Abstract
Background
Intraoperative characterization of peritoneal nodules can be challenging. Probe-based confocal laser endomicroscopy (pCLE) is an innovative technique enabling real-time microscopic analysis. This study aimed to assess the role of pCLE in the discrimination of benign versus malignant peritoneal nodules during laparoscopic staging.
Materials and methods
During this prospective trial, pCLE was performed ex vivo on fresh samples of peritoneal nodules in 30 consecutive patients, after topical application of indocyanine green. The final diagnosis was obtained histologically, as per standard of care. pCLE image criteria for normal versus inflammatory versus malignant nodules were established (phase I); these criteria were tested retrospectively on selected videos by two examiners (phase II). The primary endpoints were values of accuracy in diagnosing malignant nodules.
Results
pCLE criteria for malignant nodules defined in phase I were: strongly fluorescent irregular clusters of cancerous cells, nonfluorescent nuclei of cancerous cells, and substantially lower fluorescence of the extracellular matrix fluorescence compared with cancerous clusters. In phase II, the detection rate of these criteria was significantly higher in malignant compared with benign nodules. Overall sensitivity, specificity, positive and negative predictive values to detect malignant nodules were 75, 100, 100 and 89 %, respectively. Interobserver agreement was substantial (kappa 0.69).
Conclusion
These preliminary results suggest that pCLE is a valuable tool to discriminate between benign and malignant peritoneal nodules, with a high positive predictive value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Probe-based confocal laser endomicroscopy (pCLE) is an innovative imaging technique allowing real-time microscopic assessment of mucosa [1]. In this technique, a 488-nm laser beam excites the tissue of interest at a certain confocal depth (0–70 microns). Due to this excitation, the tissue emits a fluorescence that is captured and processed by the system. A fluorescent dye may be needed to enhance contrast [2]. pCLE was first introduced in gastrointestinal endoscopy to help target biopsies, increase diagnostic accuracy and guide therapeutic orientation [3–7]. Further applications have been described in pulmonology for the characterization of peripheral pulmonary lesions [8, 9] and in urology for examination of the bladder and upper urinary tract [10, 11].
Intraoperative characterization of peritoneal nodules represents a challenge for surgeons. Despite significant improvements in preoperative imaging techniques, some patients present with nonspecific nodules located on the peritoneum. During laparoscopic staging, biopsies of suspicious, visible peritoneal lesions are performed and the samples sent for frozen section. This analysis is crucial to detect the presence of nonvisible metastases, thus redirecting patients toward chemotherapy [12]. However, this strategy has several weak points. First, the accuracy of the frozen sections is limited by the slightly lower quality of the histological slides compared to standard methods and also by the impossibility of carrying out histochemical analyses. Secondly, biopsies may be sampled at an inappropriate location in the lesion, missing the cancerous part, particularly if the suspicious zones are not clearly identified. Finally, in the case of diffuse peritoneal nodules, the total number of possible biopsies taken for frozen section analysis remains limited and is time consuming [13]. pCLE enables unlimited, real-time optical biopsies. This could be a valuable tool for characterizing peritoneal nodules. In this context, the aim of the present study was first to establish pCLE criteria for benign, inflammatory and metastatic nodules and then to evaluate the accuracy of these pCLE criteria in the identification of malignant nodules.
Patients and methods
Patient inclusion and exclusion criteria
Patients aged 18 years or older were eligible for the trial if they required laparoscopic assessment or surgical resection for a digestive cancer. Patients were excluded if they had an allergy to contrast agents (i.e. indocyanine green (ICG)), if they had previous life-threatening allergic reactions, if they were pregnant or breastfeeding, if they had a history of cardiopulmonary disease (including bronchial asthma), restricted renal function or if they were under beta-blocker treatment. The study was approved by the regional investigational review board (07/16/2014).
Intervention
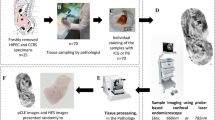
During the surgical procedures, peritoneal nodules were resected for pCLE and classical pathological analysis. For the purpose of the study, a benign peritoneal specimen could also be sampled as a control group (Fig. 1a). Fresh samples were fixed on a cork support by the surgeon and sent to the department of pathology immediately after surgical resection (Fig. 1b) in order reproduce the in vivo conditions as closely as possible.
