Abstract
Background
Laparoscopic liver resection (LLR) has now been established as a safe and minimally invasive technique that is deemed feasible for treating hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC). However, the role of LLR in treating combined hepatocellular-cholangiocarcinoma (cHCC-CC) patients has been rarely reported. This study aimed to assess the efficacy of LLR when compared with open liver resection (OLR) procedure for patients with cHCC-CC.
Methods
A total of 229 cHCC-CC patients who underwent hepatic resection (34 LLR and 195 OLR patients) from January 2014 to December 2018 in Zhongshan Hospital, Fudan University were enrolled and underwent a 1:2 propensity score matching (PSM) analysis between the LLR and OLR groups to compare perioperative and oncologic outcomes. Overall survival (OS) and recurrence-free survival (RFS) parameters were assessed by the log-rank test and the sensitivity analysis.
Results
A total of 34 LLR and 68 OLR patients were included after PSM analysis. The LLR group displayed a shorter postoperative hospital stay (6.61 vs. 8.26 days; p value < 0.001) when compared with the OLR group. No significant differences were observed in the postoperative complications’ incidence or a negative surgical margin rate between the two groups (p value = 0.409 and p value = 1.000, respectively). The aspartate aminotransferase (AST), alanine aminotransferase (ALT), and inflammatory indicators in the LLR group were significantly lower than those in the OLR group on the first and third postoperative days. Additionally, OS and RFS were comparable in both the LLR and OLR groups (p value = 0.700 and p value = 0.780, respectively), and similar results were obtained by conducting a sensitivity analysis.
Conclusion
LLR can impart less liver function damage, better inflammatory response attenuation contributing to a faster recovery, and parallel oncologic outcomes when compared with OLR. Therefore, LLR can be recommended as a safe and effective therapeutic modality for treating selected cHCC-CC patients, especially for those with small tumors in favorable location.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Combined hepatocellular-cholangiocarcinoma (cHCC-CC) is a rare and aggressive primary hepatic malignancy originating from the cells having histological characteristics of both hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) [1]. Being a rare entity, the variable incidence rate of cHCC-CC ranges from 2–5% in primary liver cancers [2].
The clinical features of cHCC-CC remain controversial as this disease shows intermediate clinical features between HCC and ICC. Several studies have shown that some features of cHCC-CC resemble the aggressive behavior of HCC, whereas other features mimic ICC characteristics [3, 4]. Previous literature demonstrated that genetic features of cHCC-CC were more similar to ICC than HCC [5], which was in contrast with another recent study that identified that cHCC-CC harbored frequent genetic aberrations similar to HCC [6]. Another multicenter study that used genomic and transcriptomic sequencing revealed that in cHCC-CC, areas with a clear boundary between the HCC and ICC components demonstrated robust ICC-like pathological features, whereas those parts without clear boundaries between the two components exhibited HCC-like features [7].
At present, no specific guidelines have been established for effective management of this disease, but surgical intervention remains one of the most effective curative approaches that yields improved desired outcomes in patients with cHCC-CC [2, 8]. LLR has been proven as a safe and effective surgical option in treating aggressive liver tumors, such as HCC, ICC, or other metastatic liver tumors, etc. [9,10,11,12,13]. However, to the best of our knowledge, there is no evidence comparing LLR and OLR as effective therapeutic interventions for cHCC-CC patients. Our study aimed to evaluate the efficacy of LLR for cHCC-CC patients and compare resultant perioperative and oncologic outcomes between the LLR and OLR groups.
