Abstract
Background
Laparoscopic gastrectomy (LG) is now a widely accepted treatment option for gastric cancer. However, there is insufficient evidence for LG for advanced gastric cancer (AGC). Many retrospective studies have shown that LG for AGC is safe and feasible, but very few studies have shown the actual outcome in general practice. The aim of this study is to analyze our last 15 years of experience in LG for AGC.
Methods
This is a retrospective review from May 2003 to May 2017 in Seoul National University Bundang Hospital. A total of 1592 patients who had LG for AGC were enrolled of which 109 patients with open conversion were excluded. We evaluated the short-term and long-term oncologic outcomes of LG for AGC.
Results
A total of 1483 patients were analyzed. There were 432 cases of total gastrectomy, 982 cases of distal gastrectomy, and 69 cases of proximal gastrectomy. The total complication rate was 9.1% (135/1483), which included wound-related complications (0.7%), postoperative bleeding (0.5%), anastomosis or stump leakage (2.2%), intestinal obstruction (0.9%), pancreatic fistula (0.1%), intra-abdominal abscess (1.6%), and lung morbidity (3.0%). The rate of Clavien-Dindo grade 3 and above complications was 4.9%. Age was the only significant risk factor in multivariate analysis (OR 1.02; 95% CI, 1.01–1.04, P = 0.01). 5-year overall survival stratified by stage was as follows: stage IB 88.9%, stage IIA 88.7%, stage IIB 84.2%, stage IIIA 71.7%, stage IIIB 56.8%, stage IIIC 45.4%, and stage IV 25%. Total recurrence rate was 14.4%, which included local recurrence (1.1%) and distant metastases (13.3%).
Conclusions
During our 15 years of experience, we have successfully performed 1483 cases of AGC with laparoscopy. Our results showed short-term and long-term oncologic outcomes that were comparable with other studies. LG is safe and feasible in general practice for advanced gastric cancer when performed by experienced surgeons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic gastrectomy (LG) is now a widely accepted treatment option for gastric cancer since its introduction by Kitano et al., especially for early gastric cancer (EGC). [1] The Japanese JCOG 0912 study reported similar complication rates after laparoscopic and open distal gastrectomy (DG) for EGC, while the Korean KLASS-01 study showed that laparoscopic DG was associated with lower incidence of overall postoperative complications [2, 3]. KLASS-01 study also proved non-inferiority of laparoscopy over open DG in long-term survival. [4] There are many retrospective studies that show that LG for advanced gastric cancer (AGC) is safe and feasible when compared with open gastrectomy (OG) [5,6,7]. However, most of these studies are limited by their small sample size. In 2008, we initiated the first prospective single arm phase 2 trial of LG for AGC [8]. We showed acceptable morbidity and mortality rate, demonstrating the safety and technical feasibility of laparoscopy for AGC. The recent Chinese CLASS-01 trial showed similar 3-year survival between laparoscopy and open. [9] The long-term results of KLASS-02 and JLSSG0901 are pending [10]. Well-controlled clinical studies are important for building evidence for laparoscopy. However, they do not reflect the actual outcome of laparoscopy for AGC in real-life practice. Therefore, we aim to analyze all AGC patients who received laparoscopic gastrectomy for the last 15 years since the opening of our institution. The endpoints studied are postoperative morbidity and long-term oncologic outcome. To our knowledge, this is the largest study to show real-life long-term outcome in general practice.
Materials and methods
Patient population
This is a retrospective review of electronic medical records from May 2003 to May 2017 in Seoul National University Bundang Hospital. A total of 1592 patients underwent LG for AGC during this 15-year time period. All patients were included in this analysis, except for 109 patients who had open conversion from laparoscopy. From 2003 to 2008, patients with serosa-exposed tumors underwent open gastrectomy. A total of 1483 patients who successfully underwent LG were analyzed. Patient consent for the use of retrospective hospital data was not necessary for this study (IRB Approval No B-1908/558-101).
