Abstract
Background
Randomized controlled trials have been unable to demonstrate noninferiority of minimally invasive surgery for rectal cancer. The aim of this study was to assess oncologic resection success, short- and long-term morbidity, and overall survival by operative approach in a homogenous early-stage rectal cancer cohort.
Methods
This is a multicenter, propensity score-weighted cohort study utilizing deidentified data from the National Cancer Database. Individuals who underwent a formal proctectomy for early-stage rectal cancer (T1-2, N0, M0) from 2010 to 2015 were included. The primary outcome was a composite variable indicating successful oncologic resection stratified by operative approach, defined as negative margins with at least 12 lymph nodes evaluated.
Results
Among 3649 proctectomies for rectal adenocarcinoma, 1660 (45%) were approached open, 1461 (40%) laparoscopically, and 528 (15%) robotically. After propensity score weighting, compared to open approach, there were no differences in odds of successful oncologic resection (ORadj = 1.07, 95% CI 0.9, 1.28 and ORadj = 1.28, 95% CI 0.97, 1.7). Open approach was associated with longer mean (± SD) length of stay compared to laparoscopic (7.7 ± 0.18 vs. 6.5 ± 0.25 days, p < 0.001) and robotic (7.7 ± 0.18 vs. 6.3 ± 0.35 days, p < 0.001) approaches. In regard to 90-day mortality, compared to open approach, laparoscopic (ORadj = 0.56, 95% CI 0.36, 0.88) and robotic (ORadj = 0.45, 95% CI 0.22, 0.94) approaches were associated with a reduced odd of 90-day mortality. This mortality benefit persists in the long-term for laparoscopic approach (p = 0.003).
Conclusion
For individuals with early-stage rectal cancer treated with proctectomy, successful oncologic resection can be achieved irrespective of technical approach. Minimally invasive approaches provide short-term reduction in morbidity. Surgical approach must be tailored to each patient based on surgeon experience and judgement in collaboration with a multi-disciplinary team.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite increased rates of screening for colorectal cancer (CRC), rectal cancer is becoming more prevalent. The estimated number of cases in 2019 in the USA was approximately 44,000 [1]. Advancements in medical and surgical care have improved survival for rectal cancer patients in recent decades. Concurrently, minimally invasive surgical techniques have become more widespread for most gastrointestinal malignancies [2]. There have been several trials that raise concern about the equivalence of laparoscopic and robotic approaches to traditional open surgery, and data on long-term oncologic outcomes are controversial [3,4,5,6,7]. Several meta-analyses have examined the rate of complete total mesorectal excision (TME) with inconsistent results. Some have shown a higher risk of incomplete TME with minimally invasive techniques and others have suggested equivalent oncologic resection success rates [8, 9]. Additionally, translation of these outcomes to both surgeons and patients outside of clinical trials has not been evaluated.
Differences in care pathways and treatment response patterns confound evaluation of surgical technique. Inclusion of patients in whom margin positivity is related to tumor-specific factors rather than surgical quality creates a heterogeneous dataset and variation in neoadjuvant treatment patterns are known to effect oncologic outcomes [10]. In order to better understand the role of surgical technique on oncologic outcomes, this study intentionally focused on individuals with early-stage disease who did not receive neoadjuvant therapy.
The aim of this study is to evaluate oncologic resection success in early-stage rectal cancer, and to compare short- and long-term outcomes of laparoscopic, robotic, and open surgical approaches.
Materials and methods
This multicenter cohort study evaluated incidence of successful oncologic resection and short- and long-term outcomes by operative approach. Propensity score weighting was utilized to account for potential bias associated with known pre-operative variables.
Data source
The 2016 American College of Surgeons National Cancer Database (ACS NCDB) from 2010–2015.
