Abstract
Background
Although laparoscopic liver resection (LLR) has advanced into a safe and effective alternative to conventional open liver resection (OLR), it has not been widely accepted by surgeons. This article aimed to investigate the perioperative and long-term benefits of LLR versus OLR for hepatocellular carcinoma (HCC) in selected patients with well-preserved liver function and cirrhotic background.
Methods
A retrospective study was conducted on 1085 patients with HCC who underwent liver resection at Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University from July 2010 to July 2015, and 346 patients with well-preserved liver function and cirrhotic background were selected. A 1:1 propensity score matching (PSM), which is the best option to overcome selection bias, was conducted to compare the surgical outcomes and long-term prognosis between LLR and OLR. After PSM, a logistic regression analysis was used to identify the predictive risk factors of posthepatectomy liver failure (PHLF).
Results
By using PSM, the two groups were well balanced with 86 patients in each group. In the LLR group, only the median operation time was significantly longer than the OLR group, but the hospital stay, overall morbidity, and the incidence of PHLF were significantly decreased compared to OLR. There were no significant differences in the overall survival and disease-free survival rates between the two groups. On multivariate analysis, OLR was identified to be the only independent risk factor for PHLF.
Conclusions
In selected HCC patients with well-preserved liver function and cirrhotic background, LLR could be a better option compared to OLR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Hepatocellular carcinoma is the fifth most common cancer [1] and is now the second [2] leading cause of cancer-related deaths worldwide and its incidence is still on the rise. Approximately 80% of HCC develop from chronic liver diseases such as hepatitis B/C virus (HBV/HCV) -associated cirrhosis [3] and non-alcoholic fatty liver disease/NASH which is becoming an important cause of HCC in developed regions [4].
Liver transplantation has been established as an effective and safe treatment for HCC patients with decompensated cirrhosis. Unfortunately, the most challenging problem in liver transplantation is the inadequate supply of donor organs [5]. Besides, many patients are deemed unsuitable candidates for liver transplantation, either because the tumor is too advanced, or the wait for a donated cadaveric liver is too long. Until now, the most conventional treatment for HCC is liver resection. The first LLR was carried out for benign liver lesions in 1991 [6]. Improvements in laparoscopic techniques and instruments, as well as accumulated experience have led to a wider acceptance of LLR by surgeons, especially after the First International Consensus Conference in 2008 [7]. LLR is gradually considered as a safe and effective approach for HCC when carried out by experienced surgeons. Furthermore, several cohort studies have suggested that laparoscopic left lateral sectionectomy should now be accepted as the standard of care [8].
Liver resection in a cirrhotic patient is technically challenging, even with the open approach [9]. This is especially so in major LLR which is still considered to be at an exploration or learning phase with incompletely defined risks [10]. The data on whether the advantages of minimally invasive surgery, such as early recovery and discharge from hospital, less postoperative pain and early oral intake can be applied in LLR [11] are limited. Several studies have suggested that LLR results in less blood loss, lower postoperative morbidity, and fewer adhesions than OLR [3, 12,13,14]. There have been several reports which compared the safety and efficacy between LLR and OLR in cirrhotic HCC patients. However, these reports all have the limitations of small patient numbers, the studies were not case-matched, and only solitary tumors were included [14,15,16,17]. The present study was conducted to compare the perioperative and long-term outcomes between LLR and OLR in HCC patients with well-preserved liver function and background cirrhosis using propensity score matching.
Patients and methods
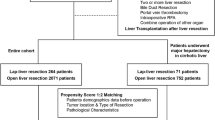
The study was approved by the medical ethical committees of Sun Yat-Sen Memorial Hospital. In this study, we retrospectively reviewed the data of 1085 consecutive HCC patients who underwent liver resection at Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University from July 2010 to July 2015.
The inclusion criteria of this study were: (1) histopathologically diagnosed as HCC with a background of cirrhosis based on the final pathology specimens and (2) well-preserved liver function which was defined as Child-Pugh class A liver function, indocyanine green (ICG) retention rate at 15 min of less than 10%, and adequate future liver remnant (FLR) of greater than 40% of the total liver volume (TLV) [18, 19]. All patients underwent routine preoperative contrast-enhanced computed tomography (CT). The arterial, portal, and venous phases of the imaging were used for CT volumetry. Patients were excluded if there was at least one of the following conditions: (1) thrombus in large vessels including the portal vein, hepatic vein or inferior vena cava; (2) clinically significant portal hypertension such as presence of esophageal varices, λ splenomegaly (> 12 cm) with a low platelet count (< 100 × 109/ml). After implementation of this exclusion criteria,a total of 346 patients were selected for this study.
