Abstract
Background
Histological analysis of surgical specimen is the gold standard for cancer classification. In particular, frozen histological diagnosis of vague peritoneal spots or uncertain excision of tumors plays a crucial role in the decision to proceed with or abandon an operation. Confocal laser microscopy (CLM) enables in-vivo and real-time high-resolution tissue analysis. This method has already been used during endoscopic assessments analyzing transformation of esophageal or colon mucosa. We examined whether a CLM device enables to distinguish between non-malignant and malignant tissue in vivo and real time and enables to assign peritoneal carcinomatosis spots to their primary tumor. In addition, we investigated whether the newly developed CLM camera device causes any tissue damage.
Methods
CC531 colon carcinoma cells were implanted on the serosa side of the colon and intraperitoneally in Wag/Rija rats via laparotomy. After 7 days of tumor growth, confocal laser microscopy in vivo was performed by re-laparotomy. Images of non-malignant and malignant tissue were characterized in terms of specific signal pattern. No fluorescent dye was used. Correlations to findings in conventional histology were systematically recorded and described. Potential tissue damage was examined by conventional histology.
Results
All animals survived the operative procedure and could be evaluated 7 days following surgery. No unexpected death occurred after surgery. Non-malignant colon is defined by small cycles of the microvilli of the colon. There is repetitive deregulated structure in colon carcinoma. Peritoneal carcinomatosis showed the same structural pattern as in primary colon carcinoma. In all examined cases, it was possible to differentiate between peritoneal carcinomatosis spots and non-malignant peritoneum. The CLM device did not cause any tissue damage.
Conclusions
The CLM camera device reported here is feasible to identify peritoneal carcinomatosis spots, assign these spots to the primary tumor, as well as distinguish between non-malignant and malignant tissue in without using any fluorescent dye.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer is the third most commonly diagnosed cancer in males and the second in females [1]. Peritoneal carcinomatosis (PC) of colon cancer depicts a progressed stage of digestive cancer and is feared as metastatic spread by patient and physician. Especially, PC is linked to significant shorter overall survival as compared with other metastatic manifestations in colon cancer [2]. In several studies, about 17.4% of patients had PC as a component of multisite disease and 2.1% of patients had PC as a sole site of disease [3]. Therefore, it is obligatory to diagnose metastasis prior to selecting therapy. In spite of great improvements of diagnostic imaging techniques such as computer tomography or magnetic resonance imaging, PC remains difficult to detect in imaging, and exploratory laparoscopy is typically performed to determine whether peritoneal metastases are involved [4].
Confocal laser microscopy (CLM) is a well-established analysis method in endoscopy based on principle of tissue fluorescence [5]. It is possible to assess pathology in gastrointestinal tissue sites, e.g., Barrett’s esophagus [6], lesions in the duodenum [7], colonic mucosa, [8] and pancreatic biliary duct [9]. A general impairment of CLM devices is the need of intravenous or local fluorescent contrast dyes, which have been reported to several side effects [10,11,12]. However, there is no CLM device for intraoperative diagnostic of tumor regions and potential metastases.
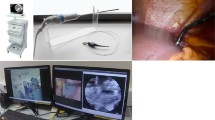
The herein reported CLM device developed for minimal-invasive surgical (MIS) approach and intraoperative diagnostic does not need any fluorescent contrast dye. This device is composed of a confocal laser scanning microscope and a rigid 5-mm laparoscope with Hopkins-rod lenses (Fig. 1). The special feature of this CLM device is that it does not require any fluorescent dye. Induced fluorescence of the tissue alone is sufficient for imaging. Introduced through a laparoscopic or thoracoscopic port, surgeons can use the CLM camera device like any other surgical instrument. Our previous study demonstrated that this device is feasible to distinguish between non-malignant and malignant mucosa ex vivo [13]. However, this CLM device for minimal-invasive surgery needs to verify assigning PC spots to their primary cancer lesion.