Patient population
Individuals greater than 18 years of age who underwent open, laparoscopic, or robotic proctectomies for invasive rectal adenocarcinoma were included in this study. Cases requiring conversion to open were included in their original cohort for analysis to reduce selection bias. Individuals were excluded if they underwent local excisions or multi-visceral resections. Only those with pre-operative early-stage disease (T1-T2, N0, M0) and who did not receive neoadjuvant chemoradiation were included in this study. These criteria were defined to create a homogenous tumor- and treatment-specific population by which to compare approach. This was an attempt to reduce bias, for example, in patients who were coded as having early-stage disease but for whom received neoadjuvant chemoradiation. Individuals who lacked values or for which data was unknown for the following data fields: insurance status, race, Hispanic origin, length of stay, tumor grade, surgical margins, lymph node examination, unplanned readmission, and 30- and 90-day mortality were excluded from this study.
Outcome variables
The primary outcome of this study was a composite variable representing successful oncologic resection as defined by negative margin status as well as pathologic evaluation of 12 or more lymph nodes [11,12,13,14,15]. ACS NCDB characterizes negative margin status as all margins grossly and microscopically uninvolved. The secondary outcomes were 30-day readmission, length of stay, 90-day or post-operative mortality, and long-term overall survival for those individuals with confirmed pathologic early-stage disease.
Estimating propensity scores
Propensity scores as regression weights were estimated using boosted regression and minimization of Kolmogorov–Smirnov (KS) statistic sums to optimize pre-treatment covariate balance between cohorts, effectively minimizing selection bias [16,17,18]. Estimated propensity score models included age, sex, insurance status, ethnicity, Charlson/Deyo co-morbidity score, T-stage, and tumor grade. Facility procedure volume for the study time period was also included in an effort to control for facility experience.
Analysis
Multinomial propensity score-weighted logistic regression models were used to estimate the odds ratios associated with a successful oncologic resection, 90-day mortality, and unplanned readmission. Multinomial propensity score-weighted linear regression models were used to calculate beta effect estimates associated with post-operative length of stay. The survival function was estimated using a weighted Kaplan–Meier estimator and survival distributions were compared using a weighted version of the log-rank test. Statistical significance, alpha, was set at 0.05. Database management and analyses were conducted using R (Version 3.6.1, R Core Team, Vienna, Austria). Propensity scores were estimated using the mnps function of the twang package. Weighted Kaplan–Meier estimates and comparison of survival distribution were performed using the svykm and survlogrank functions in the survey package. The STROBE cohort reporting guidelines were utilized [19,20,21]. The R script to reproduce the findings of this study is available at: https://github.com/wkethman/Research as “Rectal CA by Approach – NCDB.R”. The University Hospitals Institutional Review Board (IRB) deemed this study exempt and individual patient consent was not required.
Results
Among 3649 proctectomies for rectal adenocarcinoma, 1660 (45%) were approached open, 1461 (40%) laparoscopically, of which 222 required conversion to open, and 528 (15%) robotically, of which 41 required conversion to open. This represents a conversion to open rate of 15% and 7.8% for laparoscopic and robotic approaches, respectively. After propensity score weighting, there were no statistically significant differences in preoperative characteristics among the cohorts and balance was achieved without significant KS statistics (Table 1).
Successful oncologic resections were achieved in 1300 (78%), 1185 (81%), and 440 (83%) of resections performed by open, laparoscopic, and robotic approaches, respectively. Similarly, successful oncologic resections were achieved in 180 (81%) and 85% (35) of laparoscopic and robotic cases requiring conversion to open approach. Negative surgical margins were achieved in 1634 (98%), 1440 (99%), and 521 (99%) of open, laparoscopic, and robotic resections, respectively, and > 12 lymph nodes were reported in 1319 (80%), 1201 (82%), and 445 (84%) of resections. After weighting, compared to open approach, there were no differences in odds of successful oncologic resection by approach (ORadj = 1.07, 95% CI 0.9, 1.28 and ORadj = 1.28, 95% CI 0.97, 1.7) (Fig. 1). Compared to open approach, odds of achieving a negative margin (ORadj, lap = 1.0, 95% CI 0.57, 1.9 and ORadj, rob = 1.4, 95% CI 0.57, 3.4) and at least 12 lymph nodes evaluated (ORadj, lap = 1.1, 95% CI 0.9, 1.3 and ORadj, rob = 1.3, 95% CI 0.99, 1.75) were not different between approaches.
