Abstract
Background
This systematic review and meta-analysis assessed the effectiveness of robotic surgery compared to laparoscopy or open surgery for inguinal (IHR) and ventral (VHR) hernia repair.
Methods
PubMed and EMBASE were searched up to July 2022. Meta-analyses were performed for postoperative complications, surgical site infections (SSI), seroma/hematoma, hernia recurrence, operating time (OT), intraoperative blood loss, intraoperative bowel injury, conversion to open surgery, length of stay (LOS), mortality, reoperation rate, readmission rate, use of opioids, time to return to work and time to return to normal activities.
Results
Overall, 64 studies were selected and 58 were used for pooled data analyses: 35 studies (227 242 patients) deal with IHR and 32 (158 384 patients) with VHR. Robotic IHR was associated with lower hernia recurrence (OR 0.54; 95%CI 0.29, 0.99; I2: 0%) compared to laparoscopic IHR, and lower use of opioids compared to open IHR (OR 0.46; 95%CI 0.25, 0.84; I2: 55.8%). Robotic VHR was associated with lower bowel injuries (OR 0.59; 95%CI 0.42, 0.85; I2: 0%) and less conversions to open surgery (OR 0.51; 95%CI 0.43, 0.60; I2: 0%) compared to laparoscopy. Compared to open surgery, robotic VHR was associated with lower postoperative complications (OR 0.61; 95%CI 0.39, 0.96; I2: 68%), less SSI (OR 0.47; 95%CI 0.31, 0.72; I2: 0%), less intraoperative blood loss (− 95 mL), shorter LOS (− 3.4 day), and less hospital readmissions (OR 0.66; 95%CI 0.44, 0.99; I2: 24.7%). However, both robotic IHR and VHR were associated with significantly longer OT compared to laparoscopy and open surgery.
Conclusion
These results support robotic surgery as a safe, effective, and viable alternative for IHR and VHR as it can brings several intraoperative and postoperative advantages over laparoscopy and open surgery.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Inguinal (IHR) and ventral hernia repair (VHR) are common surgical procedures in adults [1, 2]. Traditionally, they are approached by open surgery, although the use of minimally invasive surgical (MIS) techniques has grown at an exponential rate over the last decades [3, 4]. The advent of robotic surgery further increased the rate of abdominal hernia repairs carried out with MIS [5,6,7,8]. In particular, the use of robotic systems brought several technical improvements, such as enhanced magnification and view, dexterity, and maneuverability, which has been seen as clear advantages over laparoscopy [6, 9,10,11,12,13]. Nevertheless, the clinical efficacy of robotic hernia repair over laparoscopic or open surgery is still matter of debate [14,15,16,17,18,19]. The present systematic review and meta-analysis was designed to provide a critical appraisal of the literature summarizing the outcomes of robotic IHR and VHR in order to answer to the following focus question: what is the effectiveness of robotic surgery compared to conventional laparoscopy or open surgery for IHR and VHR in terms of postoperative complications and hernia recurrence rate?
Materials and methods
Study design and inclusion criteria
The study protocol was registered in the PROSPERO database (provisional registration number: CRD42023413043) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statements checklist [20]. The literature search and study selection criteria were defined according to the PICOS framework:
Patients Adult patients with inguinal or ventral hernia candidate for surgical hernia repair.
Intervention Robotic abdominal wall repair. All types of hernia repair procedure were considered.
Comparison Laparoscopic and/or laparotomic abdominal wall repair.
Outcomes
-
Primary outcome: postoperative complications (at any time point) expressed as overall complication rate or by type of complication, including hernia recurrence.
-
Secondary outcomes: intraoperative variables (e.g., operating time, intraoperative blood loss, intraoperative bowel injury, conversion to open surgery), and postoperative outcomes (e.g., mortality, reoperation rate, readmission rate, postoperative use of opioids, LOS, time until return to work, and time until resume of normal activities).
Study design Any type of analytic studies (i.e., randomized and non-randomized controlled trials, prospective and retrospective studies).
Studies were included irrespective of the surgical technique (e.g., extraperitoneal or intraperitoneal IHR). Narrative and systematic reviews, meta-analysis, non-comparative studies, case reports, notes, commentaries, letters, editorials, and conference abstracts were excluded. The research was limited to human studies written in English.
Literature search strategy
A literature search was performed screening MEDLINE and EMBASE from inception to July 25, 2022. Specific research equations were designed for each database, using the following keywords and/or MeSH terms: Inguinal, Abdominal, Ventral, Incisional, Abdominal wall, Hernia, Herniorrhaphy, Hernia repair, Abdominoplasty, Wall reconstruction, Robotic surgery/robotic/robotic assisted, Laparoscopy/laparoscopic, Open surgery/laparotomy. The research equations are reported in Supplementary Table S1. In addition, the reference lists of eligible studies and pertinent review articles were crosschecked to identify potential additional records.
Study selection and risk of bias assessment
The literature search and selection were performed by two independent reviewers (CAS and NdeA). All records from the merged searches and cross-referencing were analyzed for relevance on title and abstract. To enhance sensitivity, only the records excluded by both reviewers were removed. The two reviewers further performed an independent full-text analysis of pre-selected articles. Any disagreement on study inclusion or exclusion was solved by discussion of a tiebreaker (PP). Both reviewers independently assessed the risk of bias using appropriate tools according to the study design. The Newcastle–Ottawa scale was used for case–control and cohort studies [21] and the Cochrane risk-of-bias tool (ROB-II) for randomized controlled trials [22, 23].
Data extraction and analysis
Both reviewers independently extracted and collected in a predefined excel database the following data: authors, year of publication, journal, study timeframe, design and population, patients’ demographics, length of follow-up, surgical procedure details, intraoperative and postoperative (short and long-term) outcomes, impact on patient quality of life and surgery-related costs. Data extracted from the included studies were processed for the qualitative and quantitative analyses. Cohen’s Kappa statistic was used to assess the inter-reviewers agreement during the study selection process [24]. The feasibility analysis established that, based on the final data extraction database, it was possible to conduct meta-analyses on the following outcomes: overall postoperative complications, SSI, postoperative seroma or hematoma rate, hernia recurrence, operating time, LOS, intraoperative blood loss, conversion to open surgery, mortality, reoperation rate, readmission rate, intraoperative bowel injury, postoperative use of opioids, time to return to work, and time to return to normal activities. Four different sets of analyses were conducted according to the type of hernia repair and the surgical approach: (1) Robotic vs. Laparoscopic IHR; (2) Robotic vs. Open IHR; (3) Robotic vs. Laparoscopic VHR; (4) Robotic vs. Open VHR.
