Abstract
Background
Laparoscopic rectal resection (LRR) for cancer is a challenging procedure, with conversion to open surgery being reported in up to 30% of cases. Since only a few studies with short follow-up have compared converted LRR and open RR (ORR), it is unclear if conversion to open surgery should be prevented by preferring an open approach in those patients with preoperatively known risk factors for conversion. The aim of this study was to compare early postoperative outcomes and long-term survival after completed LRR, converted LRR or ORR for non-metastatic rectal cancer.
Methods
A prospective database of consecutive curative LRRs and ORRs for rectal cancer was reviewed. Patients undergoing LRR who required conversion (CONV group) were compared with those who had primary open rectal surgery (OPEN group) and completed LRR (LAP group). A multivariate analysis was performed to identify predictors of poor survival.
Results
A total of 537 patients were included in the study: 272 in the LAP group, 49 in the CONV group and 216 in the OPEN group. There were no significant differences in perioperative morbidity, mortality and length of hospital stay between the three groups. Five-year overall survival and disease-free survival rates did not significantly differ between LAP, CONV and OPEN patients: 83.9 versus 77.8 versus 81% (P = 0.398) and 74.5 versus 62.9 versus 72.7% (P = 0.145), respectively. Similar 5-year OS and DFS rates were observed between patients who had converted LRR for locally advanced tumor or for non-tumor-related reasons: 81.2 versus 80.8% (P = 0.839) and 62.5 versus 63.7% (P = 0.970), respectively. Poor grade of tumor differentiation, lymphovascular invasion and a lymph node ratio of 0.25 or greater, but not conversion, were independently associated with poorer survival.
Conclusion
Conversion to open surgery does not impair short-term outcomes and does not jeopardize 5-year survival in patients with rectal cancer when compared to primary open surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic rectal resection (LRR) for rectal cancer is a technically demanding procedure, with conversion to open surgery being reported in up to 30% of cases [1]. During the last two decades, many efforts have been done to improve patient’s selection for LRR. However, there are no scoring models including several patient-, disease-, procedure and surgeon-related variables that are able to accurately predict the chance of conversion of LRR to open surgery [2,3,4,5,6].
Several studies have focused on short-term outcomes and survival rates after laparoscopic resection converted to open surgery for colon cancer [7,8,9,10,11,12,13,14,15,16,17] and rectal cancer [18,19,20,21,22,23,24,25,26]; however, most of them compared patients who had converted or completed laparoscopic resection, reporting controversial results.
To date, it is unclear if conversion to open surgery causes additional postoperative morbidity and impairs survival, since only a few studies with a short follow-up have specifically compared patients undergoing converted LRR or open RR (ORR) for rectal cancer [22,23,24,25,26].
The aim of this study was to compare both early postoperative outcomes and long-term survival after completed LRR, converted LRR or ORR for non-metastatic rectal cancer.
Materials and methods
This study is a retrospective analysis of a prospective database including all patients undergoing rectal resection for rectal cancer at our Institution between January 1996 and December 2011. The localization of the tumor was categorized as lower rectum (distal tumor margin less than 5 cm from the anal verge), mid-rectum (5–10 cm from the anal verge) and upper rectum (10–15 cm from the anal verge).
Exclusion criteria were: preoperative or intraoperative evidence of distant metastases, acute bowel obstruction, tumor perforation, synchronous colorectal cancers, T1–2 cancers treated with transanal endoscopic microsurgery and previous rectal surgery. Patients with a preoperatively staged T4 rectal cancer were also not considered in this study, since they were treated only with an open approach.
The first LRR in our Institution was performed in April 1992. To avoid the bias related to the learning curve, the first 40 LRRs were excluded from the study.
The use of neoadjuvant chemoradiation therapy (CRT, 45 Gy over 4 weeks in association with systemic 5-fluorouracil intravenous infusion) was discussed in an interdisciplinary tumor meeting and proposed to patients with T3-4N0-2M0 mid-lower rectal cancer. Surgery was planned 6–8 weeks after the completion of long-course neoadjuvant CRT. Both LRR and ORR with total mesorectal excision (TME) were performed for the treatment of mid- and lower rectal cancers, while patients with an upper rectal cancer underwent a partial mesorectal excision (PME). All patients with a rectal cancer invading the anal sphincter underwent abdominoperineal resection (APR). Tumor and patient characteristics were not the reasons for choosing the laparoscopic or the open approach. LRR or ORR was performed depending on the operating colorectal surgeons : the surgeons who were skilled in advanced laparoscopic colorectal surgery performed LRR, while the others chose the open approach.