Analysis of short-term post-operative outcomes revealed that compared to the open cohort, laparoscopic (ORadj = 1.1, 95% CI 0.83, 1.45) and robotic (ORadj = 1.2, 95% CI 0.8, 1.8) approaches, there were no differences in odds of unplanned readmission. Open approach was associated with longer mean (± SD) length of stay compared to laparoscopic (7.7 ± 0.18 vs. 6.5 ± 0.25 days, p < 0.001) and robotic (7.7 ± 0.18 vs. 6.3 ± 0.35 days, p < 0.001) approaches. Compared to open approach, laparoscopic (ORadj = 0.56, 95% CI 0.36, 0.88) and robotic (ORadj = 0.45, 95% CI 0.22, 0.94) approaches were associated with a reduced odd of 90-day mortality.
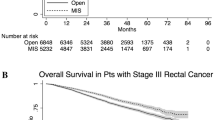
After adjusting for pathologic upstaging of disease post-operatively, 2984 proctectomies were included in our survival analysis (Fig. 2). Compared to open approach, laparoscopic approach was associated with improved long-term overall survival (p = 0.003), however, there was no significant difference (p = 0.15) in long-term overall survival when compared to robotic approach.
Discussion
This study demonstrates that open, laparoscopic, and robotic approaches to surgical resection of early-stage rectal cancer were not associated with differences in odds of successful oncologic resection. Despite this finding, open approach was associated with decreased 90-day and long-term overall survival when compared to minimally invasive approaches, even when outcomes were weighted for important confounding variables.
Short-term benefits of minimally invasive surgery must be considered in the context of longer-term oncologic outcomes for cancer patients. The COREAN and COLOR II trials demonstrated equivalent oncologic outcomes between laparoscopic and open approaches, whereas, ALaCaRT and ACOSOG Z6051 trials were unable to demonstrate noninferiority of laparoscopic approach in terms of successful oncologic resection [5, 22,23,24]. However, findings of ALaCaRT and ACOSOG Z6051 did not translate to differences in secondary outcomes of disease-free survival or locoregional recurrence at 2-years. [6, 7]. The COLOR II, COREAN, ALaCaRT and ACOSOG Z6051 trials included early and more advanced staged disease, including those who received neoadjuvant and adjuvant therapy. For example, the COREAN trial explicitly excluded patients with early-stage disease, only 1% of patients in the ACOSOG Z6051 trial had early-stage disease, ALaCaRT likely included but did not specify the percentage of patients with Stage I disease, and 28% of the COLOR II study population had clinical Stage I disease. Therefore, heterogeneity in these populations makes direct comparison to our cohort difficult, where clinical and pathologic T3-4, N1-2 staged disease was intentionally excluded to minimize the likelihood that oncologic factors would contribute to positive margins. However, regarding short-term morbidity, safety of laparoscopic approach has been well-established. Laparoscopy is associated with a reduction in infectious complications and narcotic use, as well as length of stay [25,26,27]. Considering these differences and our findings, oncologic outcomes of laparoscopic and open approaches are at least equivalent for patients with early-stage rectal cancer, and laparoscopy provides improvements in short-term morbidity.
Robotic surgery is frequently compared to laparoscopy rather than to open surgery directly. The ROLARR trial is the most notable randomized trial comparing these approaches: it demonstrated no difference in rates of conversion to open nor were there differences in secondary pathologic and quality of life outcomes [3]. A recent meta-analysis conversely found lower rates of conversion to open approach with similar length of stay, postoperative morbidity, and local recurrence rates between the two minimally invasive techniques [28,29,30]. Interestingly, it does not appear that conversion is a risk factor for worse short- or long-term outcomes and so this potential benefit of robotic approach is controversial [31]. Our study did not directly compare robotic and laparoscopic approaches, and while short-term mortality benefits were found between robotic and open approaches, this finding did not persist in the long-term, 5-year weighted overall survival analysis. This is likely related to a paucity of data extending beyond 30-months or from differences that exist between the open and robotic cohorts that are not accounted for due to the nature of this study. This evidence further strengthens the argument that, similar to laparoscopy, robotic approach is a tool that can be utilized by well-trained surgeons to provide benefits of minimally invasive surgery for select patients.