Individual study results for each outcome were pooled using fixed or random-effects models according to the clinical heterogeneity expected among the selected studies. According to the standard meta-analytical approach, continuous outcomes were analyzed as Weighted Mean Differences (WMD), while dichotomous outcomes were analyzed as Odds Ratios (OR) and Risk Differences (RD). OR is the most commonly used measure in biostatistics, but it cannot include results from studies in which no event is observed, therefore leaving out part of the evidence. For this reason, when OR measure excluded many studies due to the absence of events in dichotomous outcomes, the pooled results were reported as RD. For dichotomous outcomes, if only percentages were available, the corresponding number of patients was calculated based on these percentages and the total sample size in each group. In studies reporting data separately for unilateral and bilateral hernia or reporting LOS separately for inpatients and outpatients, overall results were derived when the numbers of patients in each group were available (if not, the data were excluded). For the 30-day outcomes (mortality, reoperation, readmission), if results were available at a later date and no event was reported in both groups (i.e., no death at all at 90 days), the results at 30-day were imputed based on the 90-day rate (0%). For the continuous outcomes, the fixed-effects models were run using the inverse variance method and the random-effects ones using the DerSimonian and Laird method, with the estimate of heterogeneity being taken from the inverse variance model. For the dichotomous outcomes, the fixed-effects models were run using the Mantel–Haenszel method and the random-effects ones using the DerSimonian and Laird method, with the estimate of heterogeneity being taken from the Mantel–Haenszel model. Heterogeneity was assessed by the Cochran’s Q test and the I2 statistics. I2 statistic was used to quantify heterogeneity, with I2 values of 25%, 50%, and 75% being considered as low, moderate, and high heterogeneity, respectively [22, 25]. The meta-analysis was performed using STATA version 14.2 (StataCorp, College Station, Texas, USA).
Results
Literature search and selection
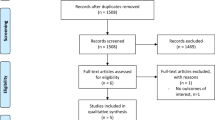
After the comprehensive stepwise literature research, 468 articles were identified, of which 385 were rejected based on the title and abstract evaluation. The remaining 83 articles underwent the full-text analysis; of these, 24 were excluded because non pertinent to the research question. No additional study was identified through cross-check of the reference lists or manual search. Finally, 64 studies were selected for the qualitative synthesis of the literature, and 58 (90.6%) were used for pooled data analyses (Fig. 1). Data on IHR and VHR were reported by 35 [4, 6, 11,12,13, 18, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] and 32 studies [7, 8, 10, 16,17,18, 36, 42, 43, 45, 56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77], respectively (3 studies concerned both IHR and VHR). The inter-reviewer percentage of agreement was 94% with a Cohen’s Kappa coefficient of 0.75, demonstrating a substantial agreement.
PRISMA Diagram. The flowchart shows the literature search and study selection process according to the PRISMA guidelines [20]
Study characteristics
The included studies were published between September 2014 and July 2022. The characteristics of the included studies are summarized in Table 1. Overall, 227 242 patients underwent IHR and 158 384 VHR. IHR was performed by robotic, laparoscopic, and open approach in 22 308 (9.8%), 46 139 (20.3%) and 158 795 (69.9%) patients, respectively. VHR was carried out by robotic, laparoscopic, and open technique in 19 225 (12.1%), 90 300 (57%) and 48 859 (30.9%) patients, respectively. Most of the studies (85.9%) have a retrospective design, of which 13 studies (23.6%) used a propensity score matching (PSM) analysis. Only 7 RCTs (10.9%) and 2 (3.1%) prospective studies were found.
The demographic and clinical characteristics of the studied patient populations are reported in Table 2. Focusing on IHR, 4 (11.4%) studies reported a higher body mass index (BMI) in the robotic group [4, 6, 42, 54] and 1 (2.8%) in the laparoscopic group [40], whereas bilateral procedures were most commonly performed in the robotic group in 3 (8.6%) studies [50, 54, 55] and in the laparoscopic group in 2 (5.7%) studies [52, 53]. Focusing on VHR, a significant between-group difference in terms of BMI, recurrent hernias, and percentage of transversus abdominis release was reported by 4 (12.5%), 2 (6.25%) and 4 (12.5%) studies, respectively. In particular, all 4 (12.5%) studies [10, 18, 36, 69] reported a higher BMI, and 3 (9.4%) studies [58, 64, 75] highlighted a higher percentage of transversus abdominis release in the robotic group.
Inguinal hernia repair
Results from pooled data analyses for the comparisons between robotic vs. laparoscopic hernia repair and between robotic vs. open hernia repair are reported in Table 3 and displayed in Fig. 2 and as Supplementary Material.
Forest plots for IHR. A Overall complications for robotic vs. laparoscopic IHR. B Overall complications for robotic vs. open IHR. C Hernia recurrence for robotic vs. laparoscopic IHR. D Hernia recurrence for robotic vs. open IHR. E Operative time for robotic vs. laparoscopic IHR. F Operative time for robotic vs. open IHR
Seventeen studies [4, 6, 11, 12, 27, 28, 35,36,37,38, 40, 42, 48, 49, 51, 52, 55] were included in the meta-analysis for overall postoperative complication rate, of which 14 (82.3%) compared robotic vs. laparoscopic IHR and 9 (52.9%) robotic vs. open IHR. Eleven (64.7%) studies reported SSI rate comparing robotic vs. laparoscopic IHR [4, 11, 35, 40, 42, 44, 46, 48, 49, 51, 52] and 8 (47%) comparing open vs. open IHR [4, 11, 12, 35, 37, 42, 52, 54]. The incidence of hematoma and/or seroma was analyzed by 11 (78.6%) [6, 26,27,28, 40, 44, 46, 48, 49, 51, 52] and 4 (28.6%) [12, 37, 45, 52] studies in the corresponding aforementioned groups. Pooled data analyses showed no significant difference in terms of overall postoperative complications, SSI and hematoma/seroma occurrence between robotic and laparoscopic or open surgery for IHR (Table 3).
Twelve studies [6, 11, 18, 26, 34, 38, 39, 44, 46, 48, 52, 54] reported the rate of hernia recurrence after IHR; of these, 11 (91.7%) concerned robotic vs. laparoscopic surgery and 5 (41.7%) robotic vs. open surgery. Pooled data analyses showed statistically significant reduced risk of hernia recurrence in the robotic surgery group compared to the laparoscopic one (OR 0.54), whereas no significant differences were noted when compared to open IHR (Fig. 2).
Operative time was significantly longer for robotic IHR compared to laparoscopy (WMD: 33.1 min) and open surgery (WMD: 41.3 min). Compared to laparoscopy, robotic IHR was associated with a higher 30-day reoperation rate (OR 4.85, Supplementary fig. S12). No statistically significant difference was noted for conversions to open surgery, LOS, 30-day hospital readmission rate, and postoperative use of opioids between robotic vs. laparoscopic IHR.
Compared to open surgery, robotic IHR was associated with a significant lower use of postoperative opioids (OR 0.46).