Therefore, in this study we asked whether this newly developed CLM device enables to distinguish in vivo between non-malignant and malignant tissue and allows assigning peritoneal tumor spots to their potential primary cancer lesion. Additionally, we examined whether this CLM device causes any tissue damage.
Materials and methods
Study design
The study was designed to evaluate the CLM device in vivo and to assess a potential tissue damage caused by the laser beam of the CLM device simultaneously. To determine whether it is possible to distinguish between non-malignant and malignant tissue as well as to assign tumor peritoneal spots to colon tumor region, we used the CC531 adenocarcinoma model in rats developed by Marquet et al. [14]. This tumor model enabled a peritoneal seeding as well as an implantation of tumor cells in the peritoneum and abdominal organs for assessment of defined regions [15, 16]. If implanted in the abdominal peritoneum, the seeding in the abdominal cavity is more slow. To determine whether the CLM device caused any tissue damage, we chose the implantation of tumor cells in the peritoneum because diffuse peritoneal seeding might prevent an assessment of healthy non-malignant tissue.
The CC531 colon adenocarcinoma cells were implanted in colon and peritoneum of male inbred WAG/Rija rats via laparotomy. After 7 days of tumor growth, we performed confocal laser microscopy in vivo using the newly developed CLM camera device via re-laparotomy. After CLM, the regions of non-malignant and malignant colon and peritoneum were resected for histological assessment of tissue damage that might be caused by the laser beam of the CLM camera device.
Animals
Ten inbred male pathogen-free WAG/Rija rats (151–308 g), delivered by Charles River, Sulzfeld, Germany, were used in this study. Maintenance and care of all animals used in this study were carried out according to the direction of national animal protection law and according to the directories of European Community Council (2010/63/E4). The animals were housed in groups of three up to four animals and had free access to food and water. They got accustomed to their new surrounding and to the investigator for at least 10 days before the first surgical intervention.
Endoscopic confocal laser microscopy camera device
The CLM camera device (Karl Storz GmbH & Co KG, Tuttlingen, Germany) is built up of a confocal laser scanning microscope (Laser class 1) and rigid 5-mm endoscope with Hopkins-rod lenses. Surgeons can use it like surgical instrument during surgery. The scanning field covers 300 µm × 300 µm with a 3-dimensional scanning depth of 80 µm. Confocal frames are collected with a scan rate of 40 frames per second. Confocal images were stored using an imaging processing software distributed by Heidelberg Engineering GmbH (Heidelberg, Germany).
Tumor suspension
CC531 moderately differentiated colon adenocarcinoma cells (Cell Lines Services GmbH, Eppelheim, Germany) were cultured in 20 ml complete RMPI 1640 medium (Cell Lines Services GmbH, Eppelheim, Germany) with 10% heat-inactivated fetal bovine serum (Cell Lines Services GmbH, Eppelheim, Germany) and 1% Penicillin/Streptomycin at 37 °C and 5% CO2 in monolayer cultures.
To prepare a tumor suspension for intraperitoneal application, the complete medium was removed. Then, after being washed with 20 ml phosphate-buffered saline, the cells were detached by 4 ml Accutase (Cell Lines Services GmbH, Eppelheim, Germany). After incubating for 10 min at 37 °C, 6 ml complete medium was added. The cells were harvested, suspended in phosphate-buffered saline, and centrifugated at 300 x g for 5 min. Vital counting was performed in a Bürker hematocytometer. The injected suspension had a density at least 2.5 × 106 vital cell/200 µl.
Tumor inoculation
All rats were anesthetized with 2% Xylazin (4–6 mg/kg body weight) and 10% S-Ketamine (2–3 mg/kg body weight) by intraperitoneal injection. Supplemental doses of S-Ketamine were given half-hourly to maintain anesthesia, when the surgical procedure exceeded 30 min.