The finding that open surgery is associated with decreased short- and long-term overall survival when compared to minimally invasive approaches likely has a complex and multifactorial explanation. Before balancing, patients in the open group tended to be older, have more co-morbidities, and were less likely to have private insurance than those in the laparoscopic and robotic groups – these factors have been implicated in worse survival. While ACS NCDB captures co-morbidities, it does so via Charlson/Deyo co-morbidity score, which does not provide the granularity to assure that the groups are similar in all ways. From a disease-specific perspective, however, this study includes only patients with T1 and T2 tumors who did not receive neoadjuvant therapy and therefore concerns regarding threatened margins or bulky tumors does not have a significant impact on our results. Since there was no difference in odds of successful oncologic resection it is more likely that survival differences between these cohorts are related to unavailable patient-specific differences.
This study has several limitations. The ACS NCDB captures approximately 70% of newly diagnosed patients with cancer treated in approximately 30% of United States hospitals accredited by the American College of Surgeons Commission on Cancer [32]. It does not capture other important confounding variables, such as body mass index (BMI), tobacco use status, and detailed co-morbidities. In addition, this dataset does not include peri-operative complications, causes of death, or recurrence which are important factors in oncologic outcomes. This study of prospectively collected data using propensity score weighting represents Level IIc evidence [33].
Despite its limitations, this study utilizes a large national database to reinforce and confirm findings seen in prospective cohort and randomized studies. The range of patients included in the ACS NCDB provides inherent strength to the study and suggests that its findings are applicable to a broad range of patients and facility types. This study also attempts to control for experience in caring for individuals with rectal cancer by including a facility volume variable in the propensity weighting. This study included patients who underwent laparoscopic or robotic approaches requiring conversion to open in an attempt to reflect real-world cohorts thus allowing more balanced comparisons of approach. By including only early-stage rectal cancer patients, this study assures that positive margins are less likely related to tumor-specific factors.
For patients with early-stage rectal cancer treated with proctectomy, this study demonstrates that successful oncologic resection can be achieved irrespective of technical approach. In addition, minimally invasive approaches provide short-term reduction in morbidity. Surgical approach must be tailored to each patient based on surgeon experience and judgement in collaboration with a multi-disciplinary team.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Konstantinidis IT, Ituarte P, Woo Y, Warner SG, Melstrom K, Kim J, Singh G, Lee B, Fong Y, Melstrom LG (2019) Trends and outcomes of robotic surgery for gastrointestinal (GI) cancers in the USA: maintaining perioperative and oncologic safety. Surg Endosc. https://doi.org/10.1007/s00464-019-07284-x
Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Pietro BP, Edlin R, Hulme C, Brown J (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer the rolarr randomized clinical trial. JAMA - J Am Med Assoc 318:1569–1580. https://doi.org/10.1001/jama.2017.7219
Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam B-H, Sohn DK, Oh JH (2018) Robot-assisted versus laparoscopic surgery for rectal cancer: A Phase II open label prospective randomized controlled trial. Ann Surg 267:243–251. https://doi.org/10.1097/SLA.0000000000002321
van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218. https://doi.org/10.1016/S1470-2045(13)70016-0