There were insufficient data to perform a meta-analysis on intraoperative blood loss, intraoperative bowel injuries, 30-day mortality, time to return to work, and time to return to normal activities.
Ventral hernia repair
Pooled data analyses of the comparisons between robotic vs. laparoscopic VHR and between robotic vs. open VHR are reported in Table 4 and displayed in Fig. 3 and as Supplementary Material.
Forest plots for VHR. A Overall complications for robotic vs. laparoscopic VHR. B Overall complications for robotic vs. open VHR. C Hernia recurrence for robotic vs. laparoscopic VHR. D Hernia recurrence for robotic vs. open VHR. E Operative time for robotic vs. laparoscopic VHR. F Operative time for robotic vs. open VHR
Data on overall complications after VHR were reported by 17 studies [7, 8, 10, 16, 17, 36, 45, 56, 58,59,60, 62, 67, 69,70,71,72], of which 11 (64.7%) compared robotic vs. laparoscopic surgery and 9 (52.9%) robotic vs. open surgery. No significant differences were found for overall complication, SSI, and seroma/hematoma occurrence between robotic and laparoscopic VHR (Table 4). Conversely, when compared to open surgery, robotic VHR was associated with fewer overall complications (OR 0.61) and less SSI (OR 0.47). No significant differences were found between robotic and laparoscopic or open VHR for hernia recurrence. Operative time was significantly longer for robotic VHR, compared to both laparoscopy (WMD: 67.3 min) and open surgery (WMD: 55.5 min).
Compared to laparoscopy, robotic VHR was associated with lower intraoperative bowel injuries (OR 0.59) and less conversions to open surgery (OR 0.51). Furthermore, when compared to open surgery, robotic VHR was associated with significantly reduced intraoperative blood loss (WMD: − 95.3 mL).
No significant difference was noted in terms of LOS, 30-day reoperation rate, 30-day hospital readmission rate, postoperative use of opioids, time to return to normal activities, and time to return to work between robotic and laparoscopic VHR (Table 4). Conversely, LOS (WMD: − 3.4 days) and 30-days readmission rate (OR 0.66) resulted significantly lower in robotic VHR compared to open VHR.
Costs
Fifteen studies [11, 18, 26, 29, 32, 33, 35, 40, 41, 47,48,49, 51, 60, 62, 64, 69, 70] reported data on costs of robotic surgery compared to laparoscopic or open approaches. A descriptive analysis was conducted to assess the financial burden of robotic surgery for IHR and VHR, considering both total hospital costs and fixed surgery-related costs per patient (Table 5). Almost all studies reported higher fixed and total hospital costs for robotic surgery compared to both laparoscopic and open approaches. Only Zayan et al. [18], who analyzed the costs of robotic surgery without distinguishing between IHR and VHR, showed that robotic abdominal wall repair was associated with lower total hospital costs (7832$ vs. 8605$) but higher fixed costs (5017$ vs. 4638$) than laparoscopy. Conversely, Petro et al. [69] reported lower ratio of fixed costs (0.97 vs. 1.00) but higher ratio of total hospital costs (1.13 vs. 0.97) for robotic VHR compared to laparoscopy.
Risk of bias assessment
Based on the NOS, only 21 (36.8%) studies were judged at low risk of bias [6, 7, 10, 13, 16, 26, 27, 30,31,32, 37, 38, 43,44,45, 49, 55, 59, 65, 77] (Table 1). Concerning the RCTs, 2 were judged at high risk of bias [39, 68], 4 with some concern [40, 66, 67, 69], and 1 at low risk of bias [70] (Fig. 4).
Discussion
The present systematic review identified 64 articles reporting on robotic IHR and VHR and comparing it to laparoscopy of open surgery. Pooled data analyses show lower hernia recurrence rate for robotic IHR over laparoscopic IHR and lower use of opioids for robotic IHR over open IHR. However, robotic IHR was associated with significantly longer OT compared to both laparoscopy and open surgery. Despite longer OT also observed for robotic VHR, the robotic approach was associated with lower bowel injuries and less conversions to open surgery compared to laparoscopy, and lower overall complication rate, less SSI, reduced intraoperative blood loss, shorter LOS, and lower 30-day readmission rate compared to open surgery. Globally, these results support the role of robotic surgery for abdominal wall repair and indicate that it can brings several intraoperative and postoperative advantages over laparoscopy and open surgery.
During the last decades, the use of robotic technology has significantly risen across various surgical disciplines, progressively entering the surgical thinking. Notably, the magnitude of the increase for robotic IHR has peaked 41-fold higher between 2012 and 2018 [3]. This trend was mirrored by a concomitant decrease in the use of open and laparoscopic surgery [3].
Focusing on studies dealing with IHR, no difference was found in terms of overall postoperative complications (including SSI and seroma/hematoma), between robotic and laparoscopic or open approaches. However, robotic IHR was associated with 46% less odds of hernia recurrence compared to laparoscopic IHR. It must be noted that the hernia recurrence rate was evaluated at different time intervals in the nine studies included for the meta-analysis, spanning from 12 [6, 34, 46] to 24 months [18, 38, 39] and more than 24 months [26, 44, 52]. Despite this, the statistical heterogeneity was nil (0%), and pooled data were derived from a large number of patients in both groups. Consistently, robotic IHR was associated with approximately 33 min and 41 min longer OT than laparoscopy and open surgery, respectively. Conversely, the 30-day reoperation rate was significantly higher for robotic IHR compared to laparoscopy. Analyzing the 30-day reoperation rate, only two studies were included in the meta-analysis. In the study by Khoraki et al. [51], 3 patients (6.7%) required reoperation in the robotic IHR group due to port-site hernia, internal hernia, and hemoperitoneum, while no event occurred in the laparoscopic group. Holleran et al. [4] reported 172 (2.84%), 148 (0.82%) and 1033 (1.02%) unplanned reoperations for robotic, laparoscopic, and open IHR, respectively, without specifying the reasons for the reintervention. The authors reported that the use of robotic platform greatly increased over the study period whereas the unplanned reoperation rate decreased from 12.5% in 2008 to 1.83% in 2019 in the IHR cohort [4]. This could probably reflect an increased surgeon’s experience with the robotic platform over the study timeframe and explain the worse outcome during the early stages of the learning curve.
