Abstract
Background
Laparoscopy-assisted gastrectomy (LAG) has been established as a low-invasive surgery for early gastric cancer. However, it remains unknown whether it is applicable also for advanced gastric cancer, mainly because the long-term results of LAG with D2 lymph node dissection for advanced gastric cancer have not been well validated compared with open gastrectomy (OG).
Methods
A retrospective cohort study was performed to compare LAG and OG with D2 lymph node dissection. For this study, 167 patients (66 LAG and 101 OG patients) who underwent gastrectomy with D2 lymph node dissection for advanced gastric cancer were reviewed. Recurrence-free survival and overall survival time were estimated using Kaplan–Meier curves. Stratified log-rank statistical evaluation was used to compare the difference between the LAG and OG groups stratified by histologic type, pathologic T status, N status, and postoperative adjuvant chemotherapy. The adjusted Cox proportional hazards regression models were used to calculate the hazard ratios (HRs) of LAG.
Results
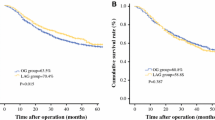
The 5-year recurrence-free survival rate was 89.6% in the LAG group and 75.8% in the OG group (nonsignificant difference; stratified log-rank statistic, 3.11; P = 0.0777). The adjusted HR of recurrence for LAG compared with OG was 0.389 [95% confidence interval (CI) 0.131–1.151]. The 5-year overall survival rate was 94.4% in the LAG group and 78.5% in the OG group (nonsignificant difference; stratified log-rank statistic, 0.4817; P = 0.4877). The adjusted HR of death for LAG compared with OG was 0.633 (95% CI 0.172–2.325).
Conclusions
The findings show that LAG with D2 lymph node dissection is acceptable in terms of long-term results for advanced gastric cancer cases and may be applicable for advanced gastric cancer treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Laparoscopy-assisted gastrectomy (LAG) for the treatment of gastric cancer was first performed by Kitano et al. [1] in 1991. Thereafter, laparoscopic gastrectomy for early gastric cancer gradually gained acceptance, and many retrospective studies have demonstrated its advantages over open gastrectomy (OG) in terms of less pain, better cosmetic results, better postoperative respiratory function, and faster recovery [2–6]. Some prospective studies have shown that operative safety and the rate of postoperative complications are comparable between LAG and OG [7–9].
Several studies investigating the long-term results of LAG for early gastric cancer are already available, demonstrating that overall survival and recurrence-free survival time are within acceptable limits. Based on the accumulation of these short- and long-term results, LAG for early gastric cancer has gained popularity and has been adopted in many medical centers in recent years.
With regard to advanced gastric cancer, however, the indication for LAG is controversial because few studies have compared the long-term results between LAG with D2 lymph node dissection and OG [10], although some studies on the short-term results are already available. Currently, standardization of the laparoscopic gastrectomy procedure and progression in the design of surgical devices have enabled us to practice LAG for advanced gastric cancer with the same confidence that we perform OG, and we hypothesized that laparoscopic surgery for gastric cancer had no negative effects on the long-term results in advanced gastric cancer cases.
This study aimed primarily to show the long-term results of LAG with D2 lymph node dissection for advanced gastric cancer, thereby validating the possibility of including LAG as a treatment for advanced gastric cancer. Additionally, the technical safety and oncologic feasibility of LAG with D2 lymph node dissection also were evaluated to confirm the short-term results of this procedure.
Patients and methods
From Jan 2000 to Dec 2009, a total of 1,099 patients with gastric cancer underwent gastrectomy at our institution according to a review of our prospective gastric cancer database and electronic medical records. Tumor node metastasis (TNM) staging was based on the Japanese Classification of Gastric Carcinoma, 2nd English edition [11].
We identified cases to be analyzed as follows. First, we extracted 485 patients who had undergone total or distal gastrectomy for gastric cancer preoperatively diagnosed clinically as T1 or T2 without distant lymph node (N3) or distant organ metastasis (M1, H1). Of the 485 cases, gastrectomy with D2 lymph node dissection was performed in 360 cases (195 cases with OG and 165 with LAG). We excluded cases with surgical findings of invasion to adjacent structures (SI), distant metastasis (M1, H1), positive washing cytology (CY1), or peritonitis carcinomatosa (P1), as well as those with other organ resection except for gallbladder or spleen. We finally identified 101 cases in the OG group and 66 cases in the LAG group that were pathologically diagnosed as stages IB to IIIB and included all of these 167 cases in this study (Fig. 1).