The abdomen was opened via midline incision and 200 µl of the tumor suspension were implanted in the colon (colon tumor region) and the peritoneum (peritoneal region) of the right abdomen (Fig. 2). The regions were marked by monofilament 5−0 polypropylene sutures (Prolene®, Ethicon, Germay). The midline incision was closed by subcutaneous polyfilament 4−0 continuous suture (Polyglectin 910, Vicryl®, Ethicon, Germany) and cutaneous monofilament 3−0 interrupted suture (polyamide, Ethilon®, Ethicon, Germany).
After surgical procedure, animals were allowed to recover from anesthesia before returning to the animal facility. They were weighted and examined for side effects (wound infection, loss of appetite, fatigue syndrome, lethargy).
In-vivo endoscopic confocal laser microscopy
After 7 days of tumor growth, re-laparotomy was performed using anesthesia as described above. Confocal laser microscopy was performed of both non-malignant and malignant tissues in colon and peritoneum. Furthermore, we analyzed the transition zone between non-malignant and malignant tissue in colon and peritoneum. Assessed regions of non-malignant colon and peritoneum as well as malignant colon and peritoneal regions were resected for histological assessment of tissue damage caused by laser beam of CLM camera system.
Analysis of data and statistical analysis
Afterward (offline) 250 virtual, laser-microscopic pictures of each region (non-malignant and malignant tissue of the colon and Peritoneum) were compared independently and blinded. We noted all morphological differences between non-malignant colon and peritoneal regions and malignant colon and peritoneal regions of each individual. In a second step, we noted all reconstructable similarities between non-malignant regions and between all malignant regions in the colon and peritoneum. Morphologic characteristics were described for each entity independently by the surgeon and the pathologist. In summary, for description only characteristics with 100% inter- and intraobserver consensus were noted. The results of the CLM were compared with histological analyses. Additionally, we noted if there were findings for any tissue damage (necrosis) in the non-malignant regions caused by the CLM laser beam.
Statistical analysis was calculated using GraphPad Prism® 6.0 statistical software. The sample size calculation is based on the tissue damage that might be performed by the laser beam of the CLM camera system. To determine if there were significant tissue damage caused by CLM laser beam (necrosis, lymphatic infiltration etc.), we performed one-way sign test. We considered no tissue damage significant at p < 0.05 with a statistical power at 1−β = 0.95.
Results
Clinical appearance
All animals survived surgical tumor inoculation and were evaluated after 7 days via re-laparotomy. Postoperatively, their body weight showed an almost steady increase in weight over the observation period. Although macroscopic examination revealed a sizeable tumor load during re-laparotomy, animals did not show any sign of suffering.
Qualitative description of CLM criteria
The regions of non-malignant and malignant regions in colon and peritoneum were inspected with the CLM camera system. We noted the characteristics of each region (Table 1).
In general, colon adenocarcinoma regions could be delineated from non-malignant colon areas. Regular round or linear patterns of colon crypts could be found in non-malignant colon tissue, whereas colon cancer regions tended to present a polymorphic and irregular pattern (Table 1).
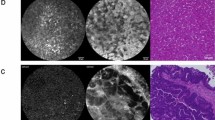
In non-malignant colon regions, we found two kinds of patterns according to crypt section line. First, regular round or oval structures were seen comparable to histological transversal sections of colon crypts (Fig. 3a). Otherwise linear patterns represent longitudinal sections of colon crypts. In most cases, it was possible to detect vessels of the colon wall.
In-vivo confocal laser microscopy of non-malignant and malignant tissue in colon and peritoneum. Confocal laser microscopy of non-malignant and malignant tissue of the colon and peritoneum with corresponding histopathologic images. In non-malignant colon regions, regular round or oval structures were seen comparable to histological transversal sections of colon crypts. In colon cancer, regular patterns were deregulated and irregular cell arrangements and morphology were seen. Observing the peritoneum, abdominal wall muscles in non-malignant areas can be identified due to scanning depth of CLM camera system. Peritoneal tumor regions showed the same pattern like primary colon cancer regions in CLM scan
In colon cancer, regular patterns were deregulated and our observations showed irregular cell arrangements and morphology. Furthermore, there were large elongated and irregular regions that presented histopathologically as glands filled with necrotic debris (Fig. 3b). We could not find any vessel structures using CLM.