Previous meta-analyses reported contrasting results about the benefits of robotic surgery for IHR. In 2019, Henriksen et al. [78] analyzed 5 retrospective studies and showed less postoperative complications after robot-assisted IHR rather than open IHR, but no differences were found compared to laparoscopic IHR. A Bayesian network meta-analysis comparing open Lichtenstein, laparoscopic trans-abdominal pre-peritoneal (TAPP), laparoscopic totally extra peritoneal (TEP), and robotic TAPP techniques showed comparable short-term outcomes for primary unilateral IHR [79]. Solaini et al. [80] and Zhao et al. [81] reported similar postoperative complications between robotic and laparoscopic surgery, whereas Qabbani et al. [82] showed significantly less complications in robotic IHR than laparoscopic IHR, as well as less hospital readmissions when compared to open IHR. A meta-analysis by Tai et al. [83] reported less hernia recurrences with fascia defect closure than with non-closure in robotic and laparoscopic direct IHR, regardless of the surgical technique. This may be potentially linked to the enhanced anatomical view, increased precision, and improved surgical dexterity of the robotic system, which surely represent important technical advantages in the complex clinical scenario of abdominal wall repair. Overall, the qualitative and quantitative syntheses of the literature demonstrate that robotic IHR is safe, feasible, and effective [84], even in an early phase of learning curve [46], with equivalent clinical effectiveness in terms of postoperative complications compared to laparoscopic and open approaches [78,79,80,81]. Nowadays, open IHR represents one of the most performed procedures in general surgery. Although there was no significant difference between open and robotic IHR, except for a longer OT and lower opioid use in the robotic group, the greater financial costs associated with robotic IHR over open IHR represent a major barrier to its widespread adoption. The choice of the surgical technique should be made on a case-by-case basis, taking into account the surgeon’s and patient’s preference, the patient’s characteristics, and the national/hospital healthcare system regulations.
Focusing on VHR, pooled data analyses indicate that robotic VHT is associated with a decreased rate of conversion to open surgery and lower intraoperative bowel injuries compared to laparoscopy, but no difference was found in terms of postoperative complications. Thus, the advantages of robotic surgery may be greater intraoperatively than on the postoperative outcomes. These findings are in agreement with those reported by Mohan et al. [85], who found a reduction in conversions to open surgery, similar postoperative complications, and equivalent hernia recurrence between robotic and laparoscopic VHR. Conversely, according to Goettman et al. [86], robotic technology allows to optimize the overlap between the mesh and the ventral hernia defect, conceivably reducing the risk of hernia recurrence compared to both laparoscopic and open VHR. Similarly, Dixit et al. [87] reported a 4% reduction of hernia recurrence after robotic procedure compared to laparoscopy. Nevertheless, previous meta-analyses did not consider data from the most recent RCTs [66,67,68, 70] published since their publication.
When compared to open VHR, robotic VHR is associated with 39% less odds of postoperative complications, 53% less SSI, less intraoperative blood loss (− 95 mL), 3.4 day shorter LOS and 34% less odds of hospital readmissions, supporting the clear advantages of performing VHR by a robotic approach. These results are in accordance with those reported by Bracale et al. [88] concerning overall complications, LOS, and operative time, despite their analysis was focused only on transversus abdominis release. Similarly, the study by Goettman et al. [86] showed less postoperative SSI occurrence for robotics. The decreasing incidence of overall postoperative complications and SSIs, the shorter LOS, the reduced blood loss, and the lower readmission rate after robotic VHR may be attributed to MIS, which reduces tissue trauma and promotes faster recovery. Indeed, the lack of significant differences between robotic and laparoscopic VHR for most of the aforementioned outcomes might be explained by the MIS nature of these two techniques. However, the lower need for conversion to open surgery and the decreased blood loss associated with robotic-assisted procedures, probably highlights once again the several technical drawbacks of laparoscopy. No statistically significant difference in terms of opioids use, time to return to work or time to return to normal activities emerged from the present pooled analysis, regardless of the type of repair and the surgical approach.
Patients’ preferences and perspectives on the diverse aspects of the health status, such as pain, mesh-related symptoms, sexual dysfunction, health-related quality of life and physical function [89], represent Patient Reported Outcome Measures (PROMs) that are of upmost importance in the evaluation of low-risk elective surgical procedures, such as IHR and VHR [90, 91]. A recent meta-analysis based on 8 studies and focused on PROMs, showed that time to return to activities of daily living and time to return to work were significantly shorter for the robotic group than the laparoscopic one, whereas no difference were found concerning postoperative pain, quality of life, body image, and patient satisfaction [87]. The present results confirmed these findings and support the use of PROMS to evaluate laparoscopic and robotic hernia repair. Nowadays, the selection of the most appropriate approach for hernia repair relies on the surgeon's expertise and caseload in MIS, but it should also be tailored on the patient’s characteristics and medical history. Further evidence is awaited to elucidate the criteria upon which define personalized surgery in order to achieve the maximum efficiency from robotic, laparoscopic, and open approach in the field of abdominal wall surgery.
For both IHR and VHR, robotic surgery was associated with significantly longer OT than laparoscopy and open surgery. This result was expected and consistently reported. Indeed, robotic docking and use is likely to prolong the OT, irrespective to the type of procedure performed and particularly during the learning curve of the surgical team. Prolonged OT has been seen as one of the main drawbacks of robotic surgery, together with the increased costs. The impact of surgery duration is obviously important from a clinical and practical perspective, potentially leading to medical risks and generating additional costs. However, the impact of OT was not systematically assessed in the selected studies and cannot be deemed from the present data. Similarly, the impact of complication rate, readmission and reoperation need on healthcare costs have not been estimated. Thus, it was not possible to further evaluate this aspect and to provide a cost-effectiveness analysis considering the differences across centers, healthcare systems, and countries. This represents a limitation of the current literature and the present systematic review. Moreover, findings must be interpreted bearing in mind the clinical and statistically heterogeneity observed among the included studies. In some studies, there were significant imbalance between the groups concerning demographic and clinical characteristics (e.g., BMI) that can represent selection criteria for the surgical approach, which was not randomized. Moreover, intraoperative and postoperative outcomes on IHR were not reported separately for unilateral and bilateral procedures, thus the pooled analysis was not conditioned depending on the type of procedure (unilateral or bilateral repair). Several other factors may impact on the pooled results, namely the type of surgical technique (i.e. extraperitoneal or intraperitoneal IHR, transversus abdominis release, intraperitoneal onlay or retromuscular mesh placement), the type of mesh used, the closure versus non-closure of the fascia defect, and the mesh fixation technique. Finally, a high variability of complications detection metrics was observed among the included studies (e.g. post-discharge follow-up as clinical examination or telephone calls). As suggested by Bittner JG, there is a compelling need for standardized definitions and uniform reporting metrics allowing to unequivocally analyze and understand the burden of hernia-specific outcomes across different studies and different healthcare systems [92].
In conclusion, the present systematic review and meta-analysis supports the use of robotic surgery for abdominal wall hernia repair. Pooled data analyses show improved outcomes for robotic surgery over laparoscopy and open surgery, particularly for VHR. Overall, these results, based on 64 studies, support robotic surgery as a safe, effective, and viable alternative to traditional open and laparoscopic surgery for IHR and VHR, and they may contribute to dismiss the residual skepticism and increase the interest towards this minimally-invasive surgical technique.