No patient received neoadjuvant chemotherapy or radiotherapy. The median age of the patients was 66 years (range, 39–88 years) in the LAG group and 65 years (range, 28–83 years) in the OG group. The follow-up period was 912 days (range, 26–1,827 days) in the LAG group and 1,604 days (range, 41–3,338 days) in the OG group.
Our guiding principle in performing lymph node dissection was that D2 dissection should be performed for potentially advanced, potentially node-positive, or undifferentiated histologic type gastric cancer even if the preoperative diagnosis is clinical T1N0. A signed informed consent was obtained preoperatively from all the patients assigned to LAG with D2 lymph node dissection after a sufficient explanation of the surgical and oncologic risks of this procedure.
The preoperative systemic workup for clinical staging at our institution consists of upper gastrointestinal endoscopy with biopsy, contrast-enhanced computed tomography, upper gastrointestinal radiographic contrast study, and abdominal ultrasonography. The postoperative surveillance schedule involves investigations every 3 months for the first 2 years, then every 6 months for the next 3 years. Periodic investigations consist of a physical examination, laboratory blood examination including the tumor markers of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), upper gastrointestinal endoscopy, abdominal ultrasonography, and computed tomography. Diagnosis of recurrence was based on imaging examination or pathologic findings of malignancy.
Recurrence-free survival and overall survival time were estimated using Kaplan–Meier curves. In this study, the stratified log-rank test was used to evaluate the differences between the LAG and OG groups, stratified by potential confounders including histologic type, pathologic T status, N status, and postoperative adjuvant chemotherapy. The Cox proportional hazards regression models were used to calculate the hazard ratios (HRs) with 95% confidence intervals (CI) for patients who underwent LAG, with adjustment for these four confounding factors. In addition, surgical outcomes (operative time, blood loss, number of dissected/metastasized lymph nodes, postoperative complications) and postoperative hospital stay also were analyzed using the Pearson χ2 test or the Wilcoxon rank-sum test.
Statistical analyses were performed using SAS software (version 9.2; SAS Institute Inc., Cary, NC, USA) and JMP software (8.0.1; SAS Institute Inc.). All values are expressed as mean ± standard deviation of the mean. All P values were two-tailed, and P values less than 0.05 were considered statistically significant.
Operative technique of laparoscopic gastrectomy
In the past, we have reported our surgical procedures of laparoscopy-assisted distal and total gastrectomy, focused primarily on reconstruction technique [12–14]. A summary of the LAG procedure at our institution is described in the following discussion.
The patient is placed on the table in a supine position with legs spread apart. The initial port is placed via a 2-cm infraumbilical incision made by the open method. A pneumoperitoneum is established with carbon dioxide insufflations at a pressure of about 8 mmHg. Four additional ports are inserted, and the operation is continued using the five-port technique. Lymph nodes in stations no. 7, 8, 9, 10, 11p, 11d, 12a, and 14v are resected by D2 lymph node dissection in addition to D1 dissection depending on tumor location [11].
In performing LAG with D2 lymph node dissection, we routinely make it a practice to check each anatomic landmark. In station no. 8 dissection, the common hepatic artery and the bifurcation of the proper hepatic artery and the gastroduodenal artery are exposed along the plane of the periarterial plexus. Next to this exposure, the lymph nodes are dissected from the retroperitoneum. After the no. 8 dissection, the left gastric artery is cut, and stations no. 7 and 9 lymph nodes are successively dissected from the periarterial plexus.
In the station no. 12a dissection, the supraduodenal artery and the right gastric artery are cut, and the proper hepatic artery is skeletonized to the line on which the left edge of the portal vein can be seen. In station no. 11p dissection, lymph nodes are dissected to the dorsal plane on which the splenic vein or pancreas can be identified. In station no. 11d dissection, the posterior gastric artery is sacrificed, and lymph nodes located between the splenic artery and vein are cleared. In station no. 10 dissection, the lymph nodes are dissected from the medial to the lateral side. To avoid surgery-related complications, we do not use combined splenectomy routinely, but only when cancer cells are likely to metastasize to station no. 10. In such cases, a clinically advanced tumor localizes mainly on the side of the greater curvature of the upper stomach [15, 16].