Patients and methods
Study population and data collection
This retrospective study was approved by the institutional review board of Zhongshan Hospital, Fudan University. Informed consent was obtained from each patient. Patient data were collected retrospectively from the electronic medical records of Zhongshan Hospital, Fudan University. From January 2014 to December 2018, 365 patients who underwent surgical treatment for cHCC-CC in our institute were identified by pathological diagnosis. Among these patients, 26 patients were excluded due to receiving liver transplantation, and 78 patients were prohibited due to preoperative anti-tumoral therapies or a repeat hepatectomy procedure for recurrence, to avoid their impact on survival and keep balance in baseline characteristics. Moreover, as LLR was not initially recommended for patients with portal vein or inferior vena cava tumor thrombosis, 32 individuals were excluded from this account. Henceforth, a total of 229 patients (34 LLR and 195 OLR) were included in this study (Fig. 1).
Baseline patient characteristics
Patients’ baseline characteristics like age, sex, body mass index (BMI), as well as comorbid disease history (hypertension, diabetes mellitus, and heart disease), were recorded. The hepatitis B/C virus (HBV/HCV) and preoperative antiviral treatment status were also identified. Preoperative laboratory examinations were obtained for each patient, which included complete blood count examination, liver function test, coagulation function test, serum tumor biomarker test, etc.
Perioperative parameters
Individual features about tumor size, number, and capsulation were obtained. Additional information like the type of resection, operation time, estimated intraoperative blood loss, blood transfusion, Pringle maneuver application, and intermittent clamping time was recorded. Anatomic liver resection is defined as systematic resection of a hepatic segment confined by tumor-bearing portal tributaries [14]. Moreover, major liver resection is defined as a resection of three or more liver segments.
Complete blood count examination and liver function test data was obtained along with the calculation of four serum component indexes that included systemic immune-inflammation (SII), platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), on the first and third postoperative day, respectively [15].
Furthermore, the percentage of positive surgical margin, as well as the resection margin value, was also calculated. The resection margin was defined as the minimum distance between the tumor and the cutting edge in the formalin-fixed tissues. Liver cirrhosis stages were recorded and confirmed by histopathological examination of paracarcinoma tissues. Moreover, tumor characteristics, including microvascular invasion status, tumor TNM staging, and Edmondson–Steiner (ES) grade, were also registered. T stage was assigned according to the 8th edition staging system of the American Joint Committee on Cancer (AJCC) for cHCC-CC cases. Additionally, metastasis status of lymph nodes was also confirmed through histopathological diagnosis in patients who underwent lymph node dissection.
According to the Clavien-Dindo classification [16], postoperative complications were evaluated and classified. The length of postoperative hospital stay was reviewed for each patient.
Follow-up
After hepatic resection, all patients were followed up at one month and then after every two or three months. Follow-up assessments included complete blood count examination, liver function test, tumor biomarker assessment, chest X-ray, abdominal ultrasonography, either computed tomography (CT), or magnetic resonance imaging (MRI) [17]. Positron emission tomography-computed tomography (PET-CT) was usually performed in patients with suspicious metastasis. Recurrence is defined as a typical imaging appearance (CT, MRI, and/or PET-CT scan). In addition, OS is defined as the period from the date of hepatectomy to death or the latest follow-up, and RFS was defined as the duration from the date of hepatectomy to recurrence or the latest follow-up. Patients who received adjuvant treatment after surgery, including preventive chemotherapies or transcatheter arterial chemoembolization, were also recorded during the follow-up period.
Statistical analysis
Continuous data were expressed as the mean [± standard deviation (SD)] or median [interquartile range (IQR)]. Normally distributed continuous variables were compared by Student’s t test, and non-normally distributed continuous data were analyzed using the Mann–Whitney U test. Categorical variables were analyzed using the Chi-square (χ2) test or Fisher’s exact test as appropriate to the situation. OS and RFS were estimated by the Kaplan–Meier method and consequently compared by the log-rank test in the PSM population. Univariate analysis was performed to identify OS- and RFS-related risk factors in all enrolled patients. Additionally, log10-transformed alpha-fetoprotein (AFP) and log10-transformed carbohydrate antigen 199 (CA19–9) were calculated to reduce the expected skewness.