Surgical techniques in laparoscopic gastrectomy for AGC
After initiation of our prospective phase II clinical trial, we gradually extended our indications for LG, with resultant increase in case numbers. Endoscopic ultrasonography for AGC was not routinely performed. CT (Computed Tomography) was performed for preoperative staging. Lymph node dissection (LND) was done for tumors from cT2 to cT4a according to the Korean and Japanese guidelines. [11, 12] D2 dissection was not possible in some cases due to patient factors, and a minimal LND (D1) was performed instead. D2 + LND is defined as the excision of additional LNs outside the D2 area, including paraaortic LNs. For DG, we performed Billroth-I, Billroth-II with or without Braun jejunojejunostomy, Roux-Y gastrojejunostomy, and uncut Roux-Y gastrojejunostomy. For all total gastrectomies (TG), Roux-Y esophagojejunostomy and jejunojejunostomy was performed. The first laparoscopic proximal gastrectomy (PG) was performed in 2004, and up to 2009, 7 patients with AGC had undergone PG with Esophago-gastrostomy (E–G stomy). After 2009, we started performing Double-Tract-Reconstruction (DTR) technique because of its lower incidence of late complications such as gastroesophageal reflux and anastomotic stenosis [13]. The anastomosis technique for each gastrectomy type was not analyzed in this study.
Definition of recurrence
Tumors can recur near the previous operative field or away, which sometimes presents as distant metastasis. We defined local recurrence as recurrence occurring at the remnant stomach (either synchronous or metachronous), anastomosis site, or regional LNs. Distant metastasis included peritoneal, solid organ, and para-aortic LN metastasis. Peritoneal metastasis was diagnosed with CT or diagnostic laparoscopy and solid organ metastasis was determined with CT, MRI, or PET/CT imaging.
Survival analysis
All Korean citizens have mandatory national health insurance where all medical cost spent and survival status is reported to the government. Therefore, survival data was obtained from the National Statistics Service of Korea, allowing us to accurately calculate the survival rate.
Statistical analysis
Continuous values are expressed as the mean and standard deviation. To identify the risk factors for postoperative morbidity, univariate and multivariate analyses were performed using Chi- square tests and binary logistic regression models. Survival analysis was done with Kaplan–Meier method, and survival probabilities were compared using log-rank test. Data were analyzed using SPSS statistics software, version 20 (IBM Inc, Armonk, NY, USA) and software R (Version 3.3.2, R Foundation for Statistical Computing, Vienna, Austria). A P value ≤ 0.05 was considered to be statistically significant for all analyses.
Results
Clinicopathologic characteristics according to gastrectomy types
Table 1 shows the patient demographics of all the patients according to gastrectomy types. DG was the most common type of surgery (66.2%) followed by TG (29.1%) and PG (4.7%). TG had the longest operation time and the most estimated blood loss (EBL). There were no D2 or D2 + LND in PG due to the absence of D2 LND by definition [12]. 17 cases of D1 LND were performed for palliative gastrectomy cases. The mean number of LNs retrieved was about 61.4 ± 24.8. The most common histologic types were tubular adenocarcinoma moderately differentiated, followed by poorly differentiated, and then poorly cohesive carcinoma. 11.5% were classified as others, which contained mixed and neuroendocrine components. The mean tumor size was the largest in TG (7.1 ± 3.7 cm). All the stages were updated according to AJCC (8th edition). T and N stages are shown in the table. The most common T and N stage was T2 and N0 in total, but for TG, T3, T4a, and N3b were most common. According to our TNM stage data, IB (T2N0) was the most common (20.6%), and then IIA (17.9%), IIIA (17.7%), IIB (14.3%), IIIB (13.4%), IIIC (11.8%), and stage IV (4.2%).
Postoperative morbidity
Postoperative morbidity was defined as complications that occurred within 30 days of surgery. All complications were graded with the Clavien–Dindo (CD) scale [14]. The total complication rate was 9.1% (135/1483) (Table 2). The complications included wound complications (8%), postoperative bleeding (5.2%), anastomosis or stump leakage (23.7%), intestinal obstruction (10.4%), severe pancreatic fistula (0.7%), intra-abdominal abscess (17%), and lung morbidity (32.6%). Also, there was one case of remnant gastric infarction and two cases of colitis. 54% (73 cases) of all complications which were CD grade 3 and above were classified as severe. Four cases of wound complications needed repair with either local or general anesthesia, six postoperative bleeding patients had surgical exploration, and one patient died due to complications after bleeding. Radiologic intervention was done in 27 patients with anastomosis and duodenal stump leakage and in 21 patients with intra-abdominal abscess. Six patients underwent percutaneous drainage for pleural effusion, and one case of remnant gastric infarction received re-operation and conversion to total gastrectomy.
