Abstract
Background
Liver re-resection plays a paramount role in treatment of patients with posthepatectomy hepatocellular carcinoma (HCC) recurrence. Laparoscopic liver resection has been a feasible alternative to open surgery. However, whether laparoscopic liver re-resection for posthepatectomy HCC recurrence is better than open liver re-resection remains unknown.
Method
From January 2008 to December 2015, 30 patients with recurrent HCC after prior liver resection underwent laparoscopic liver re-resection in our center. To minimize any confounding factors, a propensity score matching study using a patient ratio of 1:1 was conducted to compare the short- and long-term outcomes of patients who underwent laparoscopic or open liver re-resection.
Result
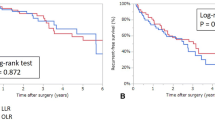
With the open surgery group compared laparoscopic group, operative time was 207.50 versus 200.5 min (p = 0.903), blood loss was 400 versus 100 ml (p = 0.000196), blood transfusion rate was 43.3 versus 0.0% (p = 0.000046), complication rates were 30.0 versus 6.7% (p = 0.01), and hospital stay was 13.5 versus 9.5 days (p = 0.000008). The median follow-up was 35 months. The 1-year, 3-year, 5-year disease-free survival rates were 79.0, 51.0, and 31.9%, versus 78.3, 57.4, and 43.0%, respectively (p = 0.474). The 1-year, 3-year, and 5-year overall survival rates were 89.4, 75, and 67.5%, versus 96.7, 85.0, and 74.4%, respectively (p = 0.413).
Conclusion
Laparoscopic liver re-resection for patients with posthepatectomy HCC recurrence provided comparable perioperative and oncological outcomes as open liver re-resection and can be a safe alternative to open procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatic resection is an effective and commonly used treatment for hepatocellular carcinoma (HCC) [1]. Tumor recurrence after liver resection remains a bottleneck to improve long-term prognosis. Cumulative 5-year tumor recurrence rates after R0 resection of HCC have been reported to be up to 70%, and intrahepatic recurrence accounts for about 80% of these patients [2]. At present, appropriate treatment strategies for recurrent HCC aiming at cure include liver re-resection, liver transplantation (LT), and radiofrequency ablation (RFA). Liver re-resection is a potentially curative treatment for HCC recurrence although factors such as multiple liver metastases, impaired liver function and poor general condition of patients affect its feasibility. The resectability rate is less than 30% [3]. LT in selected patients shows impressive results, but its role as a first line treatment for patients with recurrent HCC is limited by the insufficient supply of donor organs [4]. Radiofrequency ablation is a minimal invasive and repeatable treatment and it has achieved similar effectiveness as hepatic resection in recent studies. Nevertheless, the long-term outcomes of radiofrequency ablation is inferior to liver resection, due to more common tumor recurrence as a consequence of residual tumor, tumor seeding alone needle tracts, and possible induction of tumor dissemination into adjacent portal vessels by increased intratumoral pressure [5]. TACE is the most frequently used treatment for HCC recurrence, but the curative role of TACE is questionable because of its low 5-year survival rate [6].
Surgical progress in the past decade has established the role of laparoscopic liver resections (LLRs) in the primary treatment of HCC. In addition to its advantages of less invasiveness, it produces similar oncologic clearance with no compromise in survival as compared with open liver resection. It has gained worldwide acceptance for management of malignant liver diseases [6, 7]. LLRs has now been extended from minor wedge resections to complicated liver resections, including hemihepatectomies, extended right and left hepatectomies, and central sectionectomies [8]. Laparoscopic liver re-resection for recurrent HCC presents more technical challenges than primary liver resection because of presence of adhesions, change in anatomy, formation of collateral circulation, and impaired liver function due to surgical loss of liver parenchyma in patients with chronic liver diseases. Only a few studies have reported the feasibility and safety of laparoscopic liver re-resection (LR-R) for recurrent HCC [9,10,11,12]. A lot remains to be learned on the role of LR-R in the treatment of patients with posthepatectomy HCC recurrence.
