Abstract
Background
This study examined utilization and conversion rates for robotic and laparoscopic approaches to non-metastatic rectal cancer. Secondary aims were to examine short- and long-term outcomes of patients who underwent conversion to laparotomy from each approach.
Methods
The National Cancer Database (NCDB) was reviewed for all cases of non-metastatic adenocarcinoma of the rectum or rectosigmoid junction who underwent surgical resection from 2010 to 2016. Utilization rates of robotic, laparoscopic, and open approaches were examined. Patients were split into cohorts by approach. Subgroup analyses were performed by primary tumor site and surgical procedure. Multivariable analysis was performed by multivariable logistic regression for binary outcomes and multivariable general linear models for continuous outcomes. Survival analysis was performed by Kaplan–Meier and multivariable cox-proportional hazards regression.
Results
From 2010 to 2016, there was a statistically significant increase in utilization of the robotic and laparoscopic approaches over the study period and a statistically significant decrease in utilization of the open approach. The conversion rates for robotic and laparoscopic cohorts were 7.0% and 15.7%, p < 0.0001. Subgroup analysis revealed statistically lower conversion rates between robotic and laparoscopic approaches for rectosigmoid and rectal tumors and for LAR and APR. Converted cohorts had statistically significant higher odds of short term mortality than the non-converted cohorts (p < 0.05).Laparoscopic conversion had statistically higher odds of positive margins (p < 0.0001) and 30-day unplanned readmission (p < 0.0001) than the laparoscopic non-conversion. Increased adjusted mortality hazard was seen for converted laparoscopy relative to non-converted laparoscopy (p = 0.0019).
Conclusion
From 2010 to 2016, there was a significant increase in utilization of minimally invasive approaches to surgical management of non-metastatic rectal cancer. A robotic approach demonstrated decreased conversion rates than a laparoscopic approach at the rectosigmoid junction and rectum and for LAR and APR. Improved outcomes were seen in the minimally invasive cohorts compared to those that converted to laparotomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The management of rectal adenocarcinoma is highly individualized, involving various sequences of chemoradiotherapy based both on the initial clinical stage and final pathologic diagnosis [1]. Despite advances in neoadjuvant and adjuvant therapies, curative surgical resection remains the cornerstone of treatment. While the principles of a total mesorectal excision have not changed, evolving minimally invasive techniques represent a paradigm shift in the management of this disease.
With regard to such post-operative metrics as analgesia use, length of stay, and return of bowel function, laparoscopic approaches to rectal cancer have clear advantages over traditional laparotomy [2]. However, the technical demands of a laparoscopic pelvic dissection for rectal cancer have been attributed with increased risk of conversion to open [3]. Additionally, technical limitations of limited range of motion, loss of dexterity, and two-dimensional instrument articulations have led to scrutiny of the appropriateness of laparoscopy for the management of rectal cancer in several trials [2, 4,5,6].
Robot-assisted proctectomy was developed to overcome the technical shortcomings of laparoscopy, while maintaining the benefits of a minimally invasive approach. Benefits offered by the robotic platform include improved ergonomics, three dimensional, high resolution imaging, and fully articulating instruments. Published in 2017, the Robotic Versus Laparoscopic Resection for Rectal Cancer (ROLARR) trial sought to examine the difference in conversion rate between a robotic and a laparoscopic rectal cancer resection [4]. Their study revealed conversion to open rates from robotic and laparoscopic approaches of 8.1% vs 12.2%, respectively [4]. This difference in conversion rates, the authors concluded, was not significant enough to justify its use over laparoscopy in rectal cancer surgery [4].
Despite various conclusions in the literature, the utilization of minimally invasive techniques has continued to increase, and robotic surgery has eclipsed laparoscopy in certain centers [7]. Rate of conversion to laparotomy is often cited as a surrogate for proficiency and open conversions have been shown to have both short and long oncologic implications [8,9,10]. However, to date no large population study has analyzed how conversion rates have changed as utilization of minimally invasive techniques continue to increase. Moreover, no large population studies have examined short-and long-term oncologic consequences of conversion from a minimally invasive approach to laparotomy. Thus, the primary aim of this study was to examine trends in minimally invasive technique utilization and rates of conversion to open in non-metastatic rectal cancer. Furthermore, this study aimed to investigate potential oncologic consequences in patients who undergo conversion to laparotomy from a robotic or laparoscopic approach.