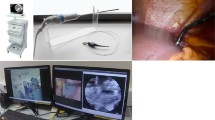
a Laparoscopic view of a peritoneal nodule (the red line indicates the resection margins comprising the suspect nodule surrounded by normal peritoneum). b Fresh sample of peritoneum fixed on a cork support immediately after surgical resection. c Probe-based confocal laser endomicroscopy (pCLE) system and micropositioning device. The UHD confocal miniprobe is placed in direct contact with the surface of the sample
Ex vivo pCLE analysis
For the purpose of this clinical investigation, the investigators used a prototype confocal system (Mauna Kea Technologies, Paris, France) made of a laser scanning unit capable of exciting fluorophores either at 640 or 785 nm and a fiber-based flexible objectives (miniprobes). The system was able to collect, process and display 10 images per second with a transverse resolution of 1 µm and optical sectioning capability of 10 µm, at a 40-µm depth and 240 µm field of view. This technology derives from the commercially available Cellvizio®, which performs in a similar way at 488 nm excitation.
All peritoneal samples were analyzed by a physicist (AP) using a CLE system comprising a laser unit emitting light at 785 nm and scanning the tissue, a UHD confocal miniprobe and a processor containing imaging software processing the images and displaying them on the monitor in real time (Mauna Kea Technologies, Paris, France). The UHD miniprobe was designed to focus a 10-µm-thick section of the sample at 40-µm depth with a field of view of 240 µm and lateral resolution of 1 µm. ICG was applied topically onto specimens. Several concentrations (0.25, 1.25 and 2.5 mg/ml) were tested in order to determine the optimal staining protocol. Confocal imaging was performed 1 min after staining the sample. The probe was placed in direct contact with the surface of the sample, with moderate compression using a device enabling micropositioning of the probe (Fig. 1c). Some nodules were then cut transversally to enable pCLE examination inside the nodule. Dynamic real-time sequences were acquired. Particular attention was paid to observation of the extracellular matrix (ECM) as this information is not provided by histology. The sectional plane of the histology was orthogonal to the plane of the pCLE images obtained, as shown by the red arrow in Fig. 2d.
Study design and endpoints
The primary endpoint was the accuracy of pCLE to diagnose malignant versus nonmalignant peritoneal nodules. The study was conducted in two phases:
Phase I: establishment of pCLE interpretation criteria
All peritoneal specimens were stratified into three groups on the basis of the definitive pathological analysis: inflammatory nodule/malignant nodule/control group (sampling of normal peritoneum). All pCLE video recordings were evaluated openly in comparison with histological results by a physicist and an expert digestive pathologist (AP, AB), in order to define interpretation criteria.
Phase 2: evaluation of pCLE interpretation criteria
The pCLE video from each of the peritoneal nodules was coded and randomized. One surgeon (DF) and one expert digestive pathologist (PV) individually reviewed one representative pCLE video per nodule. The analysis process allowed the reviewers to stop the video at any chosen time point and to visualize the video as many times as required. The reviewers were blinded to the histological data. Test characteristics were determined independently for each reviewer and combined. The combined evaluation relied on all scores that were identical between examiners. For the per-patient analysis, the discrepant cases were discussed and a consensus diagnosis was reached. Test characteristics were established from these consensus opinions. A set of 11 videos (5 from benign nodules, 3 from inflammatory nodules and 3 from cancerous nodules) was used for training purposes. These videos were not included in the review set.
Four key criteria were assessed in all pCLE videos: (1) the proportion of positive malignant pCLE criteria detected in each nodule; (2) the presence of a malignant nodule in each recording selected for the review, to calculate accuracy; (3) sensitivity, specificity, positive, and negative predictive values; and (4) the level of confidence of the diagnosis concluded from the pCLE images only.
Statistical analysis
This study is reported in accordance with the Standards for Reporting of Diagnostic Accuracy (STARD) guidelines [14]. For normally distributed variables, the mean is reported, and for nonnormally distributed variables, the median is given. Test characteristics are presented as accuracy, sensitivity, specificity, and positive and negative predictive values. The diagnostic accuracy overall was determined using the consensus review. The interobserver variability was calculated using multirater Fleiss’s kappa statistic with the following classification: poor <0.2, fair 0.21–0.4, moderate 0.41–0.6, substantial 0.61–0.8, and excellent 0.81–1.