Conversion to open surgery was defined as an unplanned laparotomy or as a wound incision larger than the incision needed to remove the specimen.
The following variables were prospectively collected in the database: patient’s characteristics (age, gender, ASA score, comorbidities), operative variables (operative time from skin incision to the application of dressings, complications and conversion rate to open surgery in case of LRR), use of neoadjuvant treatment, pathologic examination, short-term outcomes (resumption of gastrointestinal functions, morbidity classified according to the Clavien–Dindo classification [27] and length of postoperative hospital stay) and long-term oncologic results.
The following pathologic parameters were considered: tumor stage according to the TNM classification [28], number of lymph node harvested, lymph node ratio (LNR = number of positive nodes divided by total nodes harvested) and resection margins (longitudinal and circumferential).
Adjuvant chemotherapy was proposed within 8 weeks after surgery to all patients who had neoadjuvant CRT and to those patients with a postoperative diagnosis of stage 2–3 rectal cancer.
Follow-up protocol consisted of clinical examination, proctoscopy, serum carcinoembryonic antigen assay every 3 months and liver ultrasound every 6 months for the first 2 years, then annually. A CT scan of chest, abdomen and pelvis was obtained every year. A colonoscopy was performed at 1 year after surgery and then every 3 years.
Oncologic outcomes were overall survival (OS), disease-free survival (DFS), local recurrence (LR) and distant metastases rates.
Statistics
Quantitative data are provided as median and range, while categorical data are given as percentages. Proportions are compared using the χ2 test or the Fisher exact test, where appropriate. Student’s t test was used to compare normally distributed variables. Univariable OS and DFS rate analyses were performed using the Kaplan–Meier method, and the differences between the groups were assessed with the log rank test. OS and DFS were calculated from the date of surgery to the date of death from any cause or to the date of recurrence, respectively. Patients alive with or without recurrence were censored at the date of last examination. Time to LR or distant metastases was calculated from the time of surgery to date of evidence of relapse. We also performed a multivariable Cox regression analysis to identify predictors of poor OS and poor DFS. The included variables were: age, gender, surgical approach (LAP vs. CONV vs. OPEN), type of surgical procedure (AR vs. APR), grade of tumor differentiation, pT staging, number of lymph node harvested, LNR, lymphovascular invasion and adjuvant chemotherapy. Explanatory variables with univariable P ≤ 0.200 were included in the multivariable analysis in order to evaluate all potential predictors in the final modeling process.
A level of 5% was set as the criterion for statistical significance. The data were collected in an Excel spreadsheet. The statistical analysis was performed using SYSTAT version 10 (Copyright © SPSS Inc., 2000).
The appropriate sample size was calculated based on the assumption of a difference of 12% in overall recurrence after ORR or converted LRR. This difference was considered relevant based on previous studies [26], and a sample size of 247 patients (46 CONV and 201 OPEN patients) was needed to prove this difference (α set at 0.05; β set at 0.2; power = 80%).
Results
Between January 1996 and December 2011, a total of 537 patients underwent elective rectal resection for non-metastatic rectal cancer: 216 patients were treated with an open approach (OPEN group), and 321 patients had a laparoscopic resection. In 49 (15.3%) patients, LRR was converted to open surgery (CONV group).
Table 1 summarizes baseline patients’ characteristics, showing no significant differences in sex, age, body mass index, ASA score, number of comorbidities, Charlson comorbidity index, tumor location and use of neoadjuvant CRT between the three groups.
Intraoperative results
Table 2 reports the type of procedures performed in the three groups. Among patients undergoing anterior resection, a protective stoma was constructed in a similar rate of patients in the three groups.