Stevenson ARL, Solomon MJ, Brown CSB, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Wilson K, Hague W, Simes J, Australasian Gastro-Intestinal Trials Group (AGITG) ALaCaRT investigators (2019) Disease-free survival and local recurrence after laparoscopic-assisted resection or open resection for rectal cancer: the australasian laparoscopic cancer of the rectum randomized clinical trial. Ann Surg 269:596–602. https://doi.org/10.1097/SLA.0000000000003021
Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, Peters WR, Maun DC, Chang GJ, Herline A, Fichera A, Mutch MG, Wexner SD, Whiteford MH, Marks J, Birnbaum E, Margolin DA, Larson DW, Marcello PW, Posner MC, Read TE, Monson JRT, Wren SM, Pisters PWT, Nelson H (2019) Disease-free survival and local recurrence for laparoscopic resection compared with open resection of stage II to III rectal cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg 269:589–595. https://doi.org/10.1097/SLA.0000000000003002
Martínez-Pérez A, Carra MC, Brunetti F, De’Angelis N, (2017) Pathologic outcomes of laparoscopic vs open mesorectal excision for rectal cancer: A systematic review and meta-analysis. JAMA Surg 152:e165665
Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, Jimenez-Rodriguez RM, Gurrado A, Strippoli GFM (2018) Robotic versus laparoscopic minimally invasive surgery for rectal cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Surg 267:1034–1046. https://doi.org/10.1097/SLA.0000000000002523
Kolarich A, George TJ, Hughes SJ, Delitto D, Allegra CJ, Hall WA, Chang GJ, Tan SA, Shaw CM, Iqbal A (2018) Rectal cancer patients younger than 50 years lack a survival benefit from NCCN guideline–directed treatment for stage II and III disease. Cancer 124:3510–3519. https://doi.org/10.1002/cncr.31527
Leo E, Belli F, Miceli R, Mariani L, Gallino G, Battaglia L, Vannelli A, Andreola S (2009) Distal clearance margin of 1 cm or less: a safe distance in lower rectum cancer surgery. Int J Colorectal Dis 24:317–322. https://doi.org/10.1007/s00384-008-0604-z
Grosek J, Velenik V, Edhemovic I, Omejc M (2017) The influence of the distal resection margin length on local recurrence and long- term survival in patients with rectal cancer after chemoradiotherapy and sphincter- preserving rectal resection. Radiol Oncol 51:169–177. https://doi.org/10.1515/raon-2016-0030
Fitzgerald TL, Brinkley J, Zervos EE (2011) Pushing the envelope beyond a centimeter in rectal cancer: oncologic implications of close, but negative margins. J Am Coll Surg 213:589–595. https://doi.org/10.1016/j.jamcollsurg.2011.07.020
Tepper JE, O’Connell MJ, Niedzwiecki D, Hollis D, Compton C, Benson AB, Cummings B, Gunderson L, Macdonald JS, Mayer RJ (2001) Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol Off J Am Soc Clin Oncol 19:157–163. https://doi.org/10.1200/JCO.2001.19.1.157
Nagtegaal ID, Quirke P (2008) What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol Off J Am Soc Clin Oncol 26:303–312. https://doi.org/10.1200/JCO.2007.12.7027
Friedman J (2001) Greedy function approximation: a gradient boosting machine. Ann Stat. https://doi.org/10.1214/aos/1013203451
Efron B, Hastie T, Johnstone I (2004) Least angle regression. Ann Stat. https://doi.org/10.1214/009053604000000067
McCaffrey D, Ridgeway G, Morral A (2004) Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods 9(4):403–425
McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF (2013) A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 32:3388–3414. https://doi.org/10.1002/sim.5753
Ridgeway G, McCaffrey D, Morral A, Burgette L, Ann Griffin B (2017) Toolkit for Weighting and Analysis of Nonequivalent Groups: A tutorial for the twang package
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE (2014) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg Lond Engl 12:1495–1499. https://doi.org/10.1016/j.ijsu.2014.07.013
Jeong S-Y, Park JW, Nam BH, Kim S, Kang S-B, Lim S-B, Choi HS, Kim D-W, Chang HJ, Kim DY, Jung KH, Kim T-Y, Kang GH, Chie EK, Kim SY, Sohn DK, Kim D-H, Kim J-S, Lee HS, Kim JH, Oh JH (2014) Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 15:767–774. https://doi.org/10.1016/S1470-2045(14)70205-0
Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PWT, Nelson H (2015) Effect of laparoscopic-assisted resection vs open resection of stage II or III Rectal cancer on pathologic outcomes: The ACOSOG Z6051 randomized clinical trial. JAMA 314:1346–1355. https://doi.org/10.1001/jama.2015.10529