References
Beadles CA, Meagher AD, Charles AG (2015) Trends in emergent hernia repair in the United States. JAMA Surg 150:194–200. https://doi.org/10.1001/jamasurg.2014.1242
Matthews RD, Neumayer L (2008) Inguinal hernia in the 21st century: an evidence-based review. Curr Probl Surg 45:261–312. https://doi.org/10.1067/j.cpsurg.2008.01.002
Sheetz KH, Claflin J, Dimick JB (2020) Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open 3:e1918911. https://doi.org/10.1001/jamanetworkopen.2019.18911
Holleran TJ, Napolitano MA, Sparks AD, Duncan JE, Garrett M, Brody FJ (2022) Trends and outcomes of open, laparoscopic, and robotic inguinal hernia repair in the veterans affairs system. Hernia 26:889–899. https://doi.org/10.1007/s10029-021-02419-3
Sanchez A, Rodriguez O, Jara G, Sanchez R, Vegas L, Rosciano J, Estrada L (2018) Robot-assisted surgery and incisional hernia: a comparative study of ergonomics in a training model. J Robot Surg 12:523–527. https://doi.org/10.1007/s11701-017-0777-y
Kudsi OY, McCarty JC, Paluvoi N, Mabardy AS (2017) Transition from laparoscopic totally extraperitoneal inguinal hernia repair to robotic transabdominal preperitoneal inguinal hernia repair: a retrospective review of a single surgeon’s experience. World J Surg 41:2251–2257. https://doi.org/10.1007/s00268-017-3998-3
Prabhu AS, Dickens EO, Copper CM, Mann JW, Yunis JP, Phillips S, Huang LC, Poulose BK, Rosen MJ (2017) Laparoscopic vs robotic intraperitoneal mesh repair for incisional hernia: an Americas Hernia Society Quality Collaborative analysis. J Am Coll Surg 225:285–293. https://doi.org/10.1016/j.jamcollsurg.2017.04.011
Bittner JGT, Alrefai S, Vy M, Mabe M, Del Prado PAR, Clingempeel NL (2018) Comparative analysis of open and robotic transversus abdominis release for ventral hernia repair. Surg Endosc 32:727–734. https://doi.org/10.1007/s00464-017-5729-0
Ye L, Childers CP, de Virgilio M, Shenoy R, Mederos MA, Mak SS, Begashaw MM, Booth MS, Shekelle PG, Wilson M et al (2021) Clinical outcomes and cost of robotic ventral hernia repair: systematic review. BJS Open 5:zrab098. https://doi.org/10.1093/bjsopen/zrab098
Collins CE, Renshaw S, Huang LC, Phillips S, Gure TR, Poulose B (2021) Robotic vs open approach for older adults undergoing retromuscular ventral hernia repair. Ann Surg. https://doi.org/10.1097/SLA.0000000000005260
Charles EJ, Mehaffey JH, Tache-Leon CA, Hallowell PT, Sawyer RG, Yang Z (2018) Inguinal hernia repair: is there a benefit to using the robot? Surg Endosc 32:2131–2136. https://doi.org/10.1007/s00464-017-5911-4
Kolachalam R, Dickens E, D’Amico L, Richardson C, Rabaza J, Gamagami R, Gonzalez A (2018) Early outcomes of robotic-assisted inguinal hernia repair in obese patients: a multi-institutional, retrospective study. Surg Endosc 32:229–235. https://doi.org/10.1007/s00464-017-5665-z
Bittner Iv JG, Cesnik LW, Kirwan T, Wolf L, Guo D (2019) Correction to: Patient perceptions of acute pain and activity disruption following inguinal hernia repair: a propensity-matched comparison of robotic-assisted, laparoscopic, and open approaches. J Robot Surg 13:191. https://doi.org/10.1007/s11701-018-0831-4
Telem DA (2018) Is robotic surgery the future for abdominal wall hernia repair? Not so fast. Ann Surg 267:218–219. https://doi.org/10.1097/SLA.0000000000002336
Belyansky I, Weltz AS, Sibia US, Turcotte JJ, Taylor H, Zahiri HR, Turner TR, Park A (2018) The trend toward minimally invasive complex abdominal wall reconstruction: is it worth it? Surg Endosc 32:1701–1707. https://doi.org/10.1007/s00464-017-5850-0
Martin-Del-Campo LA, Weltz AS, Belyansky I, Novitsky YW (2018) Comparative analysis of perioperative outcomes of robotic versus open transversus abdominis release. Surg Endosc 32:840–845. https://doi.org/10.1007/s00464-017-5752-1
Altieri MS, Yang J, Xu J, Talamini M, Pryor A, Telem DA (2018) Outcomes after robotic ventral hernia repair: a study of 21,565 patients in the state of New York. Am Surg 84:902–908
Zayan NE, Meara MP, Schwartz JS, Narula VK (2019) A direct comparison of robotic and laparoscopic hernia repair: patient-reported outcomes and cost analysis. Hernia 23:1115–1121. https://doi.org/10.1007/s10029-019-01943-7
Sheetz KH, Dimick JB (2019) Is it time for safeguards in the adoption of robotic surgery? JAMA 321:1971–1972. https://doi.org/10.1001/jama.2019.3736
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605. https://doi.org/10.1007/s10654-010-9491-z
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Fam Med 37:360–363
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Aghayeva A, Benlice C, Bilgin IA, Bengur FB, Bas M, Kirbiyik E, Aytac E, Baca B (2020) Laparoscopic totally extraperitoneal vs robotic transabdominal preperitoneal inguinal hernia repair: assessment of short- and long-term outcomes. Int J Med Robot 16:e2111. https://doi.org/10.1002/rcs.2111
Muysoms F, Van Cleven S, Kyle-Leinhase I, Ballecer C, Ramaswamy A (2018) Robotic-assisted laparoscopic groin hernia repair: observational case-control study on the operative time during the learning curve. Surg Endosc 32:4850–4859. https://doi.org/10.1007/s00464-018-6236-7
Gerdes S, Burger R, Liesch G, Freitag B, Serra M, Vonlanthen R, Bueter M, Thalheimer A (2022) Results of robotic TAPP and conventional laparoscopic TAPP in an outpatient setting: a cohort study in Switzerland. Langenbecks Arch Surg 407:2563–2567. https://doi.org/10.1007/s00423-022-02552-2
Quilici PJ, Wolberg H, McConnell N (2022) Operating costs, fiscal impact, value analysis and guidance for the routine use of robotic technology in abdominal surgical procedures. Surg Endosc 36:1433–1443. https://doi.org/10.1007/s00464-021-08428-8