Materials and methods
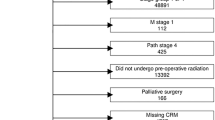
This was a retrospective cohort study of clinical data from The National Cancer Database NCDB registry from 2010 to 2016. The NCDB is a clinical oncology database, sourced from hospital registry data collected from over 1500 Commission on Cancer accredited facilities across the United States. The NCDB contains de-identified data, and therefore this study was deemed exempt by our institutional review board. No written consent was required. The NCDB Rectum Participant User File (PUF) was reviewed for patients diagnosed with invasive adenocarcinoma, identified using histology ICDO-3 code 8140/3, who underwent partial or total proctectomy (Procedural Codes 30 and 50, respectively), which included low anterior resection and abdominoperineal resection. The database began collecting data for surgical approach in 2010, and thus diagnoses prior to 2010 and those with an open or missing surgical approach data were excluded. Patients with Stage IV disease were excluded as were cases without valid staging, treatment, or follow-up data (Fig. 1).
Baseline demographics and clinicopathologic characteristics included age, sex, Charlson score, year of diagnosis, insurance, high school degree %, median income, facility type, clinical stage, systemic chemotherapy surgery sequence, and systemic radiation therapy surgery sequence. Outcomes assessed in univariate and multivariate analyses were number of regional nodes examined, positive margins, negative circumferential margin, 30-day unplanned readmission, 30-and 90-day mortality, and days to receipt of chemotherapy in those who received adjuvant chemotherapy. Baseline demographics, clinicopathologic characteristics, and unadjusted outcomes were compared by way of Chi-square or Fisher’s exact test for adequate cell count categorical variables and low cell count categorical variables (> 25% of expected cell counts < 5), respectively. Independent samples t-test was used for parametric continuous variable comparisons and Mann–Whitney U test was used for nonparametric continuous variable comparisons. Variables with corresponding univariate p value < 0.2 were entered into multivariable models and adjusted for as potential confounding covariates following a backward stepwise selection procedure with stay criteria α = 0.1. Covariates for adjustment included age, sex, race, primary site, procedure, Charlson score, insurance, facility type, clinical stage, systemic chemotherapy surgery sequence, and systemic radiation therapy surgery sequence. Multivariable logistic regression was used for categorical outcomes and natural logarithm (ln) transformed multivariable general linear models were used for positively skewed continuous outcomes. Survival analysis was performed using Kaplan–Meier estimation with corresponding Log-Rank at the univariate level, followed by multivariable Cox-proportional hazards regression. Analysis of multicollinearity in multivariable models was performed by way of a variance inflation factor analysis (VIF) with VIF < 2 considered acceptable.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and two-sided p value less than 0.05 was considered statistically significant. The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB were the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Results
For patients diagnosed with rectal adenocarcinoma between 2010 and 2016, 7947 patients underwent a robotic approach, 16,772 patients underwent a laparoscopic approach, and 26,136 patients underwent an open approach. The utilization of robotic and laparoscopic approaches significantly increased while the utilization of an open approach significantly decreased over the duration of the study (p < 0.0001; Fig. 2). Among the patients who underwent a minimally invasive approach, no statistically significant staging differences existed between the robotic and laparoscopic cohort, nor were there any differences in income or education. Relative to laparoscopic approach, robotic approach was significantly associated with a lower proportion of females, black patients, lower Charlson scores, and a higher proportion of private insurance, academic facility type, receipt of radiation therapy and chemotherapy (all respective p < 0.05; Table 1).