PSM analysis was used to control confounding bias [18]. Various covariates like age, sex, BMI, HBV status, preoperative antiviral treatment, preoperative liver functions, tumor markers (log10AFP, carcinoembryonic antigen (CEA), and log10CA19–9), resection type, cirrhosis stage, tumor characteristics (tumor size, number, capsulation, microvascular invasion status, tumor T stage, ES grade, and lymph node metastatic status) along with any received adjuvant treatment were used for achieving the propensity score. A 1:2 nearest neighbor match paradigm was used for ensuring a close match. The covariate balance was assessed using the standardized difference between the two groups after PSM analysis [19]. Furthermore, prognostic factors for OS and RFS were compared by the Cox proportional hazards regression model in the matched population. The multivariate Cox hazards regression model and inverse probability of treatment weighting (IPTW) of the propensity score model were used as the sensitivity analysis in the unmatched cohort. The hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated for LLR vs. OLR in cHCC-CC patients.
The PSM analysis was performed by the R package of MatchIt [20], and IPTW was used by the R package of ipw [21], followed by the calculation of all statistical computations by using R (version 3.5.2). A two-tailed p value < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 229 patients who underwent hepatic resection for cHCC-CC were enrolled, out of which, 34 and 195 patients underwent LLR and OLR, respectively. The flowchart for study enrollment is shown in Fig. 1. The demographic and clinical characteristics of both groups before the PSM analysis are presented in Supplementary Table S1. Some baseline differences between the two groups were identified that included the serum level of gamma-glutamyl transpeptidase (GGT) (p value = 0.034) and tumor size (p value < 0.001). No differences were found in other parameters between the two groups.
After a 1:2 PSM analysis, 34 and 68 patients who underwent LLR and OLR were enrolled and compared, respectively. The standardized differences for all matched covariates were less than 0.15. Differences in the patient characteristics between the two groups were alleviated and comparable after PSM analysis (Table 1).
Perioperative outcomes after PSM
After PSM analysis, the mean operative time was 260.59 and 197.36 min in the LLR and OLR group (p value = 0.002, Table 2), respectively. The Pringle maneuver was used for 12 patients (35.3%) in the LLR group vs. 46 patients (67.6%) in the OLR group (p value < 0.003). The mean clamping time was 24.78 min in the LLR group vs. 18.31 min in the OLR group (p value = 0.020; Table 2). The blood loss was 124.38 mL and 154.12 mL in the LLR and the OLR groups (p value = 0.251), respectively. No significant difference was observed between the two groups in the blood transfusion rate (p value = 1.000). In the LLR group, 2 of 34 laparoscopic procedures (5.9%) were converted because of adhesions caused by previous upper abdomen operations.
No significant difference was observed in the incidence of postoperative complications between the matched groups (p value = 0.409). However, the mean length of postoperative hospital stay was significantly shorter in the LLR group than in the OLR group (6.61 days vs. 8.26 days, p value < 0.001, Table 2).
There were no differences in the rate of negative surgical margin (p value = 1.000), and the resection margin’s average values between the two matched groups were comparable (LLR vs. OLR, 0.88 vs. 0.86 cm, p value = 0.931, Table 3).
Postoperative trends of serum indicators in the matched patients
No differences were observed in the baseline serum markers between the LLR and OLR groups (Table 4). Several serum markers, including the white blood cell (WBC) count, neutrophil and lymphocyte percentage, neutrophil as well as monocyte count, ALT, AST, NLR, and MLR, displayed slight differences in favor of the LLR groups on the first and third postoperative days (Table 4). These results further supported our findings that LLR could provide a better inflammatory response attenuation and liver function damage when compared to OLR.
In addition, we compared the impact of Pringle maneuver on postoperative serum markers. Our results showed that ALT and AST displayed slight differences in favor of the no clamping group after surgery (Supplementary Table S2). However, these factors have a negligible effect on short-term prognosis. We further explore the impact of intermittent clamping duration (≤ 15 or > 15 min) on postoperative serum markers. No significant differences except SII and PLR were observed between the groups with clamping ≤ 15 and > 15 min after surgery (Supplementary Table S3). These findings suggested that the usage or duration of Pringle maneuver has negligible effect on postoperative serum markers.