Tatarian T, Nie L, McPartland C, Brown AM, Yang J, Altieri MS, Spaniolas K, Docimo S, Pryor AD (2021) Comparative perioperative and 5-year outcomes of robotic and laparoscopic or open inguinal hernia repair: a study of 153,727 patients in the state of New York. Surg Endosc 35:7209–7218. https://doi.org/10.1007/s00464-020-08211-1
Janjua H, Cousin-Peterson E, Barry TM, Kuo MC, Baker MS, Kuo PC (2020) The paradox of the robotic approach to inguinal hernia repair in the inpatient setting. Am J Surg 219:497–501. https://doi.org/10.1016/j.amjsurg.2019.09.012
Janjua H, Cousin-Peterson E, Barry TM, Kuo MC, Baker MS, Kuo PC (2020) Robotic approach to outpatient inguinal hernia repair. J Am Coll Surg 231:61–72. https://doi.org/10.1016/j.jamcollsurg.2020.04.031
Abdelmoaty WF, Dunst CM, Neighorn C, Swanstrom LL, Hammill CW (2019) Robotic-assisted versus laparoscopic unilateral inguinal hernia repair: a comprehensive cost analysis. Surg Endosc 33:3436–3443. https://doi.org/10.1007/s00464-018-06606-9
AlMarzooqi R, Tish S, Huang LC, Prabhu A, Rosen M (2019) Review of inguinal hernia repair techniques within the Americas Hernia Society Quality Collaborative. Hernia 23:429–438. https://doi.org/10.1007/s10029-019-01968-y
Pokala B, Armijo PR, Flores L, Hennings D, Oleynikov D (2019) Minimally invasive inguinal hernia repair is superior to open: a national database review. Hernia 23:593–599. https://doi.org/10.1007/s10029-019-01934-8
LeBlanc KA, Gonzalez A, Dickens E, Olsofka J, Ortiz-Ortiz C, Verdeja JC, Pierce R, Prospective Hernia Study Group (2021) Robotic-assisted, laparoscopic, and open incisional hernia repair: early outcomes from the Prospective Hernia Study. Hernia 25:1071–1082. https://doi.org/10.1007/s10029-021-02381-0
Gamagami R, Dickens E, Gonzalez A, D’Amico L, Richardson C, Rabaza J, Kolachalam R (2018) Open versus robotic-assisted transabdominal preperitoneal (R-TAPP) inguinal hernia repair: a multicenter matched analysis of clinical outcomes. Hernia 22:827–836. https://doi.org/10.1007/s10029-018-1769-1
Tonelli CM, Lorenzo I, Bunn C, Kulshrestha S, Abdelsattar ZM, Cohn T, Luchette FA, Baker MS (2022) Contemporary matched-cohort comparison of surgical approach to inguinal hernia repair: are minimally invasive approaches associated with higher rates of recurrence? J Am Coll Surg 235:119–127. https://doi.org/10.1097/XCS.0000000000000235
Miller BT, Prabhu AS, Petro CC, Beffa LRA, Carbonell AM, Hope W, Warren J, Higgins RM, Jacob B, Blatnik J et al (2023) Laparoscopic versus robotic inguinal hernia repair: 1- and 2-year outcomes from the RIVAL trial. Surg Endosc 37:723–728. https://doi.org/10.1007/s00464-022-09320-9
Prabhu AS, Carbonell A, Hope W, Warren J, Higgins R, Jacob B, Blatnik J, Haskins I, Alkhatib H, Tastaldi L et al (2020) Robotic inguinal vs transabdominal laparoscopic inguinal hernia repair: the RIVAL randomized clinical trial. JAMA Surg 155:380–387. https://doi.org/10.1001/jamasurg.2020.0034
Waite KE, Herman MA, Doyle PJ (2016) Comparison of robotic versus laparoscopic transabdominal preperitoneal (TAPP) inguinal hernia repair. J Robot Surg 10:239–244. https://doi.org/10.1007/s11701-016-0580-1
Shenoy R, Mederos MA, Jacob RL, Kondo KK, DeVirgilio M, Ward R, Kansagara D, Shekelle PG, Maggard-Gibbons M, Girgis MD et al (2022) Robot-assisted general surgery procedures at the Veterans Health Administration: a comparison of surgical techniques. J Surg Res 279:330–337. https://doi.org/10.1016/j.jss.2022.06.032
Shah PC, de Groot A, Cerfolio R, Huang WC, Huang K, Song C, Li Y, Kreaden U, Oh DS (2022) Impact of type of minimally invasive approach on open conversions across ten common procedures in different specialties. Surg Endosc 36:6067–6075. https://doi.org/10.1007/s00464-022-09073-5
Kudsi OY, Bou-Ayash N, Kaoukabani G, Gokcal F (2023) Comparison of perioperative and mid-term outcomes between laparoscopic and robotic inguinal hernia repair. Surg Endosc 37:1508–1514. https://doi.org/10.1007/s00464-022-09433-1
Dewulf M, Hiekkaranta JM, Makarainen E, Saarnio J, Vierstraete M, Ohtonen P, Muysoms F, Rautio T (2022) Open versus robotic-assisted laparoscopic posterior component separation in complex abdominal wall repair. BJS Open 6:zrac057. https://doi.org/10.1093/bjsopen/zrac057
Ephraim K, Haggai B, Mohammad A, Dan A, Yehonatan N, Lior S, Dina O, David H (2022) Learning curve of robotic inguinal hernia repair in the hands of an experienced laparoscopic surgeon: a comparative study. J Robot Surg 16:1307–1312. https://doi.org/10.1007/s11701-021-01362-w
Glasgow RE, Mulvihill SJ, Pettit JC, Young J, Smith BK, Vargo DJ, Ray DM, Finlayson SRG (2021) Value analysis of methods of inguinal hernia repair. Ann Surg 274:572–580. https://doi.org/10.1097/SLA.0000000000005063
Gundogdu E, Guldogan CE, Ozmen MM (2020) Bilateral inguinal hernia repair: robotic TAPP versus laparoscopic TEP. Surg Laparosc Endosc Percutan Tech 31:439–443. https://doi.org/10.1097/SLE.0000000000000890
Muysoms F, Vierstraete M, Nachtergaele F, Van Garsse S, Pletinckx P, Ramaswamy A (2021) Economic assessment of starting robot-assisted laparoscopic inguinal hernia repair in a single-centre retrospective comparative study: the EASTER study. BJS Open 5:zraa046. https://doi.org/10.1093/bjsopen/zraa046
Kakiashvili E, Bez M, Abu Shakra I, Ganam S, Bickel A, Merei F, Drobot A, Bogouslavski G, Kassis W, Khatib K et al (2021) Robotic inguinal hernia repair: is it a new era in the management of inguinal hernia? Asian J Surg 44:93–98. https://doi.org/10.1016/j.asjsur.2020.03.015
Khoraki J, Gomez PP, Mazzini GS, Pessoa BM, Browning MG, Aquilina GR, Salluzzo JL, Wolfe LG, Campos GM (2020) Perioperative outcomes and cost of robotic-assisted versus laparoscopic inguinal hernia repair. Surg Endosc 34:3496–3507. https://doi.org/10.1007/s00464-019-07128-8