A standardized operative protocol was adopted by all the operating surgeons in both LLR and OLR. The surgeons were all experienced and skillful in both the traditional open and minimally invasive liver surgeries. The choice of LLR or OLR fully depended on the comprehensive assessment by surgeons with informed consent from the patients. LLR is the first choice for HCC patients unless there was some condition contraindicating or significantly complicating the use of laparoscopic approach, including the patients cannot tolerate pneumoperitoneum, severe adhesion, the tumor was too close to major vessels, the tumor was too large to safely perform laparoscopy, the tumor was located in posterior segments, and was difficult to complete resection via the laparoscopic approach or extensive hepatic lymph node dissection is required [20].
Preoperative investigations included CT, magnetic resonance imaging (MRI), abdominal ultrasonography, or shear-wave elastography (SWE, a method to assess the extent of fibrosis), and blood tests (blood routine examination, liver function test, and alpha feto-protein (AFP) levels). ICG retention rate at 15 min was also performed.
The terminology of liver resection was adopted as defined by the Brisbane 2000 classification [21]. Patients who were converted to open resection were included in the group of LLR [22]. Postoperative complications were graded according to the Clavien–Dindo Classification [23]. PHLF was defined and graded according to the criteria as proposed by the International Study Group of Liver Surgery (ISGLS). Accordingly, PHLF was defined as an increased in international normalized ratio (INR) and hyperbilirubinemia on or after the 5th postoperative day. PHLF grade A represented a postoperative deterioration but did not require a change in the patient’s clinical management. PHLF grade B was diagnosed when there was any deviation from the regular postoperative clinical pathway but the patients could be managed without any invasive treatment. PHLF grade C indicated that patients required an invasive procedure. Postoperative mortality was based on any death occurring within 90 days after surgery.
Propensity score matching
A 1:1 PSM was conducted. The propensity score analysis model was used to eliminate any potential bias of case-match selection. The caliper of 0.01 was used in the model. Potential confounding variables that were unrelated to the exposure but related to the outcome were included in the propensity score model to decrease the variance of an estimated exposure effect without increasing the bias [24]. Accordingly, the balanced variables included baseline characteristics such as sex, age, body mass index (BMI), cause of cirrhosis (HBV, HCV or NBNC), American Society of Anesthesiology (ASA) score, comorbidities; preoperative diagnosed cirrhosis; preoperative laboratory data including total bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), platelet, albumin, creatinine, INR, and MELD (Model for end-stage liver disease) scores; variables related to resection included intraoperative procedure, extent of resection (major or minor), types of resection and Pringle maneuver; variables on tumor characteristics included tumor size, number of tumor (solitary or multiple), microvascular invasion, and tumor stage (Edmonson Steiner grade).
Risk factors of PHLF
Variables that might affect the postoperative outcomes were incorporated into the univariate analysis to identify the risk factors of PHLF, including sex, age, the surgical approach (OLR or LLR), etiology of cirrhosis, intraoperative procedure, comorbidities, extent of hepatectomy, Pringle maneuver, tumor size, number of lesions, microvascular invasion, operation time, blood loss, blood transfusion, level of AFP, MELD score, and Edmonson Steiner grade. The variables were included into the multivariate analysis of logistic regression (for p values of less than 0.05) to identify the independent risk factors of PHLF. The univariate and multivariate analyses were both performed on the entire cohort of patients after PSM.
Postoperative care and follow-up
The protocols of enhanced recovery after surgery (ERAS) was applied to our patients, blood tests were carried out on day 1, 3, 5, and 7, and an abdominal CT was carried out on day 8. The follow-up visits were carried out once a month after hospital discharge and then once every 3 months during the first year, once every 6 months for the following 2 years and once a year, thereafter [25]. The followed up assessments included routine blood tests, liver function tests, AFP, and abdominal ultrasonography or CT/MRI.