In this study, we analyzed our experience of using LR-R to treat these patients and to study the feasibility and safety of LR-R.
Patients and methods
This study was conducted in the context of informed consents from the patients and under the approval of the Ethics Committee of the Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University. Surgery was performed with patient’s written consent. From January 2008 to December 2015, all patients who underwent laparoscopic liver re-resection for recurrent hepatocellular carcinoma after initial curative liver resection in the Department of Hepatobiliary Surgery, Sun Yat-sen Memorial Hospital, Guangzhou, China were included into this study. To minimize case selection bias, a propensity score matching study was conducted. Patients who underwent open liver re-resection for posthepatectomy HCC during the study period were considered to be selected into the control group. All patients were evaluated before surgery through blood tests, abdominal ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI). Functional liver reserve was assessed using indocyanine green retention rate at 15 min (ICG15) and CT volumentry. The selection criteria for patients to undergo liver re-resection included well-compensated liver function (Child-Pugh A), absence of gross ascites, no clinical signs of severe portal hypertension, no extrahepatic metastasis, no major blood vessel tumor invasion, and a solitary tumor of less than 5 cm. Lesions located in any liver segments were included.
Morbidity was graded according to the Clavien–Dindo classification [13]. The grade of adhesion was assessed according to the study of Becker et al.: Grade 0: no adhesion; Grade I: a thin layer of adhesion that can be separated by blunt dissection; Grade II: a thin layer of adhesion that can be separated easily by sharp dissection; Grade III: a wide range of vascular adhesion requiring careful sharp dissection; Grade IV: dense adhesion that may result in visceral injury [14].
Follow-up visits were carried out at one month after hospital discharge and then every three monthly in the first year, every six monthly in the following two years and once a year thereafter. Followed up evaluation included routine blood tests, liver function tests, coagulation function tests, serum alpha fetoprotein (AFP), chest X-ray, and abdominal ultrasound. For patients with concomitant HBV infection, anti-virus treatment was routinely given.
Surgical procedure
For open liver re-resection, a subcostal incision with an upward midline incision, through a previous surgical scar if present, was used. Intraoperative ultrasonography (Hitachi Aloka Medical, Tokyo, Japan) was routine. Hepatic parenchymal transection was performed using a harmonic scalpel (Ethicon Endo-surgery, Norderstedt, Germany) and Cavitron ultrasonic surgical aspirator (Integra LifeSciences, Sragh, Ireland). Hepatic inflow occlusion was only used when there was excessive bleeding. Hemostasis was performed using electrocautery and suturing. A drainage tube was placed close to the cut surface of the liver.
For laparoscopic liver re-resection, patients were placed either in a supine position or a left decubitus position (left decubitus position for tumors located in the right liver and supine position for other locations). The primary surgeon stood between the legs, and the other two assistants stood on each side of the patient. The first trocar was inserted using an open method. Pneumoperitoneum was established at a pressure of 12 mmHg. After introduction of a flexible laparoscope, the other four working ports were inserted under vision, depending on the location of the tumor. Adhesiolysis was performed using a harmonic scalpel (Ethicon Endo-surgery, Norderstedt, Germany) and monopolar coagulation. Intraoperative ultrasonography (Hitachi Aloka Medical, Tokyo, Japan) was used. Hepatic pedicles of the liver corresponding segment(s) to be resected were isolated and clamped with absorbable clips to occlude the blood flow. Liver parenchymal transection was then performed with a harmonic scalpel (Ethicon Endo-surgery, Norderstedt, Germany) and Cavitron ultrasonic surgical aspirator (Integra LifeSciences, Sragh, Ireland). Hemostasis was performed by monopolar coagulation, Biclamp (ERBE, Tubingen, Germany), clips and suturing. The resected specimen was placed in a plastic bag and removed through a small incision. A drainage tube was routinely used.