Examination of the peritoneum by the endoscopic CLM enabled us to scan the peritoneal layer as well as the abdominal wall muscles in non-malignant areas due to scanning depth of CLM camera system in comparison to the one cell layer of the peritoneum. Thus, non-malignant peritoneal regions showed a striated pattern with white and black bands and indistinct longitudinal stripes (Fig. 3c).
Tumor regions of the peritoneum showed the same CLM pattern as the tumor regions in the colon wall with large elongated and irregular regions as well as irregular cell arrangements in CLM scan (Fig. 3d). We could not detect the abdominal wall muscles anymore. Due to the detection of the same tumor pattern in different tissues, a potential assignment to each other is possible.
Scanning the transition from non-malignant to malignant tissue in the colon, the transition zone showed a beginning of washed out structure and irregular morphology (Fig. 4a). The transition zone between non-malignant and malignant tissue is more defined in the peritoneum. We could find abdominal wall muscle pattern next to the beginning of large elongated and irregular pattern that is characteristic for the tumor zone (Fig. 4b).
In-vivo confocal laser microscopy of transition zone between non-malignant and malignant tissue in colon and peritoneum. The transition zone in the colon showed a beginning of washed out structure and irregular morphology (A). The transition zone between non-malignant and malignant tissue is more defined in the peritoneum. Abdominal wall muscle patterns are located next to the beginning of large elongated and irregular pattern that is characteristic for the tumor zone (B)
Analysis of tissue damage
Using H&E staining, we analyzed tissue damage resulted from by the CLM laser beam in non-malignant tissue. Exposure time was defined by 5 min CLM scanning colon and peritoneal tissue. We were not able to detect any cell necrosis, lymphatic infiltration, or other inflammation or tissue damage signs on subsequent histological examination. Risk of tissue damage caused by CLM camera system was calculated with p < 0.0001 (Table 1).
Discussion
This study in a modified in-vivo colon adenocarcinoma rodent model shows that the newly developed endoscopic CLM device for MIS enables to distinguish between non-malignant and malignant tissue and enables to assign peritoneal tumor spots to their potential primary cancer lesion without performing any tissue damage.
In this study, we report the application of new developed endoscopic camera system for MIS—composed of confocal laser scanning microscope and a rigid endoscope—without fluorescent dye application. The possible field of application of this CLM camera system has been demonstrated in three ways in this study.
First, it is possible to interpret CLM images with high concordance to conventional histology. Colon and intraabdominal wall tissue show distinctive pattern, so that it is possible to distinguish between different organs. Our results are consistent with recent studies from other groups [17]. In this context, the advantage of CLM tissue examination without fluorescent dye is a rapid and repetitive measurement and avoidance of possible side effects of fluorescent dyes. Although fluorescein considered being a safe fluorescent dye, its application requires a waiting period of 8 min after intravenous application for best contrast and image quality. In addition, the window for observations is limited after dye application. Compared to fresh frozen section, it is possible to perform repetitive assessments and surgeons might reach a quick intraoperative decision because operation is not interrupted by waiting for the frozen section results.
Second, we delivered evidence that non-malignant and malignant tissue could be distinguished by the new endoscopic CLM system. These findings are in accordance with results of study Pierangelo et al. [18]. In this study, the authors characterized peritoneal spots ex vivo using indocyanine green through a confocal endomicroscope. They were able to make a distinction between malignant and non-malignant tissue. The transition zone is more defined and easier to analyze in the peritoneum. It is possible to detect the crypts of the rat colon due to scanning depth of the CLM camera system and the minimal thickness of the colon wall. Additionally, CC531 tumor cells are similar to the normal non-malignant colon mucosa cells and might complicate to define the transition zone. Depending on the tumor type and tissue species, it may differ to determine to transition zone like in the pathohistology.
