to-treat nephrotic syndrome: its effects on disease progression and growth. Front Pediatr 7:313
Sato M, Ito S, Ogura M, Kamei K (2014) Impact of rituximab on height and weight in children with refractory steroid-dependent nephrotic syndrome. Pediatr Nephrol 29:1373–1379
Acknowledgements
We would like to acknowledge the NEPTUNE sites.
Members of the Nephrotic Syndrome Study Network (NEPTUNE)
NEPTUNE enrolling centers
Cleveland Clinic, Cleveland, OH: K Dell*, J Sedor**, M Schachere#, J Negrey#
Children’s Hospital, Los Angeles, CA: K Lemley*, J Scott#
Children’s Mercy Hospital, Kansas City, MO: T Srivastava*, S Morrison#
Cohen Children’s Hospital, New Hyde Park, NY: C Sethna*, M Pfaiff#
Columbia University, New York, NY: P Canetta*, A Pradhan#
Emory University, Atlanta, GA: L Greenbaum*, C Wang**, E Yun#
Harbor-University of California Los Angeles Medical Center: S Adler*, J LaPage#
John H. Stroger Jr. Hospital of Cook County, Chicago, IL: A Athavale*, M Itteera
Johns Hopkins Medicine, Baltimore, MD: M Atkinson*, T Dell#
Mayo Clinic, Rochester, MN: F Fervenza*, M Hogan**, J Lieske*, G Hill#
Montefiore Medical Center, Bronx, NY: F Kaskel*, M Ross*, P Flynn#
NIDDK Intramural, Bethesda MD: J Kopp*
New York University Medical Center, New York, NY: L Malaga-Dieguez*, O Zhdanova**, F Modersitzki#, L Pehrson#
Stanford University, Stanford, CA: R Lafayette*, B Yeung#
Temple University, Philadelphia, PA: I Lee*, S Quinn-Boyle#
University Health Network Toronto: H Reich *, M Hladunewich**, P Ling#, M Romano#
University of Miami, Miami, FL: A Fornoni*, C Bidot#
University of Michigan, Ann Arbor, MI: M Kretzler*, D Gipson*, A Williams#, C Klida#
University of North Carolina, Chapel Hill, NC: V Derebail*, K Gibson*, A Froment#, F Ochoa-Toro#
University of Pennsylvania, Philadelphia, PA: L Holzman*, K Meyers**, K Kallem#, A Swenson#
University of Texas Southwestern, Dallas, TX: K Sambandam*, K Aleman#, M Rogers#
University of Washington, Seattle, WA: A Jefferson*, S Hingorani**, K Tuttle**§, L Manahan#, E Pao#, A Cooper#§
Wake Forest University Baptist Health, Winston-Salem, NC: JJ Lin*, Stefanie Baker#
Data analysis and coordinating center: M Kretzler*, L Barisoni**, C Gadegbeku**, B Gillespie**, D Gipson**, L Holzman**, L Mariani**, M Sampson**, J Sedor**, J Zee**, G Alter, H Desmond, S Eddy, D Fermin, M Larkina, S Li, S Li, CC Lienczewski, T Mainieri, R Scherr, A Smith, A Szymanski, A Williams
Digital pathology committee: Carmen Avila-Casado (University Health Network, Toronto), Serena Bagnasco (Johns Hopkins University), Joseph Gaut (Washington University in St Louis), Stephen Hewitt (National Cancer Institute), Jeff Hodgin (University of Michigan), Kevin Lemley (Children’s Hospital of Los Angeles), Laura Mariani (University of Michigan), Matthew Palmer (University of Pennsylvania), Avi Rosenberg (Johns Hopkins University), Virginie Royal (University of Montreal), David Thomas (University of Miami), Jarcy Zee (University of Pennsylvania) Co-Chairs: Laura Barisoni (Duke University) and Cynthia Nast (Cedar Sinai)
*Principal Investigator; **Co-investigator; #Study Coordinator
§Providence Medical Research Center, Spokane, WA
Funding
The Nephrotic Syndrome Study Network (NEPTUNE) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). NEPTUNE is funded under grant number U54DK083912 as a collaboration between NCATS and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Additional funding and/or programmatic support is provided by the University of Michigan, NephCure Kidney International, and the Halpin Foundation. RDCRN consortia are supported by the RDCRN Data Management and Coordinating Center (DMCC), funded by NCATS and the National Institute of Neurological Disorders and Stroke (NINDS) under U2CTR002818. Dr. Sethna is supported by NIH/NHLBI 1R01HL162912-01A1 and NIH/NIDDK R01DK131091. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Consortia
Contributions
AM performed the statistical analyses and primary draft of the manuscript. CS performed statistical analysis and supervised the project. All other authors contributed to the concept and design of the study and writing/editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maniar, A., Gipson, D.S., Brady, T. et al. Growth in children with nephrotic syndrome: a post hoc analysis of the NEPTUNE study. Pediatr Nephrol 39, 2691–2701 (2024). https://doi.org/10.1007/s00467-024-06375-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-024-06375-9