Intraoperative RFA was occasionally used in some patients in the two groups. A monopolar RF instrument (AngioDynamics, Amsterdam, Netherlands) was used to ablate the edges of the resected tumors to ensure adequate resection margins. The ablation was carried out using separate punctures and ablation cycles. Each ablation cycle lasted approximately 10–12 min.
Statistical analysis
A 1:1 propensity score matching between the laparoscopy group and the open surgery group was carried out to minimize selection biases in the baseline characteristics between the two groups of patients. A logistic regression model was used to estimate the propensity score for a patient who underwent open liver re-resection to match with a patient who underwent laparoscopic liver re-resection. The following factors were included in the model: age, sex of patient, size, number of tumor, hepatitis B virus status (HBV), and degree of cirrhosis. All continuous data were expressed as median (range) and differences between groups were analyzed using the Mann–Whitney U test. Categorical variables were expressed as absolute numbers (percentages) and compared between groups using the Chi squared test. Survival curves were calculated using the Kaplan–Meier method and compared between groups by the log-rank test. A p value of less than 0.05 was considered as statistically significant. The 1:1 propensity score matching was performed by the psmatch2 procedure in Stata 13.0. Other statistical analyses were performed using the SPSS Statistics version 21.0 (IBM SPSS).
Result
During the study period, 167 patients underwent liver re-resection for recurrent HCC after initial curative liver resection. 137 patients underwent open (used as the control group in the Propensity Score Matching Study), while 30 patients underwent laparoscopic liver re-resection (LR-R), the study group.
For the 30 patients who underwent LR-R, 26 underwent a second liver resection and 4 patients had a third liver resection after twice open surgeries. After propensity score matching, the extents of previous liver resections are summarized in Table 1. Demographic parameters, liver function, and tumors characteristics of patients are summarized in Table 2. There were no significant differences between the two groups in age, sex, Child–Pugh grade, type of hepatitis, histological liver cirrhosis, histological tumor microvascular invasion. The indocyanine green retention rates at 15 min before liver re-resection were 9% (4–34%) and 8.5% (2–33%), respectively. Also, no statistical significant differences were observed in laboratory test results. The median size of tumor in open surgery group was 2.45 cm (1.0–4.3 cm) and in laparoscopic group was 2.1 cm (1.0–5.0 cm). The median interval between the last previous liver resection was 17 months in both the 2 groups. In open surgery group, three recurrent HCC located in segment I; 15 in segment II, III, Iva, V, VI; eight in segment IVb, VII, VIII, and four located bilober, whereas in laparoscopic group, one recurrent HCC located in segment I; 18 in segment II, III, Iva, V, VI; four in segment IVb, VII, VIII and seven located bilober. Two patients in open surgery group had multiple tumors, while there were five patients in laparoscopic group. There were no significant differences between two groups in tumor size, location, and tumor number. The perioperative outcomes are shown in Table 3. The operative time was similar between the two groups. The median operative time was 200.5 min (range 68–525 min) in the laparoscopy group and 207.5 min (range 105–328 min) in the open surgery group. Three patients in the open surgery group required Pringle’s maneuver for excessive bleeding during liver parenchymal transection. This was unnecessary in the laparoscopy group. The estimated blood loss (median 100 ml ranging from 10 to 600 ml) and the blood transfusion requirement (0%) in the laparoscopy group were significantly lower than that in the open surgery group (400 ml ranging from 30 to 1800 ml and 43.3%, respectively). Four patients were converted to open liver re-resection for the following reasons: dense adhesions (n = 1) and failure to progress because of tumors location in posterior segments (n = 3). Two patients in open surgery group and seven patients in the laparoscopic group required intraoperative radiofrequency ablation in an attempt to ensure adequate surgical margins.
There were no significant differences between the two groups in the grades of adhesion and types of liver re-resection. Major liver resections included two right hemihepatectomies and one left hemihepatectomy in the open surgery group and one extended left hemihepatectomy in the laparoscopic group. No microscopic involved surgical margins were detected in the 2 groups.