Results
Study population
From October 2014 to July 2015, 30 patients with a mean age of 67.1 years (male gender: 15/30) were enrolled consecutively at the Institut Mutualiste Montsouris, Paris, France. The three most frequent primary tumors in this patient set were colorectal adenocarcinoma (n = 18, 60.0 %), pancreatic adenocarcinoma (n = 6, 20.0 %), and gastric adenocarcinoma (n = 3, 10.0 %). pCLE was performed successfully in all cases; 249 video sequences were acquired on 31 nodules. Of these nodules, 6 (19 %) were normal, 12 (39 %) were inflammatory, and 13 (42 %) contained malignancy. Six nodules were excluded: Two had mucinous component, three were not suitable for analysis for technical reasons, and the video was not interpretable in 1 nodule due to the low quality of the images acquired. From the 25 remaining nodules, 211 sequences were used for open evaluation in phase I. In phase II, 11 sequences selected by the physicist (AP) were used for training and 25 additional sequences were used for validation of criteria.
Performance of pCLE
ICG concentration and quality of pCLE recordings
The optimal ICG concentration was 2.5 mg/ml, and the quality of all recordings was considered as sufficient or good.
pCLE interpretation criteria
Normal peritoneum In pCLE images, the normal peritoneum appears as a dense and unorganized reticular structure composed of strongly fluorescent fibers with a diameter of 3–4 µm, as shown in Fig. 2a and c. When the thickness of the fibrous layer was <40 µm (confocal depth of imaging), the adipose tissue was clearly visualized in the pCLE images as nonfluorescent adipocytes surrounded by a strong and uniform fluorescent extracellular matrix (ECM), as shown in Fig. 2b and c. This is consistent with conventional histology (Fig. 2d).
Inflammatory peritoneal nodules As observed in classical histology (Fig. 3b), pCLE images showed an increasing density of connective tissue, emphasized by strong fluorescence (Fig. 3c and d). The reticular architecture was altered, as observed in classical histology. The remaining adipocytes were observed as large, dark zones underlying the reticular structure (Fig. 3c). The nuclei of the inflammatory cells were observed as small, nonfluorescent points within the homogeneous connective tissue (outlined with a dashed line in Fig. 3d).
Malignant nodules In pCLE images, the irregular clusters or tubular structures of cancerous cells were strongly fluorescent. The nonfluorescent nuclei of cancerous cells were easily recognizable because they appeared as dark areas inside these strongly fluorescent structures. The ECM appeared to be substantially less dense in comparison with the control group or to the inflammatory nodules. An overall decrease in fluorescence was observed in the ECM, which was substantially darker than the clusters or tubular structures of cancerous cells (Fig. 4). The most sensitive pCLE criterion for malignant nodules was the presence of tubular glands, which were seen in up to 75 % of cases by both observers.
pCLE accuracy
One pathologist and one surgeon evaluated, independently and in a blinded manner, 25 video sequences (one video extract per nodule). An accurate diagnosis was established in 96 % of cases by the surgeon and 92 % of cases by the pathologist. The respective sensitivities, specificities, positive and negative predictive values are summarized in Table 1. There was substantial interobserver agreement between both examiners (kappa 0.69). (Table 1). If the three nodules for which the examiners did not agree are excluded, sensitivity and specificity reach 100 %, with positive and negative predictive values of 100 %. For the per-patient analysis, a consensus opinion of both examiners was taken into account, leading to two nodules (8.0 %) being falsely diagnosed as benign or inflammatory and the remaining 23 nodules (92.0 %) being accurately diagnosed. The corresponding sensitivity and specificity were 75 and 100 %, respectively, with a positive predictive value of 100 % and a negative predictive value of 89 % (Table 1).
Discussion
Confocal laser endomicroscopy (pCLE) is a new technology that has emerged over the past 10 years. The aim in using this method is to overcome the inherent limitations of endoscopic sampling techniques and to provide both endoscopists and pathologists with a wider diagnostic arsenal, with the ultimate goal to rationalize and optimize subsequent patient management [15]. The introduction of pCLE as a standard modality in gastroenterology has indeed brought significant progress in management strategies, affecting many aspects of clinical care, and this requires the standardization of practice and training. The technology has been included in the American Society for Gastrointestinal Endoscopy (ASGE) guidelines, addressing the role of endoscopy in the evaluation and treatment of patients with biliary neoplasia [16], Barrett’s esophagus, colonic lesions, inflammatory bowel diseases, gastric diseases and pancreatic cysts [17].