Follow-up and survival
The PSM population revealed a median follow-up period of 34.38 months for all 102 patients, depicting 32.98 and 35.25 months for the LLR and OLR group, respectively. The recurrence after resection was observed in 15 patients (15/34, 44.1%) in the LLR and 35 patients (35/68, 51.5%) in the OLR group. The initial recurrent sites in the LLR group were found in the liver (13/15, 86.7%), lymph node (1/15, 6.7%), and adrenal gland (1/15, 6.7%). In contrast, the OLR group observed the initial recurrence sites as liver (31/35, 88.6%), lung (2/35, 5.7%), lymph node (1/35, 2.9%), and lumbar vertebra (1/35, 2.9%) (Table 3).
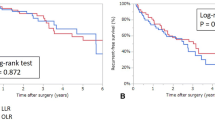
There was no significant difference between the two groups in OS after PSM analysis (p value = 0.700, Fig. 2A). The one-, two-, and three-year OS rates were 0.985, 0.926, and 0.867 in the OLR along with 1.000, 0.939, and 0.899 in the LLR group, respectively. Additionally, no significant difference was found between the two groups after PSM analysis was conducted in the Cox proportional hazards regression model (HR 1.299 95% CI 0.149–1.945, p value = 0.345, Table 5). Similar results were also obtained by sensitivity analysis in the unmatched population (multivariate analysis: HR 0.538 95% CI 0.149–1.945, p value = 0.345; IPTW: HR 0.892 95% CI 0.285–2.794, p value = 0.845) (Table 5).
Oncologic outcomes of patients with cHCC-CC after propensity score matching. A Overall survival of patients with cHCC-CC who underwent LLR (n = 34) or OLR (n = 68) after propensity score matching. B Recurrence-free survival of patients with cHCC-CC who underwent LLR (n = 34) or OLR (n = 68) after propensity score matching. LLR laparoscopic liver resection, OLR open liver resection
No significant difference was observed between the LLR and the OLR group in RFS after PSM (p value = 0.780) (Fig. 2B). The one-, two-, and three-year RFS rates were 0.676, 0.617, and 0.544 in the OLR, and 0.879, 0.657, and 0.554 in the LLR group, respectively. Moreover, the Cox proportional hazards regression model utilization after PSM analysis did not show any statistical significance (HR 1.090 95% CI 0.589–2.015, p value = 0.785, Table 5). Consistent results were observed by the sensitivity analysis in the unmatched population (multivariate analysis: HR 0.765 95% CI 0.412–1.420, p value = 0.379; IPTW: HR 0.636 95% CI 0.326–1.239, p value = 0.183; Table 5).
Identification of OS- and RFS-related risk factors
Univariate analysis was performed to identify OS- and RFS-related risk factors. Consequently, the presence of HBV (p value = 0.024), evaluated level of CA199 (p value = 0.015), major resection (p value = 0.032), large tumor size (p value < 0.001), multiple tumor numbers (p value = 0.013), absence of capsule (p value = 0.046), high T stage (p value = 0.002), or presence of MVI (p value < 0.001) were associated with high risk of death (Supplementary Table S4), while large tumor size (p value < 0.001), multiple tumor numbers (p value < 0.001), high T stage (p value < 0.001), presence of MVI (p value < 0.001), or absence of postoperative adjuvant therapy (p value = 0.007) were related to high risk of recurrence (Supplementary Table S4).