In station no. 14v dissection, lymph nodes bounded by the inferior edge of the pancreatic head and the anterior plane of the superior mesenteric vein are dissected after exposure of the root of the middle colic vein and gastrocolic trunk. In station no. 6 dissection, the right gastroepiploic vein is divided just distal to the bifurcation of the superior anterior pancreaticoduodenal vein.
In the beginning, a Billroth I reconstruction of laparoscopy-assisted distal gastrectomy (LADG) was performed via a 4-cm epigastric minilaparotomy retracted using a wound-sealing device (Alexis Wound Retractor; Applied Medical, Rancho Santa Margarita, CA, USA). However, since 2005, a 2.5 to 4-cm minilaparotomy has been performed by extending the umbilical incision, and gastroduodenostomy has been made via the umbilical laparotomy, as we reported previously [12]. In a similar fashion, Roux-en-Y reconstruction is performed via the umbilical laparotomy [13]. As for laparoscopy-assisted total gastrectomy (LATG), esophagojejunostomy is made by the efficient-purse string stapling technique, which was our original technique [14].
Results
Patient, tumor, and treatment characteristics
Patient, tumor, and treatment characteristics are summarized in Table 1. No significant differences could be observed in patient backgrounds, including age and gender. The OG group had significantly more cases with clinically advanced T status than the LAG group (P = 0.0413), although the pathologic T statuses were comparable (P = 0.2707). Other pathologic findings, histologic types, N statuses, and main locations of tumors also were comparable between the two groups.
The average tumor diameter was significantly greater in the OG group than in the LAG group (P = 0.0185). The number of distal/total gastrectomy cases was 45/21 in the LAG group and 66/35 in the OG group (nonsignificant difference; P = 0.7399). Roux-en-Y and Billroth II reconstruction were performed more frequently in the LAG group than in the OG group compared with Billroth I reconstruction (P = 0.0042). Gastrectomy with combined cholecystectomy was performed significantly more often in the OG group than in the LAG group (P = 0.0293), whereas the frequency of gastrectomy with combined splenectomy was similar in the two groups (P = 0.1650). No significant difference could be seen in the number of patients who received adjuvant chemotherapy (P = 0.5265).
Comparison of long-term results between LAG and OG
Of the 167 identified patients, 66 were treated with LAG and 101 were treated with OG. The 5-year recurrence-free survival rate was 89.6% in the LAG group and 75.8% in the OG group (Fig. 2). The recurrence-free survival time did not differ significantly between the two groups (stratified log-rank statistic, 3.11; P = 0.0777). Four cases with recurrence of peritonitis carcinomatosa were observed in the LAG group, but no local recurrence was observed. In the OG group, 22 cases with recurrence were observed including 9 liver metastases, 6 lymph node recurrences, 2 peritonitis carcinomatosa cases, 1 bone metastasis, 1 lung metastasis, 1 carcinomatous lymphangiosis, 1 carcinomatous pleuritis, and 1 local recurrence.
The 5-year overall survival rate was 94.4% in the LAG group and 78.5% in the OG group (Fig. 3). No significant difference was observed in overall survival time between the two groups (stratified log-rank test, 0.4817; P = 0.4877).
In the Cox proportional hazards regression models, adjusted for histologic type, pathologic T status, N status, and postoperative adjuvant chemotherapy, LAG and OG showed comparable HRs for gastric cancer recurrence and death. Tables 2 and 3 show that the adjusted HR was 0.389 (95% CI 0.131–1.151) for recurrence and 0.633 (95% CI 0.172–2.325) for death in the LAG group.
Comparison of surgical outcomes
The surgical outcomes for LAG and OG also were analyzed. In terms of operative indexes, LAG required a significantly longer operative time than OG (283.1 ± 57.5 vs. 225.9 ± 58.2 min; P < 0.0001). The estimated blood loss was significantly less in the LAG group (158.3 ± 249.8 ml) than in the OG group (356.3 ± 241.1 ml) (P < 0.0001).
No conversion to laparotomy was registered in the LAG group. The total number of harvested lymph nodes was 63.7 ± 26.4 in the LAG group and 44.0 ± 18.9 in the OG group (P < 0.0001). The number of metastasized lymph nodes was similar in the two groups (3.67 ± 6.47 vs. 2.77 ± 3.61; P = 0.6586).