Among the 49 patients who had LRR converted to ORR, conversion to open surgery was due to locally advanced rectal cancer in 16 (32.7%) cases. Obesity and adhesions secondary to previous abdominal operations were the reason for conversion in 15 (30.6%) and 5 (10.2%) patients, respectively (Table 2). A preemptive conversion to open surgery was performed in 46 (93.9%) patients, while a reactive conversion to an intraoperative complication occurred in only 3 cases (6.1%), due to bleeding (1 case) or small bowel injury (2 cases). No rectal perforation occurred in both groups.
Median operative time and median estimated blood losses were lower in the LAP group, while no significant differences were observed between CONV and OPEN group (Table 2).
Early postoperative outcomes
Return of bowel function was quicker after completed LRR, while no significant differences were observed between CONV and OPEN patients.
Overall 30-day postoperative morbidity rates did not differ significantly between LAP, CONV and OPEN group. In particular, similar rates of blood transfusion (4.1 vs. 2.3%, P = 0.839), wound infection (4.1 vs. 0.9%, P = 0.324), cardiac complications (0 vs. 2.8%, P = 0.517), chest infection (0 vs. 1.4%, P = 0.935), anastomotic leakage (7.5 vs. 8.1%, P = 0.850) and need for reoperation (4.1 vs. 4.6%, P = 0.831) were reported after CONV and OPEN rectal resection. Mortality rate was 0% in the LAP and CONV groups and 1.4% in the OPEN group (Table 3).
Median length of postoperative hospital stay was 2 days shorter after LAP surgery, while there were no differences between CONV and OPEN patients.
Pathologic results
The number of lymph nodes resected, the positive margin rates and the TNM stage distribution did not differ between LAP, CONV and OPEN groups (Table 4).
Long-term oncologic results
Median follow-up was 74 (range, 12–228) months for all LAP patients, 75 (range, 12–233) months for all CONV patients and 84 (range, 12–240) months for all OPEN patients (P = 0.198). Median follow-up for patients alive at the time of analysis was 100 (range, 60–228) months for LAP patients, 102 (range, 60–233) months for CONV patients and 123 (range, 60–240) months for ORR (P = 0.291). A total of 39 (7.3%) patients were lost to follow-up. As a consequence, 498 patients were considered for the long-term oncologic analysis: 251 LAP patients, 46 CONV patients and 201 OPEN patients.
Adjuvant chemotherapy was administered in 131 (52.2%) LAP patients, 29 (63%) CONV patients and in 103 (51.2%) OPEN patients (P = 0.338).
Overall recurrence rate was 24.3% in the LAP group, while it was 41.3% in the CONV group and 27.9% in the OPEN group (P = 0.107). LR developed in 12 (4.8%) LAP patients, in 5 (10.9%) CONV patients and in 13 (6.5%) OPEN patients (P = 0.264). Distant metastases rate was 19.5% (49 patients), 30.4% in the CONV group (14 patients) and 21.4% in the OPEN group (43 patients; P = 0.251). There were no significant differences in median time for local recurrence [21.5 (range 12–56) months in the LAP group, 20 (range, 13–54) months in the CONV group and 19 (range, 6–58) months in the OPEN group (P = 0.926)] and distant metastases [23.5 (range, 2–80) months in the LAP group, 17 (range, 4–83) months in the CONV group and 18 (range, 3–58) months in the OPEN group (P = 0.591)] between the three groups.
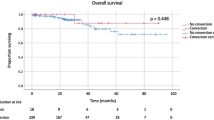
Both 5-year OS and DFS rates did not significantly differ between LAP, CONV and OPEN patients: 83.9 versus 77.8 versus 81.0% (P = 0.398) and 74.5 versus 62.9 versus 72.7% (P = 0.145), respectively. Survival curves of CONV and OPEN patients are shown in Fig. 1a, b.
Survival rates were similar among CONV patients regardless of the cause of conversion. No significant differences in 5-year OS and DFS were observed between patients who had converted LRR for a locally advanced tumor or non-tumor-related reasons: 81.2 versus 80.8% (P = 0.839) and 62.5 versus 63.7% (P = 0.970), respectively.
On univariate analysis, G3, pT3–4 rectal cancer, LNR ≥ 0.25 and lymphovascular invasion were significant risk factors for OS and DFS (Tables 5 and 6).