Discussion
cHCC-CC is a rare type of liver cancer originating from the cells having histological characteristics of both HCC and ICC. On the one hand, cHCC-CC has the characteristics of HCC, which usually occurs in conjunction with chronic hepatitis and liver cirrhosis [2, 22], but on the other hand, cHCC-CC also behaves like ICC, prevalent in some conditions such as lymph node metastasis. Radiologically, cHCC-CCs can mimic both HCC and CC imaging features due to the coexistence of both the components within the same tumor [2], making preoperative diagnosis more difficult. Recently, several studies have provided a few novel approaches for preoperative cHCC-CC diagnosis through clinical parameters or radiomic models, achieving the AUC of more than 0.70 [23, 24].
As the application of LLR for liver tumors is increasing rapidly, accumulating studies have reported the efficacy of LLR in HCC, ICC, metastatic liver tumors, etc. [9, 13]. However, the efficacy of LLR in cHCC-CC patients has been rarely reported, considering the rare nature of this entity. In this study, the investigation regarding the efficacy of LLR in cHCC-CC patients revealed that the LLR provides faster recovery and similar oncological outcomes than OLR. To the best of our knowledge, this is the first study that has compared the perioperative and oncological outcomes between the LLR and OLR groups for cHCC-CC patients.
Previous studies reported that LLR provided shorter hospital stays and a comparable mortality rate when compared to OLR in the HCC patients [10, 12]. These findings were also confirmed by our results that observed a shorter hospital stay and parallel mortality rate in the cHCC-CC patients who underwent LLR. In addition to that, our findings revealed that postoperative serum inflammatory markers (WBC count, neutrophil count, monocyte count, and PLR) were significantly lower in the LLR group than OLR group, suggesting that LLR provided a better inflammatory response attenuation when compared with the open surgical procedure. Furthermore, our study results exhibited a faster postoperative liver function recovery in the LLR group when compared to the OLR group that contributed to the fact that less surgical trauma contributed to a decreased inflammatory reaction, minor liver function damage, and a faster recovery. In addition, univariate analysis demonstrated death- and recurrence-related clinicopathological risk parameters, and closer follow-up plans and appropriate anti-tumoral treatments can be conducted for the high-risk patients.
It is an important consideration that complete tumor clearance with a negative surgical margin provides a greater prognostic benefit for patients undergoing liver cancer surgeries. Some surgeons worried that laparoscopic resection could increase the chances of positive margins due to the presence of narrow resection margins, thereby resulting in early tumor recurrence [25]. However, with the application of preoperative 3D reconstruction and intraoperative ultrasound (IOUS) that provides valuable diagnostic inputs, complete tumor clearance with a tumor-free margin can be achieved by laparoscopy. Additionally, the usage of intraoperative indocyanine green fluorescence imaging (ICG) could identify tumor localization or segmental boundaries; thus, contributing to precise liver resection [26]. In the current study, the rates of negative surgical margin and the values of minimum resection margin were comparable between the two groups, which was consistent with the previous studies [10, 27]. Notably, the OS and RFS rates were comparable between the two groups after the PSM analysis, and similar results were also identified by the sensitivity analysis. Therefore, our study data not only confirmed the previous known advantages and the comparable long-term outcomes of LLR but also provided further evidence in terms of the efficacy of LLR in treating cHCC-CC patients.
Because of its low incidence, the recurrence pattern of cHCC-CC is still controversial. Yamashita et al. reported that half of patients had an early recurrence, which included a high rate of extrahepatic recurrence (58%) [28]. Recent literary insights demonstrated that the liver (57.9%) was the most common site of recurrence, while the lymph node (15.8%) was the second-most common recurrence site in cHCC-CC [29]. In the present study, 49.0% (50/102) patients suffered from tumor recurrence; the proportion of intrahepatic recurrence (88.0%, 44/50) was higher than the extrahepatic recurrence (12.0%, 6/50), thereby suggesting that postoperative adjuvant therapies should remain focused on combating the occurrence of intrahepatic recurrences as well as metastases. Repeat liver resection, TACE, radiofrequency ablation (RFA), chemotherapy, immunotherapy, etc., were performed among the 50 recurrent or metastatic patients. Active comprehensive therapies explain this phenomenon that OS is good but RFS is unsatisfied in this study.