Statistical analysis
Categorical data were expressed as numbers (percentages). Continuous data were expressed as median (range). The Chi-squared test or Fisher’s exact test were used to compare categorical variables, and the Mann–Whitney U test was used to compare continuous variables. Initially, univariate analyses were conducted using the logistic regression analysis. Thereafter, variables found to be significantly associated with p < 0.05 were tested by multivariate analysis using a backward stepwise logistic model. The Kaplan–Meier method was used to obtain cumulative survival rates and compared using the log-rank test. A p value of less than 0.05 was considered statistically significant. All statistical analyses were performed using the statistical software SPSS Version 22.0 (IBM SPSS).
Results
During the study period, 1085 patients underwent liver resection and 346 patients were included into this study. There were 141 patients who underwent LLR and 205 patients OLR. After a 1:1 PSM, there were 86 patients in each of the groups of LLR and OLR.
Baseline and pathological characteristics
In the entire cohort (see Supplementary Table 1), there were no significant differences in the characteristics: including sex, age, etiology of cirrhosis, ASA score, comorbidities, total bilirubin, ALT, platelet, albumin, creatinine, INR, MELD scores, intraoperative procedure, extent of resection, types of resection, Pringle maneuver, tumor size, number of tumor, microvascular invasion, and tumor stage. However, the median BMI was significantly higher in the OLR group than the LLR group (23.8 vs. 22.5 kg/m2, p = 0.011). Significantly, there were less patients who were diagnosed to have cirrhosis before surgery (57.4 vs. 75.1%, p < 0.001) in the LLR group than the OLR group. The median AFP level was also significantly higher in the OLR group compared to the LLR group (172.4 vs. 26.5 ng/ml, p < 0.001). The median serum level of AST was significantly higher in the LLR group compared to the OLR group (39 vs. 25 U/l, p = 0.034).
Surgical characteristics and outcomes
There were no significant differences between the two groups in most surgical characteristics and surgical outcomes in the entire cohort (see Supplementary Table 2), including intraoperative procedure, extent of resection, types of resection, utilizing of Pringle maneuver, blood loss, blood transfusion rate, hospital stay, severe complications, and PHLF. Nevertheless, there was significantly longer operation time in the LLR group than the OLR group (230 vs. 180 min, p = 0.021). The morbidity in the LLR group tended to be lower than the OLR group but without statistical significance (8.5 vs. 15.6%, p = 0.051).
Baseline and pathological characteristics after PSM
After PSM, there were no significant differences in the baseline and pathological characteristics between the two groups as shown in Table 1.
Surgical characteristics and outcomes after PSM
Table 2 shows the surgical characteristics and surgical outcomes after PSM. The median hospital stay was significantly shorter in the LLR group than the OLR group (8 vs. 13 days, p = 0.018). The median operation time was significantly longer in the LLR group than the OLR group (210 vs. 170 min, p = 0.021). The overall morbidity was significantly lower in the LLR group than the OLR group (7.0 vs. 19.8%, p = 0.014), mainly in the incidence of ascites (0 vs. 7.0%, p = 0.029). The incidence of total PHLF was also significantly lower in the LLR group than the OLR group (15.1 vs. 32.6%, p = 0.007), mainly in grade B PHLF (5.8 vs. 17.4%, p = 0.017). There were no significant differences in the intraoperative procedure, extent of hepatectomy, resection types, and use of Pringle maneuver between the LLR and OLR groups. Besides, blood loss (150 vs. 250 ml, p = 0.224) and blood transfusion (10.5 vs. 15.1%, p = 0.361) were not significantly different between the two groups. The 90-day mortality after surgery for the LLR and the OLR groups were 0 and 2.3% (p = 0.497), respectively. The conversion rate was 9.3% (n = 8).
Risk factors of PHLF
Univariate analysis identified the surgical approach of OLR (p = 0.031), operation time ≥ 300 min (p = 0.018), and blood loss ≥ 1500 ml (p = 0.034) to be risk factors for PHLF (Table 3). Multivariate analysis identified the surgical approach of OLR (OR 2.539, 95% CI 1.127–7.851, p = 0.014) to be the only independent risk factor of PHLF.
Long-term outcomes
The median follow-up was not significantly different between the two groups, with 39.5 months in the LLR group and 37.3 months in the OLR group (p = 0.453). The 1-, 3-, and 5-year overall survival rates were 93.0, 81.4, and 69.8%, respectively, in the LLR group, and 88.4, 75.5, and 62.8%, respectively, in the OLR group (p = 0.304) (Fig. 1). The 1-, 3-, and 5-year disease-free survival rates were 75.6, 60.5, and 44.2%, respectively, in the LLR group, and 69.8, 53.5, and 38.4%, respectively, in the OLR group (p = 0.393) (Fig. 2). There were no significant differences in both the OS and DFS between the LLR and OLR groups.