On multivariate analysis, G3, LNR ≥ 0.25 and lymphovascular invasion were the only independent predictors of OS and DFS (Tables 5 and 6).
Discussion
Both early and long-term oncologic outcomes in rectal cancer patients who have a converted LRR are poorly investigated. Most studies have been designed aiming at evaluating if converted patients have worse early and oncologic outcomes than patients undergoing a laparoscopic completed rectal resection [18,19,20,21]. However, the most clinically relevant question that should be raised in order to better select rectal cancer patients for LRR or ORR and improve their outcomes is: “Are the patient’s postoperative outcomes different if he undergoes a converted LRR or a planned ORR?”. Unfortunately, there are very few and heterogeneous studies [22,23,24,25,26] that have attempted to answer this question comparing converted LRR to primary ORR, and the results are controversial. As a consequence, it is unclear if conversion leads to worse outcomes than primary ORR and therefore it should be prevented by preferring an open approach in those patients with preoperatively known risk factors for conversion.
We herein report a conversion rate of LRR to ORR of 15.3%, with a locally advanced rectal cancer being the most common reason for conversion (32.7%), followed by obesity and adhesions. These results are consistent with those reported in previous non-randomized comparative studies [21, 29] and randomized controlled trials [22, 30]. In the present study, the conversion to ORR was preemptive in most cases, while it was reactive to a complication in only 6% of patients (3 cases). We observed no significant differences in operative time and intraoperative blood loss between CONV and OPEN patients, suggesting that a preemptive conversion does not lead to adverse intraoperative outcomes. To date, the impact of preemptive and reactive conversion and the best timing for conversion of a laparoscopic colorectal resection are poorly investigated. For instance, Yang et al. [31] found in a retrospective case-match study that 60 patients after a preemptive had lower morbidity, earlier resumption of a regular diet and a shorter postoperative hospital stay than 30 patients who had a reactive conversion. However, Aytac et al. [32] did not confirm these results, reporting no statistically significant differences in rates of overall morbidity and readmission between 30 patients who had a reactive conversion and 240 patients who had a preemptive conversion. The same authors investigated the potential impact of timing of conversion on postoperative outcomes. They failed to find a threshold for conversion, reporting similar complication rates after early or late conversion. Further large studies are needed to identify a threshold for conversion in technically challenging operations and shed more light on the possible short-term effects of a late conversion occurring after a prolonged laparoscopic dissection in rectal cancer patients.
There are very limited and conflicting data in the literature about the occurrence of intraoperative complications, such as lesion to intraabdominal organs and rectal perforation in patients who have a converted LRR. The results of a national registry study [23] showed significantly higher rates of intraoperative complications such as bleeding, ureteral and splenic injury among 201 CONV patients than among 16308 OPEN rectal cancer patients, and the conversion rate was almost doubled in LRR lasting more than 180 min. Our strategy is to avoid any prolonged laparoscopic tissue dissection and any protracted manipulation of the tumor with the laparoscopic tools in order to minimize the risk of injury to intraabdominal organs and tumor spillage. In the present study, no intraoperative rectal perforation occurred, even in the presence of a locally advanced rectal tumor in more than 70% of CONV patients. This finding does not confirm the alarming results reported by Penninckx et al. [25], analyzing the PROCARE database. They found a significantly higher incidence of this feared complication among CONV patients: 21 versus 9.4% (P = 0.001). However, the interpretation of these results is challenged by the fact that even though the experience with TME and laparoscopic TME was assessed per each center, the level of training in laparoscopic TME of each surgeon participating in the PROCARE project was not known.
Some studies [22, 23] reported significantly higher rates of postoperative complications, including wound infections, pneumonia, anastomotic leakages and 30-day mortality after converted LRR than ORR for rectal cancer. For instance, among the CONV and OPEN patients enrolled in the CLASICC trial, the wound infection rate was 20 versus 12%, the chest infection rate was 15 versus 5%, while an anastomotic leak occurred in 15 versus 7%, respectively. Conversely, other series [24, 25] failed to find impaired short-term outcomes in patients who had a converted LRR when compared to OPEN patients. In the present series, overall morbidity rate was 16.3% among CONV patients and 18.9% after ORR. Minor and major complication rates according to the Clavien–Dindo classification were similar (Clavien–Dindo 1–2 10.2 vs. 10.6% and Clavien–Dindo 3–5 6 vs. 6%, respectively) and compared favorably with the literature data [24].