One patient died in the open surgery group 73 days after the operation due to liver failure. Nine complications developed in the open surgery, including bile leakage (n = 3). Moderate ascites (n = 1), mild intra-abdominal hemorrhage (n = 1), and abdominal infections (n = 4). Except one patient who developed a hepatic abscess which required reoperations, and a prolonged hospital stay of 150 days, the remaining patients were treated successfully with conservative treatment ± percutaneous interventional procedures. Only two complications developed in the laparoscopic group: a patient with intra-abdominal hemorrhage who responded to conservative treatment and a patient with biliary leakage who required percutaneous drainage. The difference in the rates of complication between the two groups was significant. The median hospital stay after operation in the open surgery group was 4 days longer than the laparoscopy group (13.5 days ranging from 8 to 150 days vs 9.5 days ranging from 5 to 29 days) and this difference was significant.
The impact of the initial liver resection on liver re-resection, whether it was carried out laparoscopically or with open liver resection was further analyzed (Table 4). There were no significant differences in the estimated blood loss, operative time, conversion rate, hospital stay and complication, although initial laparoscopic liver resection gave better results than initial open liver resection. Furthermore, Grade 3 and 4 adhesions were only found in the initial open liver resection group and one of them required conversion from laparoscopic to open liver re-resection. Again, there was no significant difference in the grade of adhesion between the two groups.
At a median follow-up of 35 months (range from 2 to 80 months) in the two groups, the 1-year, 3-year, and 5-year disease-free survival rates were 79.0, 51.0, and 31.9%, respectively. The corresponding rates in the laparoscopy group were 78.3, 57.4, and 43.0%, respectively, (p = 0.474) (Fig. 1). The 1-year, 3-year, and 5-year overall survival rates for the open liver re-resection were 89.4, 75, and 67.5%, respectively. The corresponding rates for the laparoscopy group were 96.7, 85, and 74.4%, respectively, (p = 0.413) (Fig. 2).
Discussion
Abdominal adhesions develop in 67–93% of patients after surgery [15]. Dealing with densely or vascular-rich adhesions, particularly those around the hepatic hilum or major vessels, is a technical challenge for surgeons performing laparoscopic liver re-resection. Recent developments in minimal invasive surgery allow safe and effective adhesiolysis in laparoscopic re-do surgery [16,17,18]. In our study, similar results were obtained in laparoscopic when compared with open liver re-resection. These results are contradictory to conventional concepts and could be attributed to precise dissection under optical magnification offered by laparoscopy. Moreover, pneumoperitoneum tenses up adhesion bands to facilitate the operation and to reduce surgical time. The first trocar was introduced away from any previous surgical scars by an open method (Hasson) and other trocars were inserted individually under direct vision to avoid injury. However, there was one patient with conversion to open surgery due to dense adhesions. This patient had a history of severe intra-abdominal infection after the previous hepatic resection. There was a trend towards milder adhesion formation after previous laparoscopic surgery which supports the theory that laparoscopic surgery results in decreased postoperative adhesion formation and facilitates subsequent treatment [19, 20]. However, the lack of a statistical significance can be a result of a type 2 statistical error in our study as a consequence of a small sample size.
The change in gross anatomy of the liver can be a consequence of hepatic atrophy-hypertrophy complex secondary to previous liver resection and to underlying liver diseases. Increased intraoperative blood loss and perioperative blood transfusions have been reported to be major factors influencing tumor recurrence and postoperative lethal complications such as liver failure [21,22,23,24,25]. The fear of uncontrollable bleeding followed by adverse outcomes has impeded the development of laparoscopic liver resection. Improvements in blood loss and transfusion using the laparoscopic approach have been highlighted by experts at the 2nd International Consensus Conference on Laparoscopic Liver Resection [21]. The reasons were summarized in a recent report giving credits to the positive pressure of CO2 pneumoperitoneum, state of the art transection devices, facilitation of liver inflow and outflow control, and proficient laparoscopic skills [21, 26]. Our findings showed that these benefits can be extended to laparoscopic liver re-resections. The routine use of intraoperative ultrasound (IOUS) and advanced transection devices as well as our experience in laparoscopic surgery are the other reasons for the good results in our study.