Discussion
Liver resection and liver transplantation are the standard curative therapies for HCC. Liver transplantation treats both the cancer and the underlying liver disease. The best candidates for liver transplantation are those within the Milan Criteria (solitary tumor ≤ 5 cm and up to three nodules ≤ 3 cm each) [2, 26]. Liver transplantation is the only surgical option for patients with decompensated cirrhosis. Unfortunately, severe donor organ shortage significantly limits its application. Consequently, liver resection remains the first line treatment for the majority of HCC patients with compensated liver function. However, the conventional open liver resection requires a large abdominal incision.
Since the first laparoscopic liver resection for benign liver lesions in 1991, with advances in surgical anatomy, laparoscopic skills and devices, and postoperative care [27], the indications of laparoscopic liver resection have gradually extended to include cirrhotic liver resection [28]. The advantages of laparoscopic surgery include the ability to perform surgery without a large incision, with the advantages of minimal invasiveness, and early recovery [29].
Nevertheless, cirrhotic liver resection is challenging even for experienced surgeons. The challenges include difficulties in hemostasis during liver parenchymal transection and postoperative hemorrhage, which are associated with low platelet counts, coagulopathy, and portal hypertension in these patients. Careful selection of cirrhotic HCC patients for surgery is of vital importance. In the present study, all the included patients had well-preserved liver functions, and had adequate volumes of the FLR. Wakabayashi and Ribero both recommended an FLR of > 40% of TLV to be the safe limit of liver resection for cirrhotic HCC patients [18, 19]. Ferrero [30] proposed an FLR of > 31% for patients with impaired liver function. Kim [31] suggested a standardized liver future remnant (sFLR) of ≥ 25% in non-cirrhotic patients and an sFLR of ≥ 25% with an sFLR:ICG R15 ratio of > 1.9 in patients with cirrhosis to be acceptable for safe hepatic resection. To reduce life-threatening PHLF and mortality, at least 40% of TLV was defined to be an adequate FLR in the present study. For patients whose FLR was less than 40%, preoperative portal vein embolization or a staged hepatectomy associating liver partition and portal vein ligation (ALPPS) [32] was recommended to our patients.
The median hospital stay (8 vs. 13 days, p = 0.018), incidence of complication (7.0 vs. 19.8%, p = 0.014), and incidence of PHLF (15.1 vs. 32.6%, p = 0.007) were significantly decreased after LLR compared to OLR. These results were similar to those which have been reported [9, 15, 16] or concluded in the second global review [33]. However, our operation time was longer in LLR than OLR (210 vs. 170 min, p = 0.021), which was different from the other reported studies. One possible explanation was that more difficult laparoscopic procedures were attempted in our center. There were only 10% of laparoscopic major hepatectomy in the study by Cheung [9] compared with 26.7% laparoscopic major hepatectomy in our study. Another possible explanation is the relatively high rate of conversion (9.3%) [34]. Actually, when the conversion cases (n = 8) were excluded, the significant difference no longer existed (205 vs. 180 min, p = 0.128).
Many reports on LLR have shown that LLR was associated with less blood loss and a decreased need of blood transfusion. These can be explained by the hemostatic effect of pneumoperitoneum, better magnification, and application of newly developed devices for parenchymal transection [9, 13, 24, 35]. In our study, intraoperative blood loss (150 vs. 250 ml, p = 0.224) and blood transfusion rates (10.5 vs. 15.1%, p = 0.361) tended to be decreased in the LLR group but without statistical significance. In our center, hepatic inflow occlusion was only used when there was excessive bleeding, which is the possible reason for the absence of reduction of blood loss and blood transfusion in LLR. It is our belief that although the Pringle maneuver can reduce intraoperative bleeding, the repeated ischemia and reperfusion injury using intermittent Pringle maneuver can result in impairment in liver function after hepatectomy, early recurrence, and poor survival [36]. Furthermore, a long duration of Pringle maneuver have been shown to increase tumor recurrence [37]. However, if the vascular occlusion durations are not excessive, the application of intermittent Pringle maneuver may have no adverse impact on postoperative outcomes [38, 39]. Most people believe that intraoperative bleeding and the need for blood transfusion increase the risks of postoperative morbidity and mortality, affect tumor recurrence and long-term survival in HCC patients [13, 35, 40, 41].However, by utilizing PSM to overcome the effects of co-variables, Yang et al. [42] proposed a novel finding that the associations among perioperative blood transfusion, early recurrence and decreased survivals were due to the clinical circumstances rather than the blood transfusions themselves.