It has been reported that the conversion of a laparoscopic colorectal resection is associated with poor OS and DFS [13]. However, some recent studies have shown that several variables, such as tumor-related characteristics (T stage and LNR), but not conversion per se, are independent predictors of survival, suggesting that poorer survival is more likely multifactorial [11]. The systemic inflammatory response in case of perioperative complications in these patients might also play a role in impairing long-term oncologic outcomes [13].
The evidence about oncologic outcomes after converted laparoscopic rectal resection for cancer is weak, being mainly based on studies with a short follow-up period. For instance, Rickert et al. [24] reported the oncologic outcomes in 38 CONV patients and 114 OPEN patients after a median follow-up of 34 months (range, 1–70). No significant differences were observed after CONV or OPEN rectal resection in LR rate (3 vs. 4.5%), distant metastases rate (9 vs. 10.1%) and in 3-year OS (84 vs. 85%). Similar results were reported by Penninckx et al. [25]: At 3 years after surgery, relative survival rate was 92.2% after CONV resection and 88.1% after OPEN resection. However, the evaluation of the oncologic outcomes in these patients was highly limited by the lack of follow-up data for too many patients that did not allow to assess both LR rate and DFS. The only study that reports long-term follow-up results is the CLASICC trial [33]. With a median overall follow-up of 62.9 months, Green et al. found no significant differences in OS and DFS after CONV or OPEN rectal resection after adjustment for prognostic factors, age, sex and TNM stage, suggesting that conversion does not have adverse impact on survival.
To the best of our knowledge, our study comparing converted LRR and ORR for rectal cancer has the longest follow-up. We were able to include in the oncologic analysis 46 CONV patients and 201 OPEN patients with a median follow-up of 75 months in the CONV group and 84 months in the OPEN group. We observed slightly higher rates of LR and distant metastases in the CONV group, but the differences did not reach the statistical significance. As a consequence, there was a trend toward a lower 5-year DFS in CONV patients (62.9 vs. 72.7%, P = 0.104), while 5-year OS rates were very similar (77.8 vs. 81%). The multivariate analysis showed that G3, LNR ≥ 0.25 and lymphovascular invasion, but not conversion, were the independent predictors of OS and DFS. However, the trend toward a lower 5-year DFS requires a careful consideration, and further studies are needed to confirm these data. Interestingly, survival rates were similar among CONV patients regardless of the cause of conversion. It might be argued that similar OS rates may be secondary to a shorter follow-up interval and more frequent postoperative outpatient evaluations of CONV patients who have higher postoperative morbidity rate than OPEN patients. However, in the present study there were no differences in postoperative morbidity among the two groups. We feel that the good results achieved after converted LRR in this study are related to our attitude to consider early conversion in locally advanced rectal cancers, thus reducing prolonged operative times, avoiding the risk of suboptimal oncologic dissection and reducing the rates of postoperative complications.
The present study has some limitations. First, this study was conducted at a single large academic institution; as a consequence, the results may not be generalized. Second, LRR and ORR were performed by different surgeons. However, the ORRs were performed by skilled surgeons in colorectal surgery, while all LRRs were performed by surgeons who were highly experienced in both colorectal and laparoscopic surgeries; furthermore, the first 40 laparoscopic resections were excluded to avoid the effect of the learning curve [34, 35]. As previously reported [11], conversion rate to open surgery does not seem to change even after more than 100 laparoscopic procedures. This fact might be due to the attitude to early convert the laparoscopic resection in challenging cases, such as in obese patients and in the presence of bulky tumors. Third, it is a retrospective study. Nevertheless, this is an analysis of a prospectively collected database that included three homogeneous groups of patients followed up for a median period of time longer than 6 years. In addition, there were no missing data in the three groups regarding intraoperative, early and late postoperative outcomes, and the study was powered to detect possible significant differences in the overall recurrence rate.