Postoperative complications occurred for 16 LAG patients (24.2%) and for 23 OG patients (22.8%) (P = 0.8262). The complications in the LAG group included one pancreatic fistula, two ileuses, one leakage from the duodenal stump, two intraabdominal abscesses, and three stenoses. In the OG group, we recorded one pancreatic fistula, two ileuses, four anastomotic leakages, four intraabdominal abscesses, and two stenoses. The two groups did not differ significantly. The postoperative hospital stay was 19.8 ± 18.4 days in the LAG group and 23.5 ± 15.6 days in the OG group (P < 0.0001).
Discussion
Laparoscopy-assisted gastrectomy for gastric cancers has been used more widely in many medical centers since Kitano et al. [1] first performed LADG with Billroth I reconstruction for early gastric cancer in 1991. The LAG approach attracts an increasing number of patients and surgeons because of its expected low invasiveness and good cosmesis [7] despite its association with some unresolved oncologic problems.
Several prospective trials, including randomized controlled trials, have already shown postoperative advantages of LAG for the treatment of early gastric cancer such as postoperative earlier recovery of bowel movement and ambulation, less incisional pain, better postoperative pulmonary function, a lower rate of pulmonary complications, and fewer intraoperative complications [7–9, 17, 18]. Katai et al. [9] reported the acceptable data for postoperative complications including anastomotic leakage or pancreatic fistula of LADG with D1 plus suprapancreatic node dissection for clinical stage I gastric cancer in a multicenter, prospective trial. Recently, some retrospective studies have shown that the long-term results of LAG were acceptable in the management of early gastric cancer [19–21]. Based on these evidences, LAG has been recognized as an acceptable treatment for early gastric cancer.
For the treatment of advanced gastric cancer, however, LAG has not been established as the surgical procedure of choice. From the standpoint of advanced gastric cancer curability, gastrectomy with D2 lymph node dissection is required according to the Japanese Gastric Cancer Association (JGCA) guidelines [10, 22–24], but performing this operation by laparoscopic surgery requires a highly advanced technique. Some surgeons with prominent techniques have already practiced LAG with D2 lymph node dissection [25, 26]. Since Jan 2001, when we started performing LAG for early gastric cancer at our institution, we also have gradually extended the indication for LAG to advanced gastric cancer graded clinically as T2N1, which requires D2 lymph node dissection according to the JGCA guidelines [27].
Several publications have already described the short-term results of LAG with D2 dissection as equivalent to those of OG, and we similarly have observed less intraoperative blood loss and a longer operative time. The data in our study are comparable with other published data for advanced gastric cancer including intraoperative blood loss (range, 96.5–333.3 ml with LAG versus 215–440.6 ml with OG) and operative time (range, 252–282.84 min with LAG vs 180–267.8 min with OG) [3, 5, 6, 10, 28]. Careful manipulation enabled by the magnified vision of the laparoscope and the hemostatic effect of pneumoperitoneum induced by carbon dioxide insufflations supposedly contribute to lower blood loss [29].
Some publications have reported the number of retrieved lymph nodes in LAG with D2 dissection as similar to that in OG with D2 dissection [3, 10, 28], including the number of group 2 lymph nodes, according to subset analysis [30]. The number of lymph nodes in our analysis also showed that LAG could produce satisfactory lymph node dissection, suggesting that oncologically appropriate D2 lymph node dissection could be carried out with laparoscopic surgery.
We observed that the postoperative complications were comparable between LAG and OG, including anastomotic leakage and pancreatic fistula. We observed a shorter postoperative hospital stay in the LAG group than in the OG group, but we cannot simply accept this result as evidence that the postoperative recovery from surgery was faster with LAG because the historical backgrounds of the two groups were different. We believe that the postoperative recovery was possibly superior with LAG, but this assumption should be validated in a prospective randomized trial. We attribute the relatively long hospital stays in both groups to the difference between Japan’s medical insurance system and that of other countries. In fact, a publication from Japan also reported postoperative hospital stays similar to ours (16.7 ± 5.6 days with LADG versus 21 ± 11.4 days with ODG) [3].
For early gastric cancer, comparison of long-term results between LADG and ODG demonstrated that LADG was a feasible approach [19]. With regard to the long-term results of LAG for advanced gastric cancer, some retrospective reports on overall survival and recurrence-free survival time also have been published, but most of these reports involved only one-arm studies or analyzed the long-term results coupled with those for early gastric cancer. [10, 31–34].