The Louisville consensus [7] claimed that conversion from laparoscopy to open laparotomy should be considered to be a prudent surgical practice rather than a failure. Conversion should be performed in difficult cases and for patient safety. The overall reported conversion rates are around 4.1% [33]. The conversion rate of our study is 9.3% (n = 8), which is equal to the conversion rate reported by Xiang [43], lower than the 23.3% reported by Kim [16] but higher than the 6.9% reported by Siddiqi [44] and the 2.3% reported by Long [45]. Massive haemorrhage and difficulty in hemostasis were the main reasons for conversion (n = 4). One of these patients had a tumor larger than 10 cm and numerous supplying vascular leading to excessive bleeding. Followed by severe adhesions (n = 2) that generated after previous abdominal operation. The other two conversions were due to tumor location (n = 2). One was located at segment 4a and was too close to the diazoma and one was located at segment 7/8. A tumor which is located at the postero-superior segment is identified to be an independent risk factor of conversion in LLR [46].
Consistent with the published literature [45, 47], our morbidities were significantly lower after LLR than OLR (7.0 vs. 19.8%, p = 0.014), especially for postoperative ascites. The less invasiveness of laparoscopy significantly mitigated damage to the abdominal wall, thus avoiding interruption of collateral veins and lymphatic circulation. Less exposure of the abdominal viscera may be the other important reason [11, 48] for the reduced incidence of ascites. Additionally, a CO2 pneumoperitoneum, which has been demonstrated to reduce local immune responses against surgical stress [14], could be another explanation. There were also other possible explanations which include less mobilization and manipulation of the liver and less intraoperative fluid requirements in LLR [13].
Being less invasive, LLR has been demonstrated to be associated with improved postoperative outcomes, including less operative morbidity and PHLF, without compromising the long-term survival outcomes. Both the 1-, 3-, and 5-year OS (p = 0.304) and the 1-, 3-, and 5-year DFS (p = 0.393) of LLR were comparable to OLR. Therefore, LLR for HCC with a background of cirrhosis have been suggested to be a good choice of treatment in selected patients [49].
As shown in Table 2,the incidence of PHLF was significantly lower in LLR than in OLR (15.1 vs. 32.6%, respectively, p = 0.007). To further confirm the advantage of LLR for reducing the incidence of PHLF, a multivariate analysis was conducted and we identified the surgical approach of OLR (OR 2.539, 95% CI 1.127–7.851, p = 0.014) to be the only independent risk factor of PHLF, implying that LLR is a better surgical approach in selected patients with cirrhosis.
In conclusion, with reduced hospital stay and lower incidence of complications and PHLF, LLR could be a better option for HCC in selected patients with well-preserved liver function and cirrhotic background.