In the majority of patients in the robotic and laparoscopic groups, primary tumor site was classified C20.9 (“rectum”). Primary tumor site was less frequently classified as C19.9 ("rectosigmoid") in the robotic cohort than the laparoscopic cohort (14.2% vs 25.2%, p < 0.0001). In both groups, the procedure was classified as NCDB Procedure Code 30 (partial proctectomy, low anterior resection [LAR]) more frequently than Procedure Code 50 (total proctectomy, abdominal perineal resection [APR]). The approach was more frequently classified as APR in the robotic group than the laparoscopic group (22.0% vs 16.8%, p < 0.0001) (Table 1).
The difference in rates of conversion to laparotomy between robotic and laparoscopic approaches was statistically significant (7.0% vs 15.7%, p < 0.0001; Fig. 4). The trends in conversion rates for each approach are seen in Fig. 3. The robotic conversion to open rate did not significantly change over time (p = 0.4599), however, the laparoscopic conversion to open rate throughout the years significantly decreased (p < 0.0001).
Separate conversion subgroup analyses were performed for each approach with regard to primary tumor site and procedure. There were fewer conversions from a robotic approach than a laparoscopic approach at the rectosigmoid (6.2% vs 13.2%, p < 0.0001) and at the rectum (6.1% vs 12.7%, p < 0.0001; Fig. 4). Additionally, there were fewer conversions from a robotic approach than a laparoscopic approach for LAR (7.2% vs 14.9%, p < 0.0001) and APR (6.3% vs 17.0%, p < 0.0001; Fig. 4).
Univariate analysis for binary outcomes between robotic, robotic converted, laparoscopic, and laparoscopic converted approaches prior to adjusting for potential confounding covariates is seen in Table 2. Subsequent multivariable analysis showed that robotic conversion was significantly associated with increased adjusted odds of 30-day mortality (p = 0.0288) and 90-day mortality (p = 0.0046) compared to the non-converted robotic group. No difference was seen in regional nodes examined, positive margins, negative circumferential resection margins, or unplanned readmission between the robotic conversion and robotic cohorts (Table 3). In those who received adjuvant chemotherapy, no difference was seen in days from surgery to receipt of chemotherapy between the non-converted robotic and converted robotic cohort (p = 0.1337; Tables 4, 5).
Relative to the non-converted laparoscopic group, laparoscopic conversion was significantly associated with increased adjusted odds of 12 or more regional lymph nodes examined (p = 0.0013), positive margins (p < 0.0001), 30-day unplanned readmission (p < 0.0001), and 90-day mortality (p < 0.0222; Table 3). Increased adjusted odds of 30-day mortality trended towards significance (p = 0.0582) and no difference was detected in negative circumferential resection margins (p = 0.5096). In those who received adjuvant chemotherapy, those who received laparoscopic conversion had 6.0% ± 2.7% longer time from surgery to chemotherapy relative to those who received non-converted laparoscopy (reverse transformed mean ± standard error; p = 0.0306; Tables 4, 5).
No significant differences in adjusted odds of 12 or more nodes examined, negative circumferential margin, 30-day unplanned readmission, 30-day mortality, or 90-day mortality were detected between the converted robotic and converted laparoscopic cohorts. A trend-level significant observation with a clinically relevant effect size was observed to show decreased adjusted odds of positive margin status in those who underwent robotic conversion relative to those who underwent laparoscopic conversion (p < 0.1; Table 3).
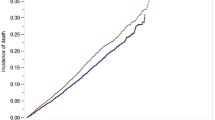
Kaplan Meier estimation illustrates survival in Fig. 5. Five-year overall survival for the non-converted robotic, converted robotic, non-converted laparoscopic, converted laparoscopic, and open cohorts were 74.1%, 67.7%, 73.6%, 69.7%, and 67.4%, respectively. Multivariable Cox-proportional hazards regression further elucidated increased adjusted mortality hazard for converted laparoscopy relative to non-converted laparoscopy (p = 0.0019). No difference in adjusted mortality hazard was detected between converted and non-converted robotic cohorts or converted laparoscopy and converted robotic cohorts (Table 6).