The pursuit for liver parenchyma preservation without compromising any oncological principle is another challenge in liver re-resection. In laparoscopy surgery, tumor resection margin is more difficult to define due to the loss of tactile sensation, especially for those located in posterosuperior liver segments where poor visualization, angled transection line, and difficult manipulation limited by costal margin and kinetics diaphragm may result in insufficient tumor clearance. Involved surgical margins have been documented to have a major influence on survival [27, 28]. In our study, a monopolar radiofrequency device was used to encircle the tumor before parenchymal transection in some patients with unclearly defined tumor borders. This technique ensures an ablated margin left behind and improves oncological radicality. In addition, such a precoagulation process can be performed percutaneously without any violation of the minimally invasiveness and benefits to the subsequent laparoscopic liver resection [27,28,29]. This approach is not routinely used by us for fear of complications such as vessel thrombosis, biliary injury and bleeding on withdrawal of the needle [30].
Salvage liver transplantation (SLT) has gained popularity in recent years for recurrent HCC because of its total removal of tumors and cure for underlying cirrhosis. In the literature, SLT offered a 5-year disease-free rate of 67%, 5-year overall survival rate of 62%, with morbidity rate of 34% and morality rate of 6.34%, which confirmed its safety, feasibility, and efficacy [31, 32]. In addition, comparable survival outcomes were observed between SLT and repeat liver resection in a recent study [5]. The improvement in SLT therefore raise a question of whether repeat liver resection should be a preferred therapy for patients with preserved liver function and resectable recurrent HCC within Milan criteria. In our study, short- and long-term outcomes of both open and laparoscopic liver resection appear not to be inferior to that of SLT [31,32,33,34]. However, these results could not differentiate whether SLT or RR offers maximum benefits for patients with recurrent HCC. Selection bias might have existed in this study because most of patients had a recurrence-free interval more than 1 year, less microvascular invasion, small tumor size, and relatively well-preserved liver function. Currently, no standardized treatment criteria for patients with resectable and transplantable recurrent HCC are available. Considering the similar long-term survival with minimal invasion, safety, and readily accessibility, LR-R should be considered as a choice of treatment. Further studies are warranted to confirm a decisional algorithm for patients with resectable and transplantable recurrent HCC.
Our long-term results were also superior to that reported by Lu and his colleagues who treated recurrent HCC by percutaneous thermal ablation [35]. Percutaneous thermal ablation, indeed, has been recommended to be an alternative to repeat resection with its acceptable effectiveness and minimal invasiveness [36]. However, incomplete necrosis and a high incidence of needle tract dissemination have rendered by some authors to be not a curative procedure [37, 38]. In our opinion, ablation is more suitable for patients with recurrent HCC who cannot tolerate surgical treatment.
In our center, we started to perform laparoscopic liver surgery in 2006, and we only began to perform LR-R after we had accumulated enough experience in laparoscopic liver resection [39]. LR-R is technically more demanding and requires the expertise in both laparoscopic and hepatic surgery. Our results are comparable to many studies on laparoscopic primary or repeat liver resection [10, 21]. The incidence of complication was significant lower in the laparoscopic than the open surgery group.
Although laparoscopic liver resection for all individual liver segments has been demonstrated to be feasible [40,41,42], three of four cases of conversion to open surgery in our study were related to poor tumor location. For laparoscopic liver re-resection, careful patient selection is important to its success.
In summary, this propensity score matching study showed that LR-R for patients with posthepatectomy HCC recurrence resulted in reduced blood loss, reduced requirement of blood transfusion, lower morbidity rate, shorter hospital stay, and satisfactory comparable oncological outcomes when compared with open liver re-resection. Previous open liver resection produced results which were not inferior to previous laparoscopic liver resection for subsequent laparoscopic re-resection. In centers with good experience in laparoscopic and liver surgery, it can be a safe alternative to open liver re-resection.