As pCLE technology was initially developed for application to endoscopy not surgery, the peritoneal cavity has until now been poorly explored. pCLE exploration of the peritoneum was in fact initially performed in 2007 by von Delius et al. [18] and Meining et al. [19] by endoscopic transgastric access after IV injection of fluorescein in porcine models.
Intraoperative differentiation between inflammatory and malignant nodules can be particularly challenging [20]. The present study evaluated the capability of pCLE technology to detect malignancy in patients with peritoneal nodules by performing ex vivo analysis immediately after surgical resection. This series suggests, for the first time, that pCLE accurately differentiates between inflammatory and malignant nodules. The methodology applied to our trial excluded most of the potential sources of bias. The results showed a good sensitivity and an excellent specificity, with a correspondingly high negative predictive value. The criteria were applied with similar accuracy by the two independent examiners, as demonstrated by the substantial interobserver agreement (kappa 0.69).
Of particular note, the present series underlines the importance of taking into account the ECM as a decisive additional parameter to improve cancer diagnosis. This important result emphasizes the fact that the presence of cancerous cells in peritoneal tissues dramatically modifies the biochemical structure of the ECM. Indeed, pCLE imaging may indirectly evaluate the biochemical changes occurring during carcinogenesis in the ECM. This could become a powerful tool and fill the gap whereby histology does not enable assessment of the ECM. Likewise, improvement in this innovative approach may allow the surgeon to detect cancer directly in vivo, without invasive and time-demanding biologic tissue sampling. The next step will be to evaluate the feasibility of performing pCLE intraoperatively and to determine whether the criteria observed ex vivo can also be retrieved in in vivo conditions. Interpretation of the images requires training. The investigator who performed the retrospective analysis has become an expert in the interpretation of pCLE images during this study, and it may be that the results are less reproducible by nonexpert physicians.
There were some limitations to the present series. Firstly, the sample size was limited, and the results should be confirmed in larger series. Secondly, only adenocarcinoma lesions were observed. A further study should evaluate the performance of pCLE in the characterization of signet cells and mucinous carcinomas. Additionally, as the tumor invades peritoneal tissues, fibrosis may occur, resulting in an increased thickness of the superficial peritoneal layer. In these conditions, the confocal depth (40 microns) selected for this first study may not be sufficient to ensure a satisfactory sensitivity of the pCLE technique.
In conclusion, these preliminary results suggest that pCLE enables good-quality microscopic imaging of peritoneal nodules that accurately correlates with histological findings. More importantly, the capacity of pCLE with a 785-nm excitation to detect biochemical changes in the ECM represents a significant development in the characterization of peritoneal nodules, which otherwise is challenging in terms of differentiating inflammatory from malignant nodules using frozen sections. The results of this study are promising, and the next step will be to include the application of the newly defined criteria in a study of the intraoperative characterization of peritoneal nodules.
References
Vercauteren T, Meining A, Lacombe F, Perchant A (2008) Real time autonomous video image registration for endomicroscopy: fighting the compromises. Three-Dimens Multidimens Microsc Image Acquis Process XV 6861:68610C
Becker V, Vieth M, Bajbouj M, Schmidt RM, Meining A (2008) Confocal laser scanning fluorescence microscopy for in vivo determination of microvessel density in Barrett’s esophagus. Endoscopy 40:888–891
Caillol F, Filoche B, Gaidhane M, Kaheleh M (2013) Refined probe-based confocal laser endomicroscopy classification for biliary strictures: the Paris classification. Dig Dis Sci 58:1784–1789
Shahid MW, Buchner AM, Coron E (2012) Diagnostic accuracy of probe-based confocal laser endomicroscopy in detecting residual colorectal neoplasia after EMR: a prospective study. Gastrointest Endosc 75:525–533
Napoléon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Filoche B, Giovannini M (2015) A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy 47:26–32