Third, this CLM device enables to assign peritoneal tumor regions to their potential primary tumor. Our results correspond with results of metastatic gastric cancer in rats [19]. In this study, the authors used a similar animal model examining CLM for gastric cancer. In this context, our animal model does not truly represent a metastatic model, but rather represents heterotrophically implanted cells in the peritoneum [20]. This model does not have the task of identifying neither tumor cells in the colon mucosa—a domain of endoscopy—nor tumor cell spread in the abdominal cavity. The aim was rather to show that a defined tumor pattern could be attributed to its metastasis (peritoneal tumor regions) and vice versa using our CLM system. Compared to the other studies, our system is the only one designed for minimally invasive surgery and allows tissue examination without fluorescent dyes.
In preparation for clinical use, H&E histology showed no tissue damage, which is an important prerequisite for in-vivo intraoperative testing in patients. Using a Laser class 1, surgeons can apply our CLM system without any special eye protection.
With regard to fresh frozen section, our CLM aims to upgrade this technology to enable examinations of peritoneal spots rapidly and repetitively during MIS. However, the range of application is not only limited to minimally invasive surgery. Discussing CLM images during surgery, pathologists and surgeons might consider in the future this novel application.
In conclusion, the CLM camera device reported in this study provides a safe examination tool for real-time and in-vivo tissue assessment during MIS. It enables the reliable differentiation between benign and malignant tissue as well as the assignment of metastasis to its origin.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108. https://doi.org/10.3322/caac.21262
Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ (2012) Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol 30(3):263–267. https://doi.org/10.1200/JCO.2011.37.1039
Cercek A, Cusack JC Jr, Ryan DP (2015) Treatment of peritoneal carcinomatosis of colorectal origin. Am Soc Clin Oncol Educ Book. https://doi.org/10.14694/EdBook_AM.2015.35.e208
Ishigami S, Uenosono Y, Arigami T, Yanagita S, Okumura H, Uchikado Y, Kita Y, Kurahara H, Kijima Y, Nakajo A, Maemura K, Natsugoe S (2014) Clinical utility of perioperative staging laparoscopy for advanced gastric cancer. World J Surg Oncol 12:350. https://doi.org/10.1186/1477-7819-12-350
Fugazza A, Gaiani F, Carra MC, Brunetti F, Levy M, Sobhani I, Azoulay D, Catena F, de’ Angelis GL, de’ Angelis N (2016) Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases: a systematic review and meta-analysis. BioMed Res Int 2016:4638683. https://doi.org/10.1155/2016/4638683
Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle PR, Neurath MF (2006) In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol 4(8):979–987. https://doi.org/10.1016/j.cgh.2006.05.010
Lim LG, Neumann J, Hansen T, Goetz M, Hoffman A, Neurath MF, Galle PR, Chan YH, Kiesslich R, Watson AJ (2014) Confocal endomicroscopy identifies loss of local barrier function in the duodenum of patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 20(5):892–900. https://doi.org/10.1097/MIB.0000000000000027
Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S, Nafe B, Galle PR, Neurath MF (2004) Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology 127(3):706–713
Fottner C, Mettler E, Goetz M, Schirrmacher E, Anlauf M, Strand D, Schirrmacher R, Kloppel G, Delaney P, Schreckenberger M, Galle PR, Neurath MF, Kiesslich R, Weber MM (2010) In vivo molecular imaging of somatostatin receptors in pancreatic islet cells and neuroendocrine tumors by miniaturized confocal laser-scanning fluorescence microscopy. Endocrinology 151(5):2179–2188. https://doi.org/10.1210/en.2009-1313