Discussion
To date, ROLARR represents the largest randomized controlled trial on robotic surgery, and is therefore considered the strongest available evidence on the platform. Despite the authors’ conclusions, many surgeons feel that a robotic approach for rectal cancer is oncologically safe and subsequent analyses have found lower conversion rates than seen in ROLARR [11]. The present study found both a lower rate of robotic to open conversions and higher rate laparoscopic to open conversions than in ROLARR. Likewise, Crippa et al. conducted a retrospective analysis of 600 patients undergoing laparoscopic and robotic rectal cancer surgery and found that robotic surgery was associated with a statistically significant reduction risk of conversion (5.0% vs 13.8%) [12]. A recent systematic review and meta-analysis of 681 patients across 12 countries demonstrated a significant difference in risk of intraoperative conversion to open surgery between robotic and laparoscopic surgery, with the risk begin lower in the robotic cohort [11]. These results were unchanged after removing the study of lowest quality in the sensitivity analysis.
Several explanations exist as to why these subsequent analyses have generated conclusions counter to ROLARR. Among the limitations stated by the authors of ROLAAR, is that “operations in that trial were performed, on average, by a surgeon considered to be an expert in conventional laparoscopic surgery and who may still have been in their learning phase for robotic surgery” [4], potentially producing falsely lower laparoscopic conversion rates and falsely higher robotic conversion rates than are seen among the surgical population as a whole. While their sensitivity analysis does address this discrepancy, a trial that included 40 surgeons considered expert in laparoscopy raises the question of generalizability of their results.
This analysis observed a significant increase in utilization of the laparoscopic approach over time and that the laparoscopic conversion rate statistically decreased over the from 2010 to 2016 (19.5% vs 13%, p < 0.0001) (Fig. 3). This has been observed in the literature as well. In the 2005 landmark CLASICC trial, the conversion rate was noted to be 34% [3] much higher than in the subsequent ROLARR trial (12.2%) [4], and this analysis (15.7%). A unique strength of this analysis is that we observed this trend within a single study, which has yet to be described. These findings contribute to the existing literature by suggesting that, as utilization of laparoscopy increases during residency and fellowship training and independent practice, improvement in skill and experience with these approaches are potentially translating to a decreasing laparoscopic conversion rates over time.
While the utilization of robotics also significantly increased and the conversion rate was lower in 2016 than 2010 (7.0% vs. 6.0%), the observable change was not statistically significant. Like the surgeons in ROLAAR, those who contributed to NCDB may have also still been in the learning phase for robotic surgery from 2011 to 2014. However, in the subsequent years, the conversion rate appeared to plateau, despite rapidly rising robotic utilization. Two explanations may account for the discrepancy between conversion trends between the laparoscopic and robotic approaches. The first is the different learning curves associated with these two approaches for rectal cancer resections. Jiménez-Rodríguez et al. conducted a systematic review of the literature and sought to investigate the learning curve in robotic rectal cancer resections compared to laparoscopy [13]. Their analysis found that most published studies observe a shorter learning curve for robotic resections versus laparoscopic resections, citing studies which determined the learning curve for robotics to be 15–35 cases, significantly lower than the 30–70 surgeries cited for a laparoscopic approach [14,15,16,17,18]. Thus, the shorter learning curve seen in robotics may translate to lower conversion rates early in the learning phase which subsequently remain stable over time [19]. Another explanation is simply that, as the conversion rate started low (7%), there may not reasonably be more room for significant improvement below this rate.
While conversion in NCDB is a binary variable, the circumstances behind conversion are often multifactorial and can involve patient, surgeon, and tumor-related factors. One limitation to the NCDB is the lack of details on the reason for and timing of conversion to laparotomy. In their series, Crippa et al. noted that poor vision, adhesions, and intra-abdominal obesity were the main reasons for conversion from laparoscopy, but not from robotic [12]. Additionally, they concluded that the reduction in risk of conversion between robotic and laparoscopic approaches was conserved in both obese and non-obese patients [12]. However, the authors did note that bleeding complications accounted for a quarter of the robotic conversions. Factors such as working on a distant console and a time-consuming undocking process likely affected these surgeons’ decision to convert from a robotic approach to laparotomy [12]. Similarly, Bhama et al. found that risk factors for laparoscopic colorectal resection included obesity, moderate adhesions, and severe adhesions [20]. Of these three risk factors, only severe adhesions were a risk factor for conversion with the robotic approach [20]. Thus, while our data demonstrates that overall conversion rates differ between approaches, published literature highlights unique challenges with regard to the decision to convert to laparotomy from either a robotic or laparoscopic approach.