Huerta S, Timmerman C, Argo M, Favela J, Pham T, Kukreja S, Yan J, Zhu H (2019) Open, laparoscopic, and robotic inguinal hernia repair: outcomes and predictors of complications. J Surg Res 241:119–127. https://doi.org/10.1016/j.jss.2019.03.046
Sheldon RR, Do WS, Weiss JB, Forte DM, Sohn VY (2019) Sage wisdom or anecdotal dictum? Equivalent opioid use after open, laparoscopic, and robotic inguinal hernia repair. Am J Surg 217:839–842. https://doi.org/10.1016/j.amjsurg.2019.02.022
Kosturakis AK, LaRusso KE, Carroll ND, Nicholl MB (2018) First 100 consecutive robotic inguinal hernia repairs at a Veterans Affairs hospital. J Robot Surg 12:699–704. https://doi.org/10.1007/s11701-018-0812-7
LeBlanc K, Dickens E, Gonzalez A, Gamagami R, Pierce R, Balentine C, Voeller G, Prospective Hernia Study Group (2020) Prospective, multicenter, pairwise analysis of robotic-assisted inguinal hernia repair with open and laparoscopic inguinal hernia repair: early results from the Prospective Hernia Study. Hernia 24:1069–1081. https://doi.org/10.1007/s10029-020-02224-4
Reeves J, Mehta S, Prabha RD, Salama Y, Mittal A (2020) Robotic versus open transversus abdominis release and incisional hernia repair: a case-control study. Laparosc Endosc Robot Surg 3:59–62. https://doi.org/10.1016/j.lers.2020.06.002
Ayuso SA, Katzen MM, Aladegbami BG, Nayak RB, Augenstein VA, Heniford BT, Colavita PD (2022) Nationwide readmissions analysis of minimally invasive versus open ventral hernia repair: a retrospective population-based study. Am Surg 88:463–470. https://doi.org/10.1177/00031348211050835
Forester B, Attaar M, Donovan K, Kuchta K, Ujiki M, Denham W, Haggerty SP, Carbray J, Linn J (2021) Short-term quality of life comparison of laparoscopic, open, and robotic incisional hernia repairs. Surg Endosc 35:2781–2788. https://doi.org/10.1007/s00464-020-07711-4
LaPinska M, Kleppe K, Webb L, Stewart TG, Olson M (2021) Robotic-assisted and laparoscopic hernia repair: real-world evidence from the Americas Hernia Society Quality Collaborative (AHSQC). Surg Endosc 35:1331–1341. https://doi.org/10.1007/s00464-020-07511-w
Dauser B, Hartig N, Vedadinejad M, Kirchner E, Trummer F, Herbst F (2021) Robotic-assisted repair of complex ventral hernia: can it pay off? J Robot Surg 15:45–52. https://doi.org/10.1007/s11701-020-01078-3
Guzman-Pruneda AF, Huang LC, Collins C, Renshaw S, Narula V, Poulose KB (2021) Abdominal core quality of life after ventral hernia repair: a comparison of open versus robotic-assisted retromuscular techniques. Surg Endosc 35:241–248. https://doi.org/10.1007/s00464-020-07386-x
Armijo P, Pratap A, Wang Y, Shostrom V, Oleynikov D (2018) Robotic ventral hernia repair is not superior to laparoscopic: a national database review. Surg Endosc 32:1834–1839. https://doi.org/10.1007/s00464-017-5872-7
Coakley KM, Sims SM, Prasad T, Lincourt AE, Augenstein VA, Sing RF, Heniford BT, Colavita PD (2017) A nationwide evaluation of robotic ventral hernia surgery. Am J Surg 214:1158–1163. https://doi.org/10.1016/j.amjsurg.2017.08.022
Warren JA, Cobb WS, Ewing JA, Carbonell AM (2017) Standard laparoscopic versus robotic retromuscular ventral hernia repair. Surg Endosc 31:324–332. https://doi.org/10.1007/s00464-016-4975-x
Carbonell AM, Warren JA, Prabhu AS, Ballecer CD, Janczyk RJ, Herrera J, Huang LC, Phillips S, Rosen MJ, Poulose BK (2018) Reducing length of stay using a robotic-assisted approach for retromuscular ventral hernia repair: a comparative analysis from the Americas Hernia Society Quality Collaborative. Ann Surg 267:210–217. https://doi.org/10.1097/SLA.0000000000002244
Petro CC, Thomas JD, Tu C, Krpata DM, Beffa LR, Rosen MJ, Prabhu AS (2022) Robotic vs laparoscopic ventral hernia repair with intraperitoneal mesh: 1-year exploratory outcomes of the PROVE-IT randomized clinical trial. J Am Coll Surg 234:1160–1165. https://doi.org/10.1097/XCS.0000000000000171
Costa TN, Abdalla RZ, Tustumi F, Junior UR, Cecconello I (2023) Robotic-assisted compared with laparoscopic incisional hernia repair following oncologic surgery: short- and long-term outcomes of a randomized controlled trial. J Robot Surg 17:99–107. https://doi.org/10.1007/s11701-022-01403-y
Dhanani NH, Olavarria OA, Holihan JL, Shah SK, Wilson TD, Loor MM, Ko TC, Kao LS, Liang MK (2021) Robotic versus laparoscopic ventral hernia repair: one-year results from a prospective, multicenter, blinded randomized controlled trial. Ann Surg 273:1076–1080. https://doi.org/10.1097/SLA.0000000000004795
Petro CC, Zolin S, Krpata D, Alkhatib H, Tu C, Rosen MJ, Prabhu AS (2021) Patient-reported outcomes of robotic vs laparoscopic ventral hernia repair with intraperitoneal mesh: the PROVE-IT randomized clinical trial. JAMA Surg 156:22–29. https://doi.org/10.1001/jamasurg.2020.4569
Olavarria OA, Bernardi K, Shah SK, Wilson TD, Wei S, Pedroza C, Avritscher EB, Loor MM, Ko TC, Kao LS et al (2020) Robotic versus laparoscopic ventral hernia repair: multicenter, blinded randomized controlled trial. BMJ 370:m2457. https://doi.org/10.1136/bmj.m2457
Chen YJ, Huynh D, Nguyen S, Chin E, Divino C, Zhang L (2017) Outcomes of robot-assisted versus laparoscopic repair of small-sized ventral hernias. Surg Endosc 31:1275–1279. https://doi.org/10.1007/s00464-016-5106-4
Gonzalez AM, Romero RJ, Seetharamaiah R, Gallas M, Lamoureux J, Rabaza JR (2015) Laparoscopic ventral hernia repair with primary closure versus no primary closure of the defect: potential benefits of the robotic technology. Int J Med Robot 11:120–125. https://doi.org/10.1002/rcs.1605