Although lymph node metastasis was considered as an independent prognosis predictor of cHCC-CC patients [22, 30], the prognostic benefit of lymph node dissection (LND) is still controversial [2]. Owing to this fact LND was not considered as a conventional therapeutic option in resectable patients with HCC [31], and regional LND was recommended as a standard surgical therapeutic intervention in ICC patients [32]. However, it was observed that routine LND procedure does not seem to impact the OS of patients with ICC [33, 34]. The extended LND is not generally recommended because it does not provide a survival benefit in ICC patients, instead causes more postoperative complications, such as lymphatic leakage [35, 36]. In our institute, LND was carried out in patients with enlarged regional lymph nodes duly assessed by the preoperative imaging examinations (CT, MRI, and/or PET-CT, etc.). Due to the presence of overlapping radiological features of HCC and CC, it is very difficult to make a preoperative radiological diagnosis; hence in clinical practice, the potential therapeutic options for cHCC-CC patients are usually based on preoperative imaging findings or tumor markers, in accordance with the HCC or ICC treatment options. In our study, none of the patients (0/34) received LND in the LLR group, while two patients (2/68) received LND in the matched OLR group. Due to the lower incidence of LND, it is extremely difficult to compare the role of LND between the two groups. The role of LND and its oncologic efficacy in cHCC-CC patients should be investigated in further studies.
There are several limitations to this study. Firstly, the present study was a retrospective and non-randomized study involving a single center, although PSM analysis was utilized to eliminate most of the confounding bias, residual confounding bias (such as selection bias) still might have existed. The mean tumor size in the OLR group was larger than that in the LLR group before PSM analysis, and it was corrected in the matched population. Therefore, the paralleled outcomes might be limited to small tumors currently. More than half of patients undergoing anatomic liver resection were performed left lateral segment resection, indicating the LLR is suitable for those with small tumors in favorable locations. Further multicenter randomized studies with larger sample sizes and longer follow-up periods, as well as prospective clinical trials, should be conducted to identify precise oncologic outcomes between the LLR and OLR groups for all cHCC-CC patients.
In conclusion, as compared to OLR, LLR showed early recovery and comparable oncologic outcomes for the patients with cHCC-CC. Therefore, LLR can be considered an effective alternative for selected cHCC-CC patients, especially for those with small tumors in favorable locations.
References
Seehawer M, D’Artista L, Zender L (2019) The Worst from Both Worlds: cHCC-ICC. Cancer Cell 35:823–824
Leoni S, Sansone V, Lorenzo S, Ielasi L, Tovoli F, Renzulli M, Golfieri R, Spinelli D, Piscaglia F (2020) Treatment of Combined Hepatocellular and Cholangiocarcinoma. Cancers (Basel) 12:794
Lee CH, Hsieh SY, Chang CJ, Lin YJ (2013) Comparison of clinical characteristics of combined hepatocellular-cholangiocarcinoma and other primary liver cancers. J Gastroenterol Hepatol 28:122–127
Vilchez V, Shah MB, Daily MF, Pena L, Tzeng CW, Davenport D, Hosein PJ, Gedaly R, Maynard E (2016) Long-term outcome of patients undergoing liver transplantation for mixed hepatocellular carcinoma and cholangiocarcinoma: an analysis of the UNOS database. HPB (Oxford) 18:29–34
Cazals-Hatem D, Rebouissou S, Bioulac-Sage P, Bluteau O, Blanche H, Franco D, Monges G, Belghiti J, Sa Cunha A, Laurent-Puig P, Degott C, Zucman-Rossi J (2004) Clinical and molecular analysis of combined hepatocellular-cholangiocarcinomas. J Hepatol 41:292–298