References
El-Serag HB (2011) Hepatocellular carcinoma. N Engl J Med 365:1118–1127
Maluccio M, Covey A (2012) Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin 62:394–399
Twaij A, Pucher PH, Sodergren MH, Gall T, Darzi A, Jiao LR (2014) Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol 20:8274–8281
Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. Lancet 391:1301–1314
Dienstag JL, Cosimi AB (2012) Liver transplantation—a vision realized. N Engl J Med 367:1483–1485
Reich H, Mcglynn F, Decaprio J, Budin R (1991) Laparoscopic excision of benign liver lesions. Obstet Gynecol 78:956–958
Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D’Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS (2009) The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg 250:825–830
Dagher I, O’Rourke N, Geller DA, Cherqui D, Belli G, Gamblin TC, Lainas P, Laurent A, Nguyen KT, Marvin MR, Thomas M, Ravindra K, Fielding G, Franco D, Buell JF (2009) Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg 250:856–860
Cheung TT, Dai WC, Tsang SH, Chan AC, Chok KS, Chan SC, Lo CM (2016) Pure laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 110 patients with liver cirrhosis: a propensity analysis at a single center. Ann Surg 264:612–620
Aldrighetti LA, Cai X, Cleary S, Chen KH, Schon R, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261:619–629
Morise Z, Kawabe N, Kawase J, Tomishige H, Nagata H, Ohshima H, Arakawa S, Yoshida R, Isetani M (2013) Pure laparoscopic hepatectomy for hepatocellular carcinoma with chronic liver disease. World J Hepatol 5:487–495
Kruger JA, Fonseca GM, Coelho FF, Jeismann V, Herman P (2017) Laparoscopic right hepatectomy for cirrhotic patients: takasaki’s hilar control and caudal approach. Ann Surg Oncol 24:558–559
Yin Z, Fan X, Ye H, Yin D, Wang J (2013) Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol 20:1203–1215
Yamashita Y, Ikeda T, Kurihara T, Yoshida Y, Takeishi K, Itoh S, Harimoto N, Kawanaka H, Shirabe K, Maehara Y (2014) Long-term favorable surgical results of laparoscopic hepatic resection for hepatocellular carcinoma in patients with cirrhosis: a single-center experience over a 10-year period. J Am Coll Surg 219:1117–1123
Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, Fan ST, Lo CM (2013) Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 257:506–511
Kim H, Suh KS, Lee KW, Yi NJ, Hong G, Suh SW, Yoo T, Park MS, Choi Y, Lee HW (2014) Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: a case-controlled study with propensity score matching. Surg Endosc 28:950–960
Yoon YI, Kim KH, Kang SH, Kim WJ, Shin M, Jung DH, Park C, Ahn CS, Moon DB, Ha TY, Song GW, Hwang S, Lee SG (2017) Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score matched analysis. Ann Surg 265:856–863
Wakabayashi H, Ishimura K, Okano K, Karasawa Y, Goda F, Maeba T, Maeta H (2002) Application of preoperative portal vein embolization before major hepatic resection in patients with normal or abnormal liver parenchyma. Surgery 131:26–33
Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN (2007) Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg 94:1386–1394
Tanaka S, Kubo S, Kanazawa A, Taked Y, Hirokawa F, Nitta H, Nakajima T, Kaizu T, Kaneko H, Wakabayashi G (2017) Validation of a difficulty scoring system for laparoscopic liver resection: a multicenter analysis by the endoscopic liver surgery study group in Japan. J Am Coll Surg 225:249–258
Pang YY (2002) The Brisbane 2000 terminology of liver anatomy and resections. HPB 4:99–100
Fuks D, Cauchy F, Fteriche S, Nomi T, Schwarz L, Dokmak S, Scatton O, Fusco G, Belghiti J, Gayet B, Soubrane O (2016) Laparoscopy decreases pulmonary complications in patients undergoing major liver resection: a propensity score analysis. Ann Surg 263:353–361
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol 63:643–650
Liu K, Chen Y, Wu X, Huang Z, Lin Z, Jiang J, Tan W, Zhang L (2017) Laparoscopic liver re-resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: a propensity score matching study. Surg Endosc. https://doi.org/10.1007/s00464-017-5556-3
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 379:1245–1255
Afaneh C, Kluger MD (2013) Laparoscopic liver resection: lessons at the end of the second decade. Semin Liver Dis 33:226–235
Cannon RM, Saggi B, Buell JF (2014) Evaluation of a laparoscopic liver resection in the setting of cirrhosis. HPB 16:164–169
Clavien PA, Barkun J (2015) Consensus conference on laparoscopic liver resection: a jury-based evaluation. Ann Surg 261:630–631