Conclusion
Conversion of LRR to ORR for non-metastatic rectal cancer does not seem to affect the short-term outcomes and jeopardize long-term survival. Based on these data and in the absence of validated models that predict the chance of a laparoscopic rectal resection to be converted to open surgery, we feel that the laparoscopic approach should be attempted even in those rectal cancer patients with preoperatively known risk factors for conversion.
References
Arezzo A, Passera R, Scozzari G, Verra M, Morino M (2013) Laparoscopy for rectal cancer reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Surg Endosc 27:1485–1502
Schlachta CM, Mamazza J, Seshadri PA, Cadeddu MO, Poulin EC (2000) Predicting conversion to open surgery in laparoscopic colorectal resections: a simple clinical model. Surg Endosc 14:1114–1117
Tekkis PP, Senagore AJ, Delaney CP (2005) Conversion rates in laparoscopic colorectal surgery: a predictive model with 1253 patients. Surg Endosc 19:47–54
Cima RR, Hassan I, Poola VP, Larson DW, Dozois EJ, Larson DR, O’Byrne MM, Huebner M (2010) Failure of institutionally derived predictive models of conversion in laparoscopic colorectal surgery to predict conversion outcomes in an independent data set of 998 laparoscopic colorectal procedures. Ann Surg 251(4):652–658
Vaccaro CA, Rossi GL, Quintana GO, Soriano ER, Vaccarezza H, Rubinstein F (2014) Laparoscopic colorectal resections: a simple predictor model and a stratification risk for conversion to open surgery. Dis Colon Rectum 57(7):869–874
Zhang GD, Zhi XT, Zhang JL, Bu GB, Ma G, Wang KL (2015) Preoperative prediction of conversion from laparoscopic rectal resection to open surgery: a clinical study of conversion scoring of laparoscopic rectal resection to open surgery. Int J Colorectal Dis 30:1209–1216
Belizon A, Sardinha CT, Sher ME (2006) Converted laparoscopic colectomy: what are the consequences? Surg Endosc 20:947–951
Franko J, Fassler SA, Rezvani M, O’Connell BG, Harper SG, Nejman JH, Zebley DM (2008) Conversion of laparoscopic colon resection does not affect survival in colon cancer. Surg Endosc 22:2631–2634
Ptok H, Kube R, Schmidt U, Köckerling F, Gastinger I, Lippert H, Colon/Rectum Carcinoma (Primary Tumor) Study Group (2009) Conversion from laparoscopic to open colonic cancer resection - associated factors and their influence on long-term oncological outcome. Eur J Surg Oncol 35:1273–1279
Li JC, Lee JF, Ng SS, Yiu RY, Hon SS, Leung WW, Leung KL (2010) Conversion in laparoscopic-assisted colectomy for right colon cancer: risk factors and clinical outcomes. Int J Colorectal Dis 25:983–988
Allaix ME, Degiuli M, Arezzo A, Arolfo S, Morino M (2013) Does conversion affect short-term and oncologic outcomes after laparoscopy for colorectal cancer? Surg Endosc 27:4596–4607
Chew MH, Ng KH, Fook-Chong MC, Eu KW (2011) Redefining conversion in laparoscopic colectomy and its influence on outcomes: analysis of 418 cases from a single institution. World J Surg 35:178–185. https://doi.org/10.1007/s00268-010-0824-6
Clancy C, O’Leary DP, Burke JP, Redmond HP, Coffey JC, Kerin MJ, Myers E (2015) A meta-analysis to determine the oncological implications of conversion in laparoscopic colorectal surgery. Colorectal Dis 17:482–490
Li J, Guo H, Guan XD, Cai CN, Yang LK, Li YC, Zhu YH, Li PP, Liu XL, Yang DJ (2015) The impact of laparoscopic converted to open colectomy on short-term and oncologic outcomes for colon cancer. J Gastrointest Surg 19:335–343
Masoomi H, Moghadamyeghaneh Z, Mills S, Carmichael JC, Pigazzi A, Stamos MJ (2015) Risk factors for conversion of laparoscopic colorectal surgery to open surgery: does conversion worsen outcome? World J Surg 39:1240–1247. https://doi.org/10.1007/s00268-015-2958-z
Yerokun BA, Adam MA, Sun Z, Kim J, Sprinkle S, Migaly J, Mantyh CR (2016) Does conversion in laparoscopic colectomy portend an inferior oncologic outcome? Results from 104,400 patients. J Gastrointest Surg 20:1042–1048