Joseph NM, Tsokos CG, Umetsu SE, Shain AH, Kelley RK, Onodera C, Bowman S, Talevich E, Ferrell LD, Kakar S, Krings G (2019) Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J Pathol 248:164–178
Xue R, Chen L, Zhang C, Fujita M, Li R, Yan SM, Ong CK, Liao X, Gao Q, Sasagawa S, Li Y, Wang J, Guo H, Huang QT, Zhong Q, Tan J, Qi L, Gong W, Hong Z, Li M, Zhao J, Peng T, Lu Y, Lim KHT, Boot A, Ono A, Chayama K, Zhang Z, Rozen SG, Teh BT, Wang XW, Nakagawa H, Zeng MS, Bai F, Zhang N (2019) Genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell 35:932-947.e938
Azizi AA, Hadjinicolaou AV, Goncalves C, Duckworth A, Basu B (2020) Update on the genetics of and systemic therapy options for combined hepatocellular cholangiocarcinoma. Front Oncol 10:570958
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, O’Rourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schon MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261:619–629
Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y (2015) Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol 63:643–650
Lee W, Park JH, Kim JY, Kwag SJ, Park T, Jeong SH, Ju YT, Jung EJ, Lee YJ, Hong SC, Choi SK, Jeong CY (2016) Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc 30:4835–4840
Wu X, Huang Z, Lau WY, Li W, Lin P, Zhang L, Chen Y (2019) Perioperative and long-term outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with well-preserved liver function and cirrhotic background: a propensity score matching study. Surg Endosc 33:206–215
Gunasekaran G, Bekki Y, Lourdusamy V, Schwartz M (2021) Surgical treatments of hepatobiliary cancers. Hepatology 73:128–136
Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M (2005) Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg 242:252–259
Fu XT, Tang Z, Chen JF, Shi YH, Liu WR, Gao Q, Ding GY, Song K, Wang XY, Zhou J, Fan J, Ding ZB (2021) Laparoscopic hepatectomy enhances recovery for small hepatocellular carcinoma with liver cirrhosis by postoperative inflammatory response attenuation: a propensity score matching analysis with a conventional open approach. Surg Endosc 35(2):910–920
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J (2018) Guidelines for diagnosis and treatment of primary liver cancer in China. Liver Cancer 7:235–260
Hwang ES, Wang X (2017) Value of propensity score matching to study surgical outcomes. Ann Surg 265:457–458
Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, Choi D, Song KD, Kwon CH, Joh JW, Paik SW, Paik YH, Ahn JH (2015) Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection-propensity score analyses of long-term outcomes. Radiology 275:908–919
Ho DE, Imai K, King G, Stuart EA (2011) MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 42:1–28
Der Wal WMV, Geskus RB (2011) ipw: an R package for inverse probability weighting. J Stat Softw 43:1–23
Tian MX, Luo LP, Liu WR, Deng W, Yin JC, Jin L, Jiang XF, Zhou YF, Qu WF, Tang Z, Wang H, Tao CY, Fang Y, Qiu SJ, Zhou J, Liu JF, Fan J, Shi YH (2019) Development and validation of a prognostic score predicting recurrence in resected combined hepatocellular cholangiocarcinoma. Cancer Manag Res 11:5187–5195
Wang T, Wang W, Zhang J, Yang X, Shen S, Wang W (2020) Development and validation of a nomogram for differentiating combined hepatocellular cholangiocarcinoma from intrahepatic cholangiocarcinoma. Front Oncol 10:598433
Peng Y, Lin P, Wu L, Wan D, Zhao Y, Liang L, Ma X, Qin H, Liu Y, Li X, Wang X, He Y, Yang H (2020) Ultrasound-based radiomics analysis for preoperatively predicting different histopathological subtypes of primary liver cancer. Front Oncol 10:1646