Ferrero A, Vigano L, Polastri R, Muratore A, Eminefendic H, Regge D, Capussotti L (2007) Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg 31:1643–1651
Kim HJ, Kim CY, Park EK, Hur YH, Koh YS, Kim HJ, Cho CK (2015) Volumetric analysis and indocyanine green retention rate at 15 min as predictors of post-hepatectomy liver failure. HPB 17:159–167
Zhang GQ, Zhang ZW, Lau WY, Chen XP (2014) Associa ting liver partition and portal vein ligation for staged hepatectomy (ALPPS): a new strategy to increase resectability in liver surgery. Int J Surg 12:437–441
Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G (2016) Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 263:761–777
Nguyen KT, Gamblin TC, Geller DA (2009) World review of laparoscopic liver resection-2,804 patients. Ann Surg 250:831–841
Goumard C, Farges O, Laurent A, Cherqui D, Soubrane O, Gayet B, Pessaux P, Pruvot FR, Scatton O (2015) An update on laparoscopic liver resection: the French hepato-bilio-pancreatic surgery association statement. J Visc Surg 152:107–112
Cho JY, Han HS, Choi Y, Yoon YS, Kim S, Choi JK, Jang JS, Kwon SU, Kim H (2017) Association of remnant liver ischemia with early recurrence and poor survival after liver resection in patients with hepatocellular carcinoma. JAMA Surg 152:386–392
Liu S, Li X, Li H, Guo L, Zhang B, Gong Z, Zhang J, Ye Q (2016) Longer duration of the Pringle maneuver is associated with hepatocellular carcinoma recurrence following curative resection. J Surg Oncol 114:112–118
van den Broek MA, Bloemen JG, Dello SA, van de Poll MC, Olde Damink SW, Dejong CH. Randomized controlled trial analyzing the effect of 15 or 30 min intermittent Pringle maneuver on hepatocellular damage during liver surgery. J Hepatol 55:337–345
Dua MM, Worhunsky DJ, Hwa K, Poultsides GA, Norton JA, Visser BC. Extracorporeal Pringle for laparoscopic liver resection. Surg Endosc 29:1348–1355
Franssen B, Alshebeeb K, Tabrizian P, Marti J, Pierobon ES, Lubezky N, Roayaie S, Florman S, Schwartz ME (2014) Differences in surgical outcomes between hepatitis B- and hepatitis C-related hepatocellular carcinoma: a retrospective analysis of a single North American center. Ann Surg 260:650–658
Liu L, Wang Z, Jiang S, Shao B, Liu J, Zhang S, Zhou Y, Zhou Y, Zhang Y (2013) Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta-analysis. PLoS ONE 8:e64261
Yang T, Lu JH, Lau WY, Zhang TY, Zhang H, Shen YN, Alshebeeb K, Wu MC, Schwartz M, Shen F. Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: a propensity score matching analysis. J Hepatol 64:583–593
Xiang L, Li J, Chen J, Wang X, Guo P, Fan Y, Zheng S (2016) Prospective cohort study of laparoscopic and open hepatectomy for hepatocellular carcinoma. Br J Surg 103:1895–1901
Siddiqi NN, Abuawwad M, Halls M, Rawashdeh A, Giovinazzo F, Aljaiuossi A, Wicherts D, D’Hondt M, Hilal MA (2017) Laparoscopic right posterior sectionectomy (LRPS): surgical techniques and clinical outcomes. Surg Endosc. https://doi.org/10.1007/s00464-017-5958-2
Long T, Bac NH, Thuan ND, Dat LT, Viet D, Chuong LCHQ (2014) Laparoscopic liver resection: 5-year experience at a single center. Surg Endosc 28:796–802
Troisi RI, Montalti R, Van Limmen JG, Cavaniglia D, Reyntjens K, Rogiers X, De Hemptinne B (2014) Risk factors and management of conversions to an open approach in laparoscopic liver resection: analysis of 265 consecutive cases. HPB 16:75–82
Morise Z, Ciria R, Cherqui D, Chen KH, Belli G, Wakabayashi G (2015) Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci 22:342–352
Truant S, Bouras AF, Hebbar M, Boleslawski E, Fromont G, Dharancy S, Leteurtre E, Zerbib P, Pruvot FR (2011) Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc 25:3668–3677
Sposito C, Battiston C, Facciorusso A, Mazzola M, Muscara C, Scotti M, Romito R, Mariani L, Mazzaferro V (2016) Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg 103:871–880
Acknowledgements
We really appreciate Wan Yee Lau, the former chairman of IHBPA, for his useful guidance and suggestion. This work was supported by the National Natural Science Foundation of China (Grant No. 81372562).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Diclosures
Drs. Xinqiang Wu, Lei Zhang, Zejian Huang, Won Yee Lau, Wenda Li, Pai Lin, and Yajin Chen have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, X., Huang, Z., Lau, W.Y. et al. Perioperative and long-term outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with well-preserved liver function and cirrhotic background: a propensity score matching study. Surg Endosc 33, 206–215 (2019). https://doi.org/10.1007/s00464-018-6296-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6296-8