Allaix ME, Furnée EJ, Mistrangelo M, Arezzo A, Morino M (2016) Conversion of laparoscopic colorectal resection for cancer: What is the impact on short-term outcomes and survival? World J Gastroenterol 22(37):8304–8313
Agha A, Fürst A, Iesalnieks I, Fichtner-Feigl S, Ghali N, Krenz D, Anthuber M, Jauch KW, Piso P, Schlitt HJ (2008) Conversion rate in 300 laparoscopic rectal resections and its influence on morbidity and oncological outcome. Int J Colorectal Dis 23:409–417
Rottoli M, Bona S, Rosati R, Elmore U, Bianchi PP, Spinelli A, Bartolucci C, Montorsi M (2009) Laparoscopic rectal resection for cancer: effects of conversion on short-term outcome and survival. Ann Surg Oncol 16:1279–1286
Yamamoto S, Fukunaga M, Miyajima N, Okuda J, Konishi F, Watanabe M (2009) Impact of conversion on surgical outcomes after laparoscopic operation for rectal carcinoma: a retrospective study of 1073 patients. J Am Coll Surg 208:383–389
Laurent C, Leblanc F, Wütrich P, Scheffler M, Rullier E (2009) Laparoscopic versus open surgery for rectal cancer: long term oncologic results. Ann Surg 250:54–61
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM, MRC CLASICC Trial Group (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Mroczkowski P, Hac S, Smith B, Schmidt U, Lippert H, Kube R (2012) Laparoscopy in the surgical treatment of rectal cancer in Germany 2000–2009. Colorectal Dis 14(12):1473–1478
Rickert A, Herrle F, Doyon F, Post S, Kienle P (2013) Influence of conversion on the perioperative and oncologic outcomes of laparoscopic resection for rectal cancer compared with primarily open resection. Surg Endosc 27:4675–4683
Penninckx F, Kartheuser A, Van de Stadt J, Pattyn P, Mansvelt B, Bertrand C, Van Eycken E, Jegou D, Fieuws S (2013) Outcome following laparoscopic and open total mesorectal excision for rectal cancer. Br J Surg 100:1368–1375
Keller DS, Khorgami Z, Swendseid B, Champagne BJ, Reynolds HL Jr, Stein SL, Delaney CP (2014) Laparoscopic and converted approaches to rectal cancer resection have superior long-term outcomes: a comparative study by operative approach. Surg Endosc 28:1940–1948
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Edge SB, Compton CC (2010) The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474
Ng KH, Chung-Kei D, Cheung HY et al (2009) Laparoscopic resection for rectal cancers: lessons learned from 579 cases. Ann Surg 249:82–86
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E, COLOR II Study Group (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372:1324–1332
Yang C, Wexner SD, Safar B, Jobanputra S, Jin H, Li VK, Nogueras JJ, Weiss EG, Sands DR (2009) Conversion in laparoscopic surgery: Does intraoperative complication influence outcome? Surg Endosc 23:2454–2458
Aytac E, Stocchi L, Ozdemir Y, Kiran RP (2013) Factors affecting morbidity after conversion of laparoscopic colorectal resections. Br J Surg 100:1641–1648
Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM (2013) Long-term follow-up of the medical research council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100:75–82
Bottger TC, Mohseni D, Beardi J, Rodehorst A (2011) Learning curve in laparoscopic rectum surgery. Zentralbl Chir 136:273–281
Park IJ, Choi GS, Lim KH, Kang BM, Jun SH (2009) Multidimensional analysis of the learning curve for laparoscopic colorectal surgery: lessons from 1000 cases of laparoscopic colorectal surgery. Surg Endosc 23:839–846
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Allaix, M.E., Furnée, E., Esposito, L. et al. Analysis of Early and Long-Term Oncologic Outcomes After Converted Laparoscopic Resection Compared to Primary Open Surgery for Rectal Cancer. World J Surg 42, 3405–3414 (2018). https://doi.org/10.1007/s00268-018-4614-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4614-x