The secondary objective of this analysis was to examine the consequences of conversion to open both in terms of short-term oncologic measures and in survival. Though our analysis showed that a robotic converted to open approach was significantly associated with increased adjusted odds of 30-day mortality (p = 0.0288) and 90-day mortality (p = 0.0046) compared to the robotic non-converted group, this did not translate to statistically significant differences in 5-year survival between the two groups (aHR [95% CI] 1.21 [0.96–1.52], 0.1086). However, significant differences in both short- and long-term outcomes were seen between the laparoscopic and laparoscopic converted groups. While laparoscopic conversion to open was significantly associated with increased adjusted odds of 12 or more regional lymph nodes examine (p = 0.0013), conversion was associated with increased odds of positive margins (p < 0.0001), 30-day unplanned readmission (p < 0.0001), and 90-day mortality (p < 0.0222). In those who received adjuvant chemotherapy, days from surgery to receipt of adjuvant chemotherapy also statistically different in multivariable analysis (p = 0.0306). Furthermore, multivariable cox-proportional hazards regression revealed increased adjusted mortality hazard for converted laparoscopy relative to non-converted laparoscopy (p = 0.0019) but not for converted robotic relative to non-converted robotic (p = 0.1086).
Allaix et al. conducted a MEDLINE search of currently available evidence on the impact of conversion in rectal cancer on both short-term outcomes and long-term survival. They found that the results after conversion from laparoscopy were less favorable than those achieved by than in the laparoscopic non-converted patients, observing both lower OS and DFS in the case of conversion in laparoscopic rectal cancer [21]. In our analysis, we observed significant differences in number of lymph nodes examined and positive margins between the laparoscopic converted and non-converted groups, but not between the robotic converted and non-converted groups, potentially impacting OS and DFS. Majbar et al. observed that conversion to laparotomy in patients undergoing rectal resection was associated with higher rates of postoperative complications, anastomotic leaks, and reoperations. Conversion was also an independent predictive factor to postoperative morbidity in their multivariate analysis.[22]. Thus, the long-term effects of conversion in these patients are likely multifactorial, related to tumor status, postoperative complication rate, and potentially the inflammatory response incited by conversion to laparotomy [23].
Kim et al. conducted an analysis to evaluate how the timing of open conversion influences short-term and oncologic outcomes after minimally invasive surgery for colorectal cancer. In their study, patients were classified into early (within 60 min of procedure start) or late (after 60 min) conversion groups. Between the early conversion and non-conversion groups, mean operative time was longer in the early conversion group, but rates of 30-day postoperative complications, time to soft diet, and hospital stay were not different statistically different. However, between the late conversion and non-converted groups, rates of 30-day postoperative complications, intensive care unit care and transfusion were significantly higher in the late conversion group. Additionally, time to soft diet and hospital stay were longer in the late conversion group compared to non-converted. Ultimately, recurrence-free and cancer-specific survival rates did not differ among the early, late conversion, and non-converted groups [24]. While our analysis could not examine the timing of conversion, this would have been helpful in identifying risks associated with post-operative outcomes in these patients, as data suggests that open conversion within 60 min of the beginning of surgery may not worsen short-term and oncologic outcomes.