In the univariate analysis, age, ASA score, operation time, estimated blood loss, and total gastrectomy showed statistical significance as risk factors of postoperative morbidity (Table 3). However, on the multivariate analysis, age was the only risk factor with significant value (1.02, 95% CI 1.01–1.04) (P = 0.01).
Survival analysis
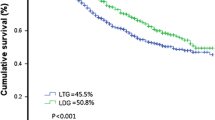
Table 4 shows the 5-year overall survival (OS) rate and Fig. 1 shows the Kaplan–Meier curve. Median follow-up was 35.1 months. 5-year OS was 88.9% for stage Ib, 88.7% for stage IIA, 84.2% for stage IIB, 60.3% for stage III (71.7% for stage IIIA, 56.8% for stage IIIB, 45.4% for stage IIIC), and 25% for stage IV.
Excluding 213 patients with other malignancy and palliative gastrectomy, including stage 4 cancer, we analyzed the disease-free survival (DFS) of the remaining 1270 patients (Table 4). 5-year DFS was 85.4% for stage IB, 87.2% for stage IIA, 78.1% for stage IIB, 65.6% for stage IIIA, 50.8% for stage IIIB, and 25.2% for stage IIIC.
Stage 4 gastric cancer
Survival data on stage 4 cancer were analyzed (Table 5). There are no reports on survival analysis of LG for stage 4 AGC cases in the literature. Among our cases, 37 cases (58.7%) had intraoperative peritoneal seeding and 13 cases (20.6%) showed postoperative malignant peritoneal cytology. Four patients had distant organ metastasis involving the liver, ovary, or appendix, which were resected together during the surgery. Some patients had paraaortic LN (N = 7) and station 14v metastasis (N = 5).
Recurrence analysis
Cancer-specific local recurrence and distant metastasis after the initial LG were analyzed (Table 6). Total recurrence rate was 14.4% (214/1483). There were 16 cases (1.1%) of local recurrence: remnant stomach (5 cases), anastomosis site (3 cases), and regional LN recurrence (8 cases). There were 198 cases (13.3%) of distant metastasis after surgery: peritoneum (78 cases), liver (35 cases), bone (14 cases), lung (6 cases), colon (12 cases), ovary (13 cases), paraaortic LN (32 cases), and other organs (small bowel, bladder, spleen, pleura, gallbladder, skin, and brain, 8 cases).
Discussion
While the incidence has been decreasing, gastric cancer is the fifth most frequently diagnosed cancer and the third leading cause of cancer death worldwide [15]. Currently, resection with appropriate lymphadenectomy is the only treatment approach that offers a chance of survival for GC patients [12]. The KLASS 01 and JCOG 0703 studies have shown non-inferiority in the safety of LG compared to OG concerning morbidity and mortality for EGC [3, 16, 17]. As for AGC, short-term morbidity, mortality, and long-term survival results of a Chinese randomized controlled trial(CLASS-01) proved LG to be safe. [9, 18].
D2 LND for TG and DG is the general recommendation in AGC [12]. In our study, there were 356 cases (24%) of D1 + LND which were not appropriate for AGC. This was due to technical difficulty during the early years and the changes in guidelines. Fifteen patients who received D1 LND had reasons such as palliative setting, severe medical co-morbidities, and uncontrollable condition under general anesthesia. Theoretically, PG is not indicated for AGC patients. S. Haruta et al. claimed that proximal gastrectomy can be applicable for AGC localized in the upper third of the stomach for less than T3 tumors that were smaller than 4 cm. [20] In our institution, we performed PG for tumors in the upper third of the stomach less than T3 and smaller than 3 cm. In nine patients who were elderly, comorbid patients with large and severe upper third tumors, we performed proximal gastrectomy for better quality of life after surgery.