Cheng D, Cross CL, Calfee G, Kirgan D, Williams SJ, Baynosa J, St Hill CR (2019) Comparing treatment patterns of hepatocellular carcinoma at academic centers and non-academic centers within the Mountain Region. Am J Surg 218:1052–1059
Wang X, Teh CSC, Ishizawa T, Aoki T, Cavallucci D, Lee SY, Panganiban KM, Perini MV, Shah SR, Wang H, Xu Y, Suh KS, Kokudo N (2021) Consensus guidelines for the use of fluorescence imaging in hepatobiliary surgery. Ann Surg 274:97–106
Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G (2016) Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 263:761–777
Yamashita YI, Aishima S, Nakao Y, Yoshizumi T, Nagano H, Kuroki T, Takami Y, Ide T, Ohta M, Takatsuki M, Nanashima A, Ishii F, Kitahara K, Iino S, Beppu T, Baba H, Eguchi S (2020) Clinicopathological characteristics of combined hepatocellular cholangiocarcinoma from the viewpoint of patient prognosis after hepatic resection: high rate of early recurrence and its predictors. Hepatol Res 50:863–870
Ishii T, Ito T, Sumiyoshi S, Ogiso S, Fukumitsu K, Seo S, Taura K, Uemoto S (2020) Clinicopathological features and recurrence patterns of combined hepatocellular-cholangiocarcinoma. World J Surg Oncol 18:319
Wakizaka K, Yokoo H, Kamiyama T, Ohira M, Kato K, Fujii Y, Sugiyama K, Okada N, Ohata T, Nagatsu A, Shimada S, Orimo T, Kamachi H, Taketomi A (2019) Clinical and pathological features of combined hepatocellular-cholangiocarcinoma compared with other liver cancers. J Gastroenterol Hepatol 34:1074–1080
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67:358–380
Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM (2015) Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 17:669–680
Li DY, Zhang HB, Yang N, Quan Y, Yang GS (2013) Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: results of a monocentric series. World J Gastroenterol 19:9084–9091
Kim DH, Choi DW, Choi SH, Heo JS, Kow AW (2015) Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery 157:666–675
Xun XD, Li Q (2016) Surgical treatment of intrahepatic cholangiocarcinoma: a retrospective study of 104 cases. Cancer Biol Med 13:469–473
Gerken ALH, Herrle F, Jakob J, Weiß C, Rahbari NN, Nowak K, Karthein C, Hohenberger P, Weitz J, Reißfelder C, Dobroschke JC (2020) Definition and severity grading of postoperative lymphatic leakage following inguinal lymph node dissection. Langenbecks Arch Surg 405:697–704
Acknowledgements
We acknowledged Dr. Yining Wang for his assistance of patient collection. This work was supported by the Major Program of National Natural Science Foundation of China (No. 82090054), National Natural Science Foundation of China (No. 81772566), Clinical Research Plan of Shanghai Hospital Development Center (No. 2020CR3004A), and Natural Science Funds of Shanghai (No. 21ZR1413800).
Funding
Major Program of National Natural Science Foundation of China, No. 82090054, Xiaoying Wang, National Natural Science Foundation of China, No. 81772566, Xiaoying Wang, Clinical Research Plan of Shanghai Hospital Development Center, No. 2020CR3004A, Xiaoying Wang, Natural Science Funds of Shanghai, No. 21ZR1413800, Kai Zhu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dan-Jun Song, Kai Zhu, Jin-peng Tan, Jia-Bin Cai, Min-Zhi Lv, Jie Hu, Zhen-Bin Ding, Guo-Ming Shi, Ning Ren, Xiao-Wu Huang, Ying-Hong Shi, Shuang-Jian Qiu, Qing-Hai Ye, Hui-Chuan Sun, Qiang Gao, Jian Zhou, Jia Fan, and Xiao-Ying Wang have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, DJ., Zhu, K., Tan, Jp. et al. Perioperative and oncologic outcomes of laparoscopic versus open liver resection for combined hepatocellular-cholangiocarcinoma: a propensity score matching analysis. Surg Endosc 37, 967–976 (2023). https://doi.org/10.1007/s00464-022-09579-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09579-y