Numerous limitations exist in this study. Common to all retrospective reviews, this study is prone to selection bias, with surgeons’ potential selection patients with more favorable BMI, tumor characteristics, or less extensive history of abdominal surgery, ultimately affecting the decision of surgical approach and threshold for conversion. While the NCDB offers the Charlson score, which is comparable to the American Society of Anesthesiologists (ASA) score, it offers no data with regard to BMI, prior abdominal surgeries, nutritional markers, specific tumor height etc. Mentioned earlier, the NCDB does not offer details on reason or timing for conversion, which as discussed, have major implications on the reason for conversion and potentially differences in outcomes between groups. Wide variation in surgeon experience with the various approaches likely existed, with no means in NCDB to control for years of experience or duration from residency or fellowship training. While this adds skill level heterogeneity to each sample, lack of descriptors prevented us from performing a skill-based sensitivity analysis between groups. Regarding the analysis of time to adjuvant chemotherapy, only those who were coded as receiving adjuvant therapy were included in this specific analysis, regardless of tumor status, node status, or post-operative mortality. Thus, those who qualified for adjuvant therapy but experienced complications that may have resulted in forgoing therapy were excluded in the analysis, introducing some error in this metric.
Additionally, the issue of power must be discussed as the low rates of robotic and laparoscopic conversion significantly reduce our sample sizes, and in turn our power, when we analyze conversions. This lends itself to increased probability of type II error, or false non-inferiority. For example, the trend-level significant (p < 0.1) difference between positive margins between converted robotic and converted laparoscopic cases would require sample sizes of 1311 robotic conversions and 6185 laparoscopic conversions to detect our observed effect size as statistically significant at p < 0.05 (α = 0.05) with minimum adequate power of 80%. This is roughly 2.5 times the conversion cases that our sample provides us. We have additionally weighed the observed adjusted effect sizes (aORs, aHRs, etc.) with our observed conclusions of statistical significance. Although, per the example, every comparison in our study is not adequately powered, we are observing clinically negligible effect sizes associated with non-significant p values for all comparisons other than trend-level significant findings. Because of this, once more years of data accrue, similar comparisons with higher power are warranted for triangulation of conclusions. Finally, certain outcomes such as disease-free survival and recurrence rates are absent from NCDB which could aid in the overall oncologic assessment of these patients.
This analysis of a large, national oncology database demonstrated that the utilization of minimally invasive approaches to rectal adenocarcinoma are increasing while rates open approach utilization and rates of conversion to laparotomy are decreasing. Additionally, a robotic approach was associated with significantly less conversions at the rectosigmoid junction and rectum and for LAR and APR. Moreover, we identified a statistically significant mortality hazard associated with laparoscopic conversion that was not seen in the robotic group. Ultimately, these factors should be considered when deciding on a minimally invasive strategy for resection of rectal adenocarcinoma.
References
Cameron JL, Cameron AM (2019) Current Surgical Therapy. Elsevier, Amsterdam
Van der Pas MHGM, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WCJ et al (2013) COlorectal cancer laparoscopic or conversion in laparoscopic and robotic rectal cancer surgery open resection II (COLOR II) study group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218
Guillou PJ, Quirke P, Thorpe H et al (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365(9472):1718–1726. https://doi.org/10.1016/S0140-6736(05)66545-2
Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J (2017) Effect of roboticassisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA. https://doi.org/10.1001/jama.2017.7219
Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, Jung KH, Kim TY, Kang GH, Chie EK, Kim SY, Sohn DK, Kim DH, Kim JS, Lee HS, Kim JH, Oh JH (2014) Open versus laparoscopic surgery for mid-rectal or lowrectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non- inferiority, randomized controlled trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(14)70205-0
Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H (2015) Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. https://doi.org/10.1001/jama.2015.10529
Baek SK, Carmichael JC, Pigazzi A (2013) Robotic surgery: colon and rectum. Cancer J. https://doi.org/10.1002/bjs.11435
Gouvas N, Georgiou PA, Agalianos C, Tzovaras G, Tekkis P, Xynos E (2018) Does conversion to open of laparoscopically attempted rectal cancer cases affect short- and long-termoutcomes? A systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A 28:117–126
Duraes LC, Steele SR, Camargo MGM, Gorgun E, Kalady MF, Valente M et al (2019) Conversion to open from laparoscopic colon resection is a marker for worse oncologic outcomes in colon cancer. Am J Surg 217:491–495
Lee YF, Albright J, Akram WM, Wu J, Ferraro J, Cleary RK (2018) Unplanned robotic-assisted conversion-to-open colorectal surgery is associated with adverse outcomes. J Gastrointest Surg 22:1059–1067
Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, Jimenez-Rodriguez RM, Gurrado A, Strippoli GFM (2018) Robotic versus laparoscopic minimally invasive surgery for rectal cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. https://doi.org/10.1097/SLA.0000000000002523
Crippa J, Grass F, Achilli P, Mathis KL, Kelley SR, Merchea A, Colibaseanu DT, Larson DW (2020) Risk factors for conversion in laparoscopic and robotic rectal cancer surgery. Br J Surg. https://doi.org/10.1002/bjs.11435
Jiménez-Rodríguez RM, Rubio-Dorado-Manzanares M, Díaz-Pavón JM, Reyes-Díaz ML, Vazquez-Monchul JM, Garcia-Cabrera AM, Padillo J, De la Portilla F (2016) Learning curve in robotic rectal cancer surgery: current state of affairs. Int J Colorectal Dis. https://doi.org/10.1007/s00384-016-2660-0
Jimenez-Rodriguez RM, Diaz-Pavon JM, de la Portilla, de Juan F, Prendes-Sillero E, Dussort HC, Padillo J (2013) Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Color Dis 28:815–821
Yamaguchi T, Kinugasa Y, Shiomi A, Sato S, Yamakawa Y, Kagawa H, Tomioka H, Mori K (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29:1679–1685
Sng KK, Hara M, Shin JW, Yoo BE, Yang KS, Kim SH (2013) The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc 27:3297–3307
Park EJ, Kim CW, Cho MS, Baik SH, Kim DW, Min BS, Lee KY, Kim NK (2014) Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3 phase learning process comparison. Surg Endosc 28:2821–2831
Foo CC, Law W (2014) The learning curve of robotic-assisted low rectal resection of a novice rectal surgeon. World J Surg 40:456–462
Koerner C, Rosen S (2019) How robotics is changing and will change the field of colorectal surgery. RLD J Gastrointest Surg 11(10):381–387
Bhama AR, Wafa AM, Ferraro J, Collins SD, Mullard AJ, Vandewarker JF, Krapohl G, Byrn JC, Cleary RK (2016) Comparison of risk factors for unplanned conversion from laparoscopic and robotic to open colorectal surgery using the michigan surgical quality collaborative (MSQC) database. J Gastrointest Surg 20:1223–1230
Allaix ME, Furnée EJ, Mistrangelo M, Arezzo A, Morino M (2016) Conversion of laparoscopic colorectal resection for cancer: what is the impact on short-term outcomes and survival? World J Gastroenterol 22(37):8304–8313. https://doi.org/10.3748/wjg.v22.i37.8304
Majbar AM, Abid M, Alaoui M et al (2016) Impact of conversion to open surgery on early postoperative morbidity after laparoscopic resection for rectal adenocarcinoma: a retrospective study. J Laparoendosc Adv Surg Tech A 26(9):697–701. https://doi.org/10.1089/lap.2016.0027
Clancy C, O’Leary DP, Burke JP, Redmond HP, Coffey JC, Kerin MJ, Myers E (2015) A meta-analysis to determine the oncological implications of conversion in laparoscopic colorectal cancer surgery. Colorectal Dis 17:482–490
Kim IY, Kim BR, Kim YW (2017) Impact of timing of conversion to open surgery on short-term and oncologic outcomes in patients undergoing minimally invasive surgery for colorectal cancer. Am Surg 83(1):71–77
Funding
This study was performed without grant support or support from other financial relationships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Salvatore Parascandola, Dr. Salini Hota, Mr. Andrew Sparks, Sameh Boulos, Kathryn Cavallo, Dr. George Kim, declares no conflicts of interest or financial ties to disclose. Dr. Vincent Obias, consultant for Medrobotics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parascandola, S.A., Hota, S., Sparks, A.D. et al. Trends in utilization, conversion rates, and outcomes for minimally invasive approaches to non-metastatic rectal cancer: a national cancer database analysis. Surg Endosc 35, 3154–3165 (2021). https://doi.org/10.1007/s00464-020-07756-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07756-5