In retrospective studies, the morbidity rate of LG for AGC ranged from 8 to 24.2% [5,6,7, 21,22,23]. Shinohara et al. reported a morbidity rate of 24.2% after LG with D2 LND for AGC (N = 186) and 1.1% (2 patients) of hospital deaths [6]. Inokuchi reported 17% morbidity rate after LG with D2 LND and showed similar types of complications [24]. Our results showed an overall complication rate of 9.1% (Table 2). Although we could not directly compare the results from different studies, our postoperative 30-day complication rates after LG with D2 lymphadenectomy for AGC showed better results than other studies. Anastomotic leakage, which is one major complication of gastric surgery, should be evaluated specifically. The anastomotic leakage rate in our study was 2.2%, which is within the range of 1.1% to 2.7% of previous reports [6, 21, 23, 25]. In our risk factor multivariate analysis, old age was the only significant value and this coincides with previous studies (Table 3) [26, 27]. In our institution’s previous clinical study, we reported that age over 70 was an independent risk factor for complications [8]. Extended surgery is still risky for the elderly especially if it is a difficult, time demanding, laparoscopic case. Finding the optimal choice of surgical access after an appropriate assessment of risks and benefits is important.
Conversion rate is an important index for laparoscopic technique. LG with D2 LND is a demanding procedure and surgeons tend to convert to open if laparoscopy becomes unsafe. Our open conversion rate was 6.8%, with the following reasons: advanced stage (54.1%), uncontrolled bleeding (17.4%), severe adhesion due to previous operation (9.2%), small abdominal cavity (3.7%), unstable vital signs after CO2 inflation (3.7%), and intraoperative pleural injury during surgery (1.8%). “Advanced stage” includes large, serosa-invading tumors, and tumors invading adjacent structures. Until 2008, if the tumor was exposed to the serosa, open conversion was routinely performed. When we analyzed the cases performed in the recent 5 years, open conversion rate was 3.4%, with advanced stage being the most common reason (1.7%) followed by uncontrolled bleeding (0.9%), severe adhesion (0.4%), unstable vital sign (0.2%), and pleural injury (0.2%). This may be due to the adoption of a standardized laparoscopic technique by the same group of surgeons. Even with many open conversions in the early years, our open conversion rate is comparable to previous reports of 2.2 to 7% [6, 8, 19].
Our survival data showed a comparable 5-year OS and DFS compared to other studies (Table 4). A multicenter retrospective data from Korea reported 5-year OS of 90.5%, 86.4%, 78.3%, 52.8%, 52.9%, and 37.5%, respectively [21]. A recent COATC 1001 study by Park et al. also showed a high 3-year DFS: 94.1% for stage I, 87.6% for stage II, and 56.6% for stage III. According to our data, stage III showed a 5-year OS of 60.3%. However, stage IIIA (71.7%), IIIB (56.8%), and IIIC (45.4%) showed a big difference in survival, so a separate analysis of these stages seems important in survival analysis. All the patients from stage II and above were sent for further chemotherapy consult and 72% (851/1178) subsequently underwent adjuvant chemotherapy. Our chemotherapy rate is low compared to other controlled trials (100%) such as COATC 1001 trials, which is expected in real-world data, but still shows good survival outcome.
There are no reports on survival analysis of LG for stage 4 AGC cases. Gastrectomy was carried out and a minimum of D1 LND was done for all these patients. In cases with paraaortic LN and station 14v metastases, we resected all LNs except for one case of paraaortic LN metastasis due to extensive disease. Aggressive attempts, albeit performed by experienced expert laparoscopic gastric cancer surgeons, to remove the primary tumor and extensive LN dissection, subject to the patients’ condition, could have resulted in the high survival rate of 25%. However, caution is required in treating patients with peritoneal seeding. Peritoneal seeding was associated with a significantly lower survival than the other stage 4 patients (5-year OS 13.1% vs. 39.4%, respectively, P = 0.0136). Therefore, patient selection is crucial before performing surgery in patients with peritoneal seeding.
In a recent long-term analysis, Li et al. reported recurrence patterns after LG for AGC [28]. Peritoneal recurrence being the most common (12.4%), locoregional recurrence was 10.7%, hematogenous recurrence was 6.1%, multiple patter recurrence was 4.1%, and distant LN metastasis was shown in 2.4%. Also, Kinoshita et al. recently reported 89 cases (89/261, 34%) of recurrence after R0 resection in the LOC-A study, with peritoneal recurrence being the most common also (42/261, 16.1%) [29]. Our study showed a comparable recurrence rate (Table 6). Distant metastasis after LG can be explained by invisible micro-metastasis during or before surgery, but local recurrence is possible associated with the adequacy of surgery. There is a great concern in clinical practice whether the application of LG for AGC has the possibility of malignant cell dissemination and local recurrence [30, 31]. However, our real-life long-term data show comparable results to other studies.