References
Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Yamamoto J, Shimada K, Kosuge T, Okada S, Takayasu K, Yamasaki S (1998) Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology 28:1241–1246
Poon RT, Fan ST, Lo CM, Liu CL, Ng IO, Wong J (2000) Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol 18:1094–1101
Sugimachi K, Maehara S, Tanaka S, Shimada M, Sugimachi K (2001) Repeat hepatectomy is the most useful treatment for recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 8:410–416
Itamoto T, Nakahara H, Amano H, Kohashi T, Ohdan H, Tashiro H, Asahara T (2007) Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery 141:589–597
Chan AC, Chan SC, Chok KS, Cheung TT, Chiu DW, Poon RT, Fan ST, Lo CM (2013) Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl 19:411–419
Minagawa M, Makuuchi M, Takayama T, Kokudo N (2003) Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg 238:703–710
Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA (2011) Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 146:348–356
Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D (2009) The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg 250:772–782
Chan AC, Poon RT, Chok KS, Cheung TT, Chan SC, Lo CM (2014) Feasibility of laparoscopic re-resection for patients with recurrent hepatocellular carcinoma. World J Surg 38:1141–1146
Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamamoto S, Yamazoe S, Ohira G, Nakajima T (2013) Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 20:512–517
Belli G, Cioffi L, Fantini C, D’Agostino A, Russo G, Limongelli P, Belli A (2009) Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: feasibility, safety, and results. Surg Endosc 23:1807–1811
Hu M, Zhao G, Xu D, Liu R (2011) Laparoscopic repeat resection of recurrent hepatocellular carcinoma. World J Surg 35:648–655
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Becker JM, Dayton MT, Fazio VW, Beck DE, Stryker SJ, Wexner SD, Wolff BG, Roberts PL, Smith LE, Sweeney SA, Moore M (1996) Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg 183:297–306
Szomstein S, Lo Menzo E, Simpfendorfer C, Zundel N, Rosenthal RJ (2006) Laparoscopic lysis of adhesions. World J Surg 30:535–540
Wu JM, Lin HF, Chen KH, Tseng LM, Tsai MS, Huang SH (2007) Impact of previous abdominal surgery on laparoscopic appendectomy for acute appendicitis. Surg Endosc 21:570–573
Yoon YS, Han HS, Choi YS, Jang JY, Suh KS, Kim SW, Lee KU, Park YH (2006) Total laparoscopic right posterior sectionectomy for hepatocellular carcinoma. J Laparoendosc Adv Surg Technol A 16:274–277
Nunobe S, Hiki N, Fukunaga T, Tokunaga M, Ohyama S, Seto Y, Yamaguchi T (2008) Previous laparotomy is not a contraindication to laparoscopy-assisted gastrectomy for early gastric cancer. World J Surg 32:1466–1472
Polymeneas G, Theodosopoulos T, Stamatiadis A, Kourias E (2001) A comparative study of postoperative adhesion formation after laparoscopic vs open cholecystectomy. Surg Endosc 15:41–43
Dowson HM, Bong JJ, Lovell DP, Worthington TR, Karanjia ND, Rockall TA (2008) Reduced adhesion formation following laparoscopic versus open colorectal surgery. Br J Surg 95:909–914
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, O’Rourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schon MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261:619–629
Takenaka K, Kanematsu T, Fukuzawa K, Sugimachi K (1990) Can hepatic failure after surgery for hepatocellular carcinoma in cirrhotic patients be prevented? World J Surg 14:123–127
Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH (2002) Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg 236:397–406
Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, Dematteo RP (2009) Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 249:617–623
Hanazaki K, Kajikawa S, Shimozawa N, Matsushita A, Machida T, Shimada K, Yazawa K, Koide N, Adachi W, Amano J (2005) Perioperative blood transfusion and survival following curative hepatic resection for hepatocellular carcinoma. Hepatogastroenterology 52:524–529