Tontini GE, Mudter J, Vieth M, Atreya R, Günther C, Zopf Y, Wildner D, Kiesslich R, Vecchi M, Neurath MF, Neumann H (2015) Confocal laser endomicroscopy for the differential diagnosis of ulcerative colitis and Crohn’s disease: a pilot study. Endoscopy 47:437–443
Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, Bajbouj M, Galmiche JP, Abrams JA, Rastogi A, Gupta N, Michalek JE, Lauwers GY, Wallace MB (2011) Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc 74:465–472
Thiberville L, Moreno-Swirc S, Vercauteren T, Peltier E, Cavé C, Bourg Heckly G (2007) In vivo imaging of the bronchial wall microstructure using fibered confocal fluorescence microscopy. Am J Respir Crit Care Med 175:22–31
Wellikoff AS, Holladay RC, Downie GH, Chaudoir CS, Brandi L, Turbat-Herrera EA (2015) Comparison of in vivo probe-based confocal laser endomicroscopy with histopathology in lung cancer: a move toward optical biopsy. Respirology 20:967–974
Wu K, Liu JJ, Adams W, Sonn GA, Mach KE, Pan Y, Beck AH, Jensen KC, Liao JC (2011) Dynamic real-time microscopy of the urinary tract using confocal laser endomicroscopy. Urology 78:225–231
Zlatev DV, Altobelli E, Liao JC (2015) Advances in imaging technologies in the evaluation of high-grade bladder cancer. Urol Clin North Am 42:147–157
Grobmyer SR, Fong Y, D’Angelica M, Dematteo RP, Blumgart LH, Jarnagin WR (2004) Diagnostic laparoscopy prior to planned hepatic resection for colorectal metastases. Arch Surg 139:1326–1330
Oberschmid B, Dietrich A, Wittekind C (2012) Frozen sections diagnostics in visceral surgery. Stomach and intestines. Pathologie 33:407–412
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, de Vet HC, Lijmer JG (2003) The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 138:W1–W12
Wang KK, Carr-Lock DL, Singh SK, Neumann H, Bertani H, Galmiche JP, Arsenescu RI, Caillol F, Chang KJ, Chaussade S, Coron E, Costamagna G, Dlugosz A, Ian Gan S, Giovannini M, Gress FG, Haluszka O, Ho KY, Kahaleh M, Konda VJ, Prat F, Shah RJ, Sharma P, Slivka A, Wolfsen HC, Zfass A (2015) Use of probe-based confocal laser endomicroscopy (pCLE) in gastrointestinal applications. A consensus report based on clinical evidence. U Euro Gastroenterol J 3:230–254
Anderson MA, Appalaneni V, Ben-Menachem T, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Fisher LR, Fukami N, Hwang JH, Ikenberry SO, Jain R, Jue TL, Khan K, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Shergill AK, Dominitz JA, Cash BD (2013) American Society for gastrointestinal endoscopy (ASGE) standards of practice committee. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc 77:167–174
ASGE Technology Committee, Chauhan SS, Abu Dayyeh BK, Bhat YM, Gottlieb KT, Hwang JH, Komanduri S, Konda V, Lo SK, Manfredi MA, Maple JT, Murad FM, Siddiqui UD, Banerjee S, Wallace MB (2014) Confocal laser endomicroscopy. Gastrointest Endosc 80:928–938
Von Delius S, Feussner H, Wilhelm D, Karagianni A, Henke J, Schmid RM, Meining A (2007) Transgastric in vivo histology in the peritoneal cavity using miniprobe-based confocal fluorescence microscopy in an acute porcine model. Endoscopy 39:407–411
Meining A, Bajbouj M, Delius S, Prinz C (2007) Confocal laser scanning microscopy for in vivo histopathology of the gastrointestinal tract. Arab J Gastroenterol 8(1):1–4
Hong JH, Song SH, Kim SE (2011) Diffuse intraabdominal fibrosis and inflammation mimicking peritoneal carcinomatosis recurred after surgery for borderline ovarian tumor misdiagnosed by 18F-fluorodeoxyglucose-positron emission tomography. Eur J Gynaecol Oncol 32:231–233
Acknowledgments
We would like to thank Pauline La Fay, Siddharth Maskara from Mauna Kea Technologies, Bérengère Bardou from Endocontrol for additional logistical support during the trial and BPI France for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The study was funded by BPI France: #I0911038 W Prof. Brice Gayet is a consultant for Mauna Kea Technologies. Mr. Pierangelo, Dr. Fuks, Dr. Validire, Prof. Gayet have received funding from Mauna Kea Technologies to support congress registration and travel fees. Dr. Benali has no conflict of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 18891 kb)
Rights and permissions
About this article
Cite this article
Pierangelo, A., Fuks, D., Benali, A. et al. Diagnostic accuracy of confocal laser endomicroscopy for the ex vivo characterization of peritoneal nodules during laparoscopic surgery. Surg Endosc 31, 1974–1981 (2017). https://doi.org/10.1007/s00464-016-5172-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5172-7