Since this is a retrospective review of hospital medical records, there are limitations. Firstly, the surgical and medical procedures were not standardized for each patient. Surgery quality, anastomosis technique, LND, patient management, and the guidelines for gastrectomy have changed over time. These are huge factors that influence the data, but they are an inescapable part of real-world data and provide a true representation of outcome in a general practice. Secondly, hospital records were not well organized in the early years of our practice. Complications, surgical records, and pathologic LN station division were not always documented clearly. To circumvent this problem with regards to survival and follow-up data, we utilized data obtained from National Statistics Service of Korea which provides reliable data on survival. Thirdly, as surgical volume increased, more surgeons were recruited and involved in the surgery. The surgeons gained experience and procedures became standardized as time passed. Analyzing only the recent few years might help to remove these confounding factors in data analysis, but we wanted to present the real-life experience of our institution in its entirety.
During our 15 years of experience, we have performed 1483 cases of laparoscopic gastrectomy for AGC. With no controlled values nor omitted data, we present in this study our complete dataset. There has been much development of procedures and instruments in the last 15 years. However, our real-life data showed comparable, yet better morbidity and long-term oncologic outcomes, which provides evidence that LG for AGC is safe and feasible in general practice.
References
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M (2017) Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer 20:699–708. https://doi.org/10.1007/s10120-016-0646-9
Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song K, Song KY, Lee SI, Ryu SY, Lee JH, Lee HJ, Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group (2016) Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg 263(1):28–35. https://doi.org/10.1097/SLA.0000000000001346
Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH, Hyung WJ, for the Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group (2019) Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer. JAMA Oncol 263(1):28–35. https://doi.org/10.1001/jamaoncol.2018.6727
Hamabe A, Omori T, Tanaka K, Nishida T (2012) Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc 26:1702–1709. https://doi.org/10.1007/s00464-011-2096-0
Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, Inaba K, Yanaga K, Uyama I (2013) Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc 27:286–294. https://doi.org/10.1007/s00464-012-2442-x
Kim K-H, Kim MC, Jung G-J, Choi H-J, Jang J-S, Kwon H-C (2012) Comparative analysis of 5 year survival results of laparoscopy-assisted gastrectomy versus open gastrectomy for advanced gastric cancer: a case-control study using a propensity score method. Dig Surg 29:165–171. https://doi.org/10.1159/000338088
Lee J-H, Son S-Y, Lee CM, Ahn S-H, Park DJ, Kim H-H (2013) Morbidity and mortality after laparoscopic gastrectomy for advanced gastric cancer: results of a phase II clinical trial. Surg Endosc 27:2877–2885. https://doi.org/10.1007/s00464-013-2848-0
Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G, Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group (2019) Effect of laparoscopic vs open distal gastrectomy on 3 year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 Randomized Clinical Trial. JAMA 321:1983–1992. https://doi.org/10.1001/jama.2019.5359
Hur H, Lee HY, Lee H-J, Kim MC, Hyung WJ, Park Y-K, Kim W, Han S-U (2015) Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer 15:355. https://doi.org/10.1186/s12885-015-1365-z
Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel (2019) Korean Practice Guideline for Gastric Cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer 19:1–48. https://doi.org/10.5230/jgc.2019.19.e8
Kodera Y, Sano T (2016) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20:1–19. https://doi.org/10.1007/s10120-016-0622-4
Ahn S-H, Jung D-H, Son S-Y, Lee CM, Park DJ, Kim H-H (2014) Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer 17:562–570. https://doi.org/10.1007/s10120-013-0303-5
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 49:509–531. https://doi.org/10.3322/caac.21492
Kim H-H, Hyung WJ, Cho GS, Kim MC, Han S-U, Kim W, Ryu S-W, Lee H-J, Song KY (2010) Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report–a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 251:417–420. https://doi.org/10.1097/SLA.0b013e3181cc8f6b
Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, Yamaue H, Yoshikawa T, Kojima K, JCOG Gastric Cancer Surgical Study Group (2010) Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer 13:238–244. https://doi.org/10.1007/s10120-010-0565-0
Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, Xue Y, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Chen P, Liu H, Zheng C, Liu F, Yu J, Li Z, Zhao G, Chen X, Wang K, Li P, Xing J, Li G (2016) Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a Randomized Controlled Trial. J Clin Oncol 34:1350–1357. https://doi.org/10.1200/JCO.2015.63.7215
Lee J, Kim W (2009) Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: analysis of consecutive 106 experiences. J Surg Oncol 100:693–698. https://doi.org/10.1002/jso.21400
Haruta S, Shinohara H, Hosogi H, Ohkura Y, Kobayashi N, Mizuno A, Okamura R, Ueno M, Sakai Y, Udagawa H (2017) Proximal gastrectomy with exclusion of no. 3b lesser curvature lymph node dissection could be indicated for patients with advanced upper-third gastric cancer. Gastric Cancer 20:528–535. https://doi.org/10.1007/s10120-016-0624-2
Park DJ, Han SU, Hyung WJ, Kim MC, Kim W, Ryu SY, Ryu SW, Song KY, Lee HJ, Cho GS, Kim HH, Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group (2012) Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc 26:1548–1553. https://doi.org/10.1007/s00464-011-2065-7
Hwang SI, Kim HO, Yoo CH, Shin JH, Son BH (2009) Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg Endosc 23:1252–1258. https://doi.org/10.1007/s00464-008-0140-5
Kim W, Song KY, Lee H-J, Han S-U, Hyung WJ, Cho GS (2008) The impact of comorbidity on surgical outcomes in laparoscopy-assisted distal gastrectomy: a retrospective analysis of multicenter results. Ann Surg 248:793–799. https://doi.org/10.1097/SLA.0b013e3181887516
Inokuchi M, Nakagawa M, Tanioka T, Okuno K, Gokita K, Kojima K (2018) Long- and short-term outcomes of laparoscopic gastrectomy versus open gastrectomy in patients with clinically and pathological locally advanced gastric cancer: a propensity-score matching analysis. Surg Endosc 32:735–742. https://doi.org/10.1007/s00464-017-5730-7
Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, Lee HJ, Cho GS, Han SU, Hyung WJ, Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group (2008) Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale korean multicenter study. Ann Surg Oncol 15:2692–2700. https://doi.org/10.1245/s10434-008-0075-z
Kodera Y, Sasako M, Yamamoto S, Sano T, Nashimoto A, Kurita A, Gastric Cancer Surgery Study Group of Japan Clinical Oncology Group (2005) Identification of risk factors for the development of complications following extended and superextended lymphadenectomies for gastric cancer. Br J Surg 92:1103–1109. https://doi.org/10.1002/bjs.4979
Park DJ, Lee H-J, Kim HH, Yang HK, Lee KU, Choe KJ (2005) Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg 92:1099–1102. https://doi.org/10.1002/bjs.4952
Li Z, Li B, Bai B, Yu P, Lian B, Zhao Q (2018) Long-term outcomes of laparoscopic versus open D2 gastrectomy for advanced gastric cancer. Surg Oncol 27:441–448. https://doi.org/10.1016/j.suronc.2018.05.022
Kinoshita T, Uyama I, Terashima M, Noshiro H, Nagai E, Obama K, Tamamori Y, Nabae T, Honda M, Abe T, LOC-A Study Group (2018) Long-term outcomes of laparoscopic versus open surgery for clinical stage II/III gastric cancer: a Multicenter Cohort Study in Japan (LOC-A Study). Ann Surg 263(5):887–894. https://doi.org/10.1097/SLA.0000000000002768
Zhang F, Lan Y, Tang B, Hao Y, Shi Y, Yu P (2017) Comparative study of laparoscopy-assisted and open radical gastrectomy for stage T4a gastric cancer. Int J Surg 41:23–27. https://doi.org/10.1016/j.ijsu.2017.01.116
Xu Y, Hua J, Li J, Shi L, Yuan J, Du J (2017) Laparoscopic versus open gastrectomy for gastric cancer with serous invasion: long-term outcomes. J Surg Res 215:190–195. https://doi.org/10.1016/j.jss.2017.03.048
Acknowledgements
There is nothing to acknowledge.
Funding
No funding was received
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Sa-Hong Min, Yongjoon Won, Guowei Kim, Yoontaek Lee, Young Suk Park, Sang-Hoon Ahn, Do Joong Park, and Hyung-Ho Kim have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Min, SH., Won, Y., Kim, G. et al. 15-year experience of laparoscopic gastrectomy in advanced gastric cancer: analysis on short-term and long-term oncologic outcome. Surg Endosc 34, 4983–4990 (2020). https://doi.org/10.1007/s00464-019-07292-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-07292-x