Han BJ, Kushner BS, Holden SE, Majumder A, Blatnik JA (2022) Transversus abdominis release with posterior component separation in patients with previously recurrent ventral hernias: a single institution experience. Surgery 171:806–810. https://doi.org/10.1016/j.surg.2021.08.067
Thomas JD, Gentle CK, Krpata DM, Prabhu AS, Fafaj A, Zolin SJ, Phillips SE, Rosenblatt S, Rosen MJ, Petro CC (2022) Comparing rates of bowel injury for laparoscopic and robotic ventral hernia repair: a retrospective analysis of the abdominal core health quality collaborative. Hernia 26:1251–1258. https://doi.org/10.1007/s10029-022-02564-3
Kudsi OY, Gokcal F, Bou-Ayash N, Chang K (2021) Comparison of midterm outcomes between open and robotic emergent ventral hernia repair. Surg Innov 28:449–457. https://doi.org/10.1177/1553350620971182
Nguyen B, David B, Shiozaki T, Gosch K, Sorensen GB (2021) Comparisons of abdominal wall reconstruction for ventral hernia repairs, open versus robotic. Sci Rep 11:8086. https://doi.org/10.1038/s41598-021-86093-6
Walker PA, May AC, Mo J, Cherla DV, Santillan MR, Kim S, Ryan H, Shah SK, Wilson EB, Tsuda S (2018) Multicenter review of robotic versus laparoscopic ventral hernia repair: is there a role for robotics? Surg Endosc 32:1901–1905. https://doi.org/10.1007/s00464-017-5882-5
Henriksen NA, Jensen KK, Muysoms F (2019) Robot-assisted abdominal wall surgery: a systematic review of the literature and meta-analysis. Hernia 23:17–27. https://doi.org/10.1007/s10029-018-1872-3
Aiolfi A, Cavalli M, Micheletto G, Lombardo F, Bonitta G, Morlacchi A, Bruni PG, Campanelli G, Bona D (2019) Primary inguinal hernia: systematic review and Bayesian network meta-analysis comparing open, laparoscopic transabdominal preperitoneal, totally extraperitoneal, and robotic preperitoneal repair. Hernia 23:473–484. https://doi.org/10.1007/s10029-019-01964-2
Solaini L, Cavaliere D, Avanzolini A, Rocco G, Ercolani G (2022) Robotic versus laparoscopic inguinal hernia repair: an updated systematic review and meta-analysis. J Robot Surg 16:775–781. https://doi.org/10.1007/s11701-021-01312-6
Zhao F, Wang B, Chen J (2021) Comparison between robotic and laparoscopic inguinal hernia repair in Caucasian patients: a systematic review and meta-analysis. Ann Transl Med 9:885. https://doi.org/10.21037/atm-21-2126
Qabbani A, Aboumarzouk OM, ElBakry T, Al-Ansari A, Elakkad MS (2021) Robotic inguinal hernia repair: systematic review and meta-analysis. ANZ J Surg 91:2277–2287. https://doi.org/10.1111/ans.16505
Tai TE, Bai GH, Shiau CH, Wu JC, Hou WH (2022) Fascia defect closure versus non-closure in minimal invasive direct inguinal hernia mesh repair: a systematic review and meta-analysis of real-world evidence. Hernia. https://doi.org/10.1007/s10029-022-02732-5
Aiolfi A, Cavalli M, Micheletto G, Bruni PG, Lombardo F, Perali C, Bonitta G, Bona D (2019) Robotic inguinal hernia repair: is technology taking over? Systematic review and meta-analysis. Hernia 23:509–519. https://doi.org/10.1007/s10029-019-01965-1
Mohan R, Yeow M, Wong JYS, Syn N, Wijerathne S, Lomanto D (2021) Robotic versus laparoscopic ventral hernia repair: a systematic review and meta-analysis of randomised controlled trials and propensity score matched studies. Hernia 25:1565–1572. https://doi.org/10.1007/s10029-021-02501-w
Goettman MA, Riccardi ML, Vang L, Dughayli MS, Faraj CH (2020) Robotic assistance in ventral hernia repair may decrease the incidence of hernia recurrence. J Minim Access Surg 16:335–340. https://doi.org/10.4103/jmas.JMAS_92_19
Dixit R, Prajapati OP, Krishna A, Rai SK, Prasad M, Bansal VK (2023) Patient-reported outcomes of laparoscopic versus robotic primary ventral and incisional hernia repair: a systematic review and meta-analysis. Hernia. https://doi.org/10.1007/s10029-022-02733-4
Bracale U, Corcione F, Neola D, Castiglioni S, Cavallaro G, Stabilini C, Botteri E, Sodo M, Imperatore N, Peltrini R (2021) Transversus abdominis release (TAR) for ventral hernia repair: open or robotic? Short-term outcomes from a systematic review with meta-analysis. Hernia 25:1471–1480. https://doi.org/10.1007/s10029-021-02487-5
Gram-Hanssen A, Tolstrup A, Zetner D, Rosenberg J (2020) Patient-reported outcome measures for patients undergoing inguinal hernia repair. Front Surg 7:17. https://doi.org/10.3389/fsurg.2020.00017
Chow A, Mayer EK, Darzi AW, Athanasiou T (2009) Patient-reported outcome measures: the importance of patient satisfaction in surgery. Surgery 146:435–443. https://doi.org/10.1016/j.surg.2009.03.019
Fry BT, Campbell DA Jr, Englesbe MJ, Vu JV (2019) Using patient-reported outcomes to enhance appropriateness in low-risk elective general surgery. Ann Surg 269:41–42. https://doi.org/10.1097/SLA.0000000000002864
Bittner JG (2019) Comment on robotic inguinal hernia repair: is technology taking over? Hernia 23:521–522. https://doi.org/10.1007/s10029-019-01984-y
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
All authors made substantiable contributions to the conception of the work, the acquisition, analysis, interpretation of data, drafted the work and revised it critically for important intellectual content, approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Disclosures
Nicola de’Angelis, Carlo Alberto Schena, David Moszkowicz, Curil Kuperas, Régis Fara, Sébastien Gaujoux, Jean-François Gillion, Caroline Gronnier, Jérôme Loriau, Muriel Mathonnet, Olivier Oberlin, Manuela Perez, Yohann Renard, Benoît Romain, Guillaume Passot, and Patrick Pessaux have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de’Angelis, N., Schena, C.A., Moszkowicz, D. et al. Robotic surgery for inguinal and ventral hernia repair: a systematic review and meta-analysis. Surg Endosc 38, 24–46 (2024). https://doi.org/10.1007/s00464-023-10545-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10545-5