De Palma GD (2009) Confocal laser endomicroscopy in the “in vivo” histological diagnosis of the gastrointestinal tract. World J Gastroenterol 15(46):5770–5775
Becker V, von Delius S, Bajbouj M, Karagianni A, Schmid RM, Meining A (2008) Intravenous application of fluorescein for confocal laser scanning microscopy: evaluation of contrast dynamics and image quality with increasing injection-to-imaging time. Gastrointest Endosc 68(2):319–323. https://doi.org/10.1016/j.gie.2008.01.033
Coda S, Thillainayagam AV (2014) State of the art in advanced endoscopic imaging for the detection and evaluation of dysplasia and early cancer of the gastrointestinal tract. Clin Exp Gastroenterol 7:133–150. https://doi.org/10.2147/CEG.S58157
Ellebrecht DB, Gebhard MP, Horn M, Keck T, Kleemann M (2016) Laparoscopic confocal laser microscopy without fluorescent injection: A pilot ex vivo study in colon cancer. Surg Innov. https://doi.org/10.1177/1553350616637690
Marquet RL, Westbroek DL, Jeekel J (1984) Interferon treatment of a transplantable rat colon adenocarcinoma: importance of tumor site. International journal of cancer Journal international du cancer 33(5):689–692
Lopes Cardozo AM, Gupta A, Koppe MJ, Meijer S, van Leeuwen PA, Beelen RJ, Bleichrodt RP (2001) Metastatic pattern of CC531 colon carcinoma cells in the abdominal cavity: an experimental model of peritoneal carcinomatosis in rats. Eur J Surg Oncol 27(4):359–363. https://doi.org/10.1053/ejso.2001.1117
Pelz JO, Doerfer J, Hohenberger W, Meyer T (2005) A new survival model for hyperthermic intraperitoneal chemotherapy (HIPEC) in tumor-bearing rats in the treatment of peritoneal carcinomatosis. BMC Cancer 5:56. https://doi.org/10.1186/1471-2407-5-56
Becker V, Wallace MB, Fockens P, von Delius S, Woodward TA, Raimondo M, Voermans RP, Meining A (2010) Needle-based confocal endomicroscopy for in vivo histology of intra-abdominal organs: first results in a porcine model (with videos). Gastrointest Endosc 71(7):1260–1266. https://doi.org/10.1016/j.gie.2010.01.010
Pierangelo A, Fuks D, Benali A, Validire P, Gayet B (2017) Diagnostic accuracy of confocal laser endomicroscopy for the ex vivo characterization of peritoneal nodules during laparoscopic surgery. Surg Endosc 31(4):1974–1981. https://doi.org/10.1007/s00464-016-5172-7
Hara H, Takahashi T, Nakatsuka R, Higashi S, Naka T, Sumiyama K, Miyazaki Y, Makino T, Kurokawa Y, Yamasaki M, Takiguchi S, Mori M, Doki Y, Nakajima K (2016) A novel approach of optical biopsy using probe-based confocal laser endomicroscopy for peritoneal metastasis. Surg Endosc 30(8):3437–3446. https://doi.org/10.1007/s00464-015-4626-7
White SB, Procissi D, Chen J, Gogineni VR, Tyler P, Yang Y, Omary RA, Larson AC (2016) Characterization of CC-531 as a rat model of colorectal liver metastases. PLoS ONE 11(5):e0155334. https://doi.org/10.1371/journal.pone.0155334
Acknowledgements
We thank KARL STORZ SE & Co. KG for their support with the laparoscopic CLM device.
Funding
This study was supported by the junior research Grant of the University of Lübeck (J02-2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. David Benjamin Ellebrecht, Christiane Kuempers, Marco Horn, Prof. Dr. Tobias Keck, and Prof. Dr. Markus Kleemann have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Ellebrecht, D.B., Kuempers, C., Horn, M. et al. Confocal laser microscopy as novel approach for real-time and in-vivo tissue examination during minimal-invasive surgery in colon cancer. Surg Endosc 33, 1811–1817 (2019). https://doi.org/10.1007/s00464-018-6457-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6457-9