Stevenson ARL, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J, ALaCaRT Investigators, (2015) Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: The ALaCaRT randomized clinical trial. JAMA 314:1356–1363. https://doi.org/10.1001/jama.2015.12009
Vennix S, Pelzers L, Bouvy N, Beets GL, Pierie J-P, Wiggers T, Breukink S (2014) Laparoscopic versus open total mesorectal excision for rectal cancer. In: Breukink S (ed) Cochrane Database of Systematic Reviews. John Wiley & Sons Ltd, Chichester UK, p CD05200
Quintana JM, Anton-Ladislao A, Lázaro S, Gonzalez N, Bare M, de Larrea NF, Redondo M, Briones E, Escobar A, Sarasqueta C, Garcia-Gutierrez S, Group for the R-C (2017) Outcomes of open versus laparoscopic surgery in patients with rectal cancer. Int J Colorectal Dis. https://doi.org/10.1007/s00384-017-2925-2
Kang S-B, Park JW, Jeong S-Y, Nam BH, Choi HS, Kim D-W, Lim S-B, Lee T-G, Kim DY, Kim J-S, Chang HJ, Lee H-S, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim D-H, Oh JH (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11:637–645. https://doi.org/10.1016/S1470-2045(10)70131-5
Trastulli S, Farinella E, Cirocchi R, Cavaliere D, Avenia N, Sciannameo F, Gullà N, Noya G, Boselli C (2012) Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis 14:e134–e156. https://doi.org/10.1111/j.1463-1318.2011.02907.x
Xiong B, Ma L, Huang W, Zhao Q, Cheng Y, Liu J (2015) Robotic versus laparoscopic total mesorectal excision for rectal cancer: a Meta-analysis of eight studies. J Gastrointest Surg 19:516–526. https://doi.org/10.1007/s11605-014-2697-8
Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, Jimenez-Rodriguez RM, Gurrado A, Strippoli GFM (2017) Robotic versus laparoscopic minimally invasive surgery for rectal cancer. Ann Surg. https://doi.org/10.1097/SLA.0000000000002523
Allaix ME, Furnée E, Esposito L, Mistrangelo M, Rebecchi F, Arezzo A, Morino M (2018) Analysis of early and long-term oncologic outcomes after converted laparoscopic resection compared to primary open surgery for rectal cancer. World J Surg 42:3405–3414. https://doi.org/10.1007/s00268-018-4614-x
Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, Thoburn K, Gress D, McKellar DP, Shulman LN, Facktor MA, Winchester DP (2017) Using the national cancer database for outcomes research a review. JAMA Oncol 3:1722–1728. https://doi.org/10.1001/jamaoncol.2016.6905
Oxford Centre for Evidence-based Medicine - Levels of Evidence (March 2009) - CEBM. https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed 21 Dec 2018
Funding
There were no sources of funding for this research.
Author information
Authors and Affiliations
Contributions
All authors substantially contributed to the conception of the manuscript. Dr. Kethman acquired and analyzed the data and Drs. Kethman, Dietz, Stein, and Steinhagen aided in the interpretation of the data and analysis. Dr. Kethman was responsible for drafting initial drafts of the manuscript and Drs. Bingmer, Ofshteyn, Charles, Stein, Dietz, and Steinhagen contributed to critically revising important intellectual content. All authors gave final approval of the published version of the manuscript and all agree to be accountable for all aspects of the manuscript.
Corresponding author
Ethics declarations
Disclosures
Drs. William Kethman, Katherine Bingmer, Asya Ofshteyn, Ronald Charles, and Emily Steinhagen have no conflicts of interest or financial ties, relevant to the material in this manuscript, to disclose. Dr. Sharon Stein has received a Physician’s Foundation grant for work unrelated to the topic of this manuscript and support from Stryker Corporation for travel expenses. Dr. David Dietz has received compensation as faculty for an educational course with Johnson & Johnson – Medical Device Business Services, Inc..
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kethman, W.C., Bingmer, K.E., Ofshteyn, A. et al. Effects of surgical approach on short- and long-term outcomes in early-stage rectal cancer: a multicenter, propensity score-weighted cohort study. Surg Endosc 36, 5833–5839 (2022). https://doi.org/10.1007/s00464-022-09033-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09033-z