One randomized prospective trial evaluated the long-term results of LADG for the treatment of advanced gastric cancer. In this trial, Huscher et al. [18] showed the 5-year overall survival and the 5-year recurrence-free survival time, concluding that LADG was a feasible and safe alternative to OG, but this study contains some problems including high-mortality and morbidity rates and different lymph node stations dissected compared with those indicated by JGCA. We cannot directly interpret this result as indicating that LAG is applicable for advanced gastric cancer.
In our retrospective cohort study, the long-term results did not differ significantly between LAG and OG performed for advanced gastric cancer. Our analysis is novel in that it is the first comparative study investigating the long-term results of LADG and LATG for advanced gastric cancer. In addition, our analysis also is valuable because it included so many cases performed using LAG with D2 dissection compared with the published studies mentioned earlier. We found no significant difference in recurrence-free survival and overall survival time between the LAG and OG groups, and these results suggest noninferiority of the long-term results for LAG compared with those for OG.
Nevertheless, this study had several limitations. First, although the analyses for HRs were calculated after adjustment for potential confounders, there could have been residual confounding because the historical background of the two procedures differed. The frequency of LAG was increasing during the entire time of the study, whereas the frequency of OG was decreasing. Therefore, during the analyzed period, OG was performed mainly in the early period and LAG in the late period. More precisely, S-1, of which drastic efficacy in adjuvant settings had been elucidated for East Asian gastric cancer patients in 2007 [35], was more frequently used for patients in the latter group than for patients in the former group, although the frequency of adjuvant therapy appeared to be very similar in the two groups.
Second, the postoperative follow-up period was shorter in the LAG group, so recurrence or death in this group may not have been observed during the time of analysis (Figs. 2 and 3).
Third, other possible prognostic factors (e.g., tumor size, a possible prognostic factor next to T stage, which was larger in the OG group than in the LAG group) might still exist even after adjustment for histology, pathologic T and N stages, and postoperative adjuvant chemotherapy. These circumstances made it difficult to understand easily the long-term results in this study and to draw definitive conclusions.
In conclusion, our analysis demonstrated that LAG accompanied by D2 lymph node dissection for advanced gastric cancer provided an acceptable prognosis. This result indicates that LAG with D2 lymph node dissection performed by a medical team skilled in laparoscopic surgery could be applicable for the treatment of advanced gastric cancer. However, randomized controlled trials comparing LAG and OG are required to elucidate the actual influence of LAG on the long-term results.
References
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Tokunaga M, Hiki N, Fukunaga T, Nohara K, Katayama H, Akashi Y, Ohyama S, Yamaguchi T (2009) Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection following standardization: a preliminary study. J Gastrointest Surg 13:1058–1063
Kawamura H, Homma S, Yokota R, Yokota K, Watarai H, Hagiwara M, Sato M, Noguchi K, Ueki S, Kondo Y (2008) Inspection of safety and accuracy of D2 lymph node dissection in laparoscopy-assisted distal gastrectomy. World J Surg 32:2366–2370
Huscher CG, Mingoli A, Sgarzini G, Brachini G, Binda B, Di Paola M, Ponzano C (2007) Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long-term results of a 100-patient series. Am J Surg 194:839–844
Ziqiang W, Feng Q, Zhimin C, Miao W, Lian Q, Huaxing L, Peiwu Y (2006) Comparison of laparoscopically assisted and open radical distal gastrectomy with extended lymphadenectomy for gastric cancer management. Surg Endosc 20:1738–1743
Weber KJ, Reyes CD, Gagner M, Divino CM (2003) Comparison of laparoscopic and open gastrectomy for malignant disease. Surg Endosc 17:968–971
Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y (2002) A randomized controlled trial comparing open versus laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 131:306–311
Lee JH, Han HS, Lee JH (2005) A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 19:168–173
Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, Yamaue H, Yoshikawa T, Kojima K, JCOG Gastric Cancer Surgical Study Group (2010) Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer 13:238–240
Hur H, Jeon HM, Kim W (2008) Laparoscopy-assisted distal gastrectomy with D2 lymphadenectomy for T2b advanced gastric cancers: three years’ experience. J Surg Oncol 98:515–519
Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma, 2nd English edition. Gastric Cancer 1:10–24
Omori T, Nakajima K, Nishida T, Uchikoshi F, Kitagawa T, Ito T, Matsuda H (2005) A simple technique for circular-stapled Billroth I reconstruction in laparoscopic gastrectomy. Surg Endosc 19:734–736
Omori T, Oyama T, Akamatsu H, Tori M, Ueshima S, Nakahara M, Toshirou N (2009) A simple and safe method for gastrojejunostomy in laparoscopic distal gastrectomy using the hemidouble-stapling technique: efficient purse-string stapling technique. Dig Surg 26:441–445
Omori T, Oyama T, Mizutani S, Tori M, Nakajima K, Akamatsu H, Nakahara M, Nishida T (2009) A simple and safe technique for esophagojejunostomy using the hemidouble stapling technique in laparoscopy-assisted total gastrectomy. Am J Surg 197:13–17
Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H, Okajima K (1995) Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 19:532–536
Sano T, Yamamoto S, Sasako M (2002) Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol 32:363–364
Dulucq JL, Wintringer P, Stabilini C, Solinas L, Perissat J, Mahajna A (2005) Laparoscopic and open gastric resections for malignant lesions: a prospective comparative study. Surg Endosc 19:933–938
Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Paola MD, Recher A, Ponzano C (2005) Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 241:232–237
Lee JH, Yom CK, Han HS (2009) Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc 23:1759–1763
Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N (2007) A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 245:68–72
Lee SW, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N (2010) Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg 211:33–40
Saka M, Morita S, Fukagawa T, Katai H (2011) Present and future status of gastric cancer surgery. Jpn J Clin Oncol 41:307–313
Kong SH, Yoo MW, Kim JW, Lee HJ, Kim WH, Lee KU, Yang HK (2010) Validation of limited lymphadenectomy for lower-third gastric cancer based on depth of tumour invasion. Br J Surg 98:65–72
Tanizawa Y, Terashima M (2010) Lymph node dissection in the resection of gastric cancer: review of existing evidence. Gastric Cancer 13:137–148
Uyama I, Sugioka A, Matsui H, Fujita J, Komori Y, Hasumi A (2000) Laparoscopic D2 lymph node dissection for advanced gastric cancer located in the middle or lower third portion of the stomach. Gastric Cancer 3:50–55
Tanimura S, Higashino M, Fukunaga Y, Takemura M, Tanaka Y, Fujiwara Y, Osugi H (2008) Laparoscopic gastrectomy for gastric cancer: experience with more than 600 cases. Surg Endosc 22:1161–1164
Japan Gastric Cancer Association (2004) Guidelines for diagnosis and treatment of carcinoma of the stomach (2nd English edn). Retrieved 30 April 2011 at http://www.jgca.jp
Hwang SI, Kim HO, Yoo CH, Shin JH, Son BH (2009) Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg Endosc 23:1252–1258
Papp A, Vereczkei, Lantos J, Horváth OP (2003) The effect of different levels of peritoneal CO2 pressure on bleeding time of spleen capsule injury. Surg Endosc 17:1125–1128
Song KY, Kim SN, Park CH (2008) Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer: technical and oncologic aspects. Surg Endosc 22:655–659
Lee J, Kim W (2009) Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: analysis of consecutive 106 experiences. J Surg Oncol 15:693–698
Pugliese R, Maggioni D, Sansonna F, Costanzi A, Ferrari GC, Di Lernia S, Magistro C, De Martini P, Pugliese F (2010) Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc 24:2594–2602
Song J, Lee HJ, Cho GS, Han SU, Kim MC, Ryu SW, Kim W, Song KY, Kim HH, Hyung WJ, Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group (2010) Recurrence following laparoscopy-assisted gastrectomy for gastric cancer: a multicenter retrospective analysis of 1, 417 patients. Ann Surg Oncol 17:1777–1786
Hwang SH, Park do J, Jee YS, Kim MC, Kim HH, Lee HJ, Yang HK, Lee KU (2009) Actual 3-year survival after laparoscopy-assisted gastrectomy for gastric cancer. Arch Surg 144:559–564
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Acknowledgment
We thank Toshimitsu Hamasaki, Associate Professor in the Department of Biomedical Statistics at Osaka University Graduate School of Medicine, who gave statistical advice for this article.
Disclosures
Toshirou Nishida has a grant from the Novartis Pharma K. K. for basic research investigating gastrointestinal stromal tumor (GIST). Atsushi Hamabe, Takeshi Omori, and Koji Tanaka have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamabe, A., Omori, T., Tanaka, K. et al. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc 26, 1702–1709 (2012). https://doi.org/10.1007/s00464-011-2096-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-011-2096-0