Tranchart H, O’Rourke N, Van Dam R, Gaillard M, Lainas P, Sugioka A, Wakabayashi G, Dagher I (2015) Bleeding control during laparoscopic liver resection: a review of literature. J Hepatobiliary Pancreat Sci 22:371–378
Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, Fan ST, Lo CM (2013) Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 257:506–511
Torzilli G, Montorsi M, Donadon M, Palmisano A, Del Fabbro D, Gambetti A, Olivari N, Makuuchi M (2005) “Radical but conservative” is the main goal for ultrasonography-guided liver resection: prospective validation of this approach. J Am Coll Surg 201:517–528
Akyildiz HY, Morris-Stiff G, Aucejo F, Fung J, Berber E (2011) Techniques of radiofrequency-assisted precoagulation in laparoscopic liver resection. Surg Endosc 25:1143–1147
Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS (1999) Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology 210:655–661
Zhu Y, Dong J, Wang WL, Li MX, Lu Y (2013) Short- and long-term outcomes after salvage liver transplantation versus primary liver transplantation for hepatocellular carcinoma: a meta-analysis. Transplant Proc 45:3329–3342
Chan DL, Alzahrani NA, Morris DL, Chua TC (2014) Systematic review of efficacy and outcomes of salvage liver transplantation after primary hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol 29:31–41
Belghiti J, Cortes A, Abdalla EK, Regimbeau JM, Prakash K, Durand F, Sommacale D, Dondero F, Lesurtel M, Sauvanet A, Farges O, Kianmanesh R (2003) Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg 238:885–892
Guerrini GP, Gerunda GE, Montalti R, Ballarin R, Cautero N, De Ruvo N, Spaggiari M, Di Benedetto F (2014) Results of salvage liver transplantation. Liver Int 34:e96–e104
Lu MD, Yin XY, Xie XY, Xu HX, Xu ZF, Liu GJ, Kuang M, Zheng YL (2005) Percutaneous thermal ablation for recurrent hepatocellular carcinoma after hepatectomy. Br J Surg 92:1393–1398
Rodriguez-Sanjuan JC, Gonzalez F, Juanco C, Herrera LA, Lopez-Bautista M, Gonzalez-Noriega M, Garcia-Somacarrera E, Figols J, Gomez-Fleitas M, Silvan M (2008) Radiological and pathological assessment of hepatocellular carcinoma response to radiofrequency. A study on removed liver after transplantation. World J Surg 32:1489–1494
Wu CC, Cheng SB, Yeh DC, Wang J, P’Eng FK (2009) Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg 96:1049–1057
Llovet JM, Vilana R, Bru C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pages M, Ayuso C, Sole M, Rodes J, Bruix J, Barcelona Clinic Liver Cancer G (2001) Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology 33:1124–1129
Zhang L, Chen YJ, Shang CZ, Zhang HW, Huang ZJ (2009) Total laparoscopic liver resection in 78 patients. World J Gastroenterol 15:5727–5731
Cho JY, Han HS, Yoon YS, Shin SH (2008) Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery 144:32–38
Ishizawa T, Gumbs AA, Kokudo N, Gayet B (2012) Laparoscopic segmentectomy of the liver: from segment I to VIII. Ann Surg 256:959–964
Yoon YS, Han HS, Cho JY, Ahn KS (2010) Total laparoscopic liver resection for hepatocellular carcinoma located in all segments of the liver. Surg Endosc 24:1630–1637
Acknowledgements
We are grateful to Wan Yee Lau, The Chinese University of Hong Kong, for writing assistance and review of our manuscript.
Funding
No funding supported for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Kairui Liu; Dr. Yajin Chen; Dr. Xiaolin Wu; Dr. Zejian Huang; Dr. Zeyu Lin; Dr. Junliang Jiang; Dr. Wenliang Tan; Dr. Lei Zhang have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Liu, K., Chen, Y., Wu, X. et al. Laparoscopic liver re-resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: a propensity score matching study. Surg Endosc 31, 4790–4798 (2017). https://doi.org/10.1007/s00464-017-5556-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5556-3