Abstract
Background
Laparoscopic surgery has become the standard option for gastrointestinal surgeries. However, laparoscopic procedures require extended training times and are difficult for inexperienced surgeons. Robot-assisted laparoscopic surgery facilitates easy adaptation of laparoscopic procedures, but robotic surgical systems are expensive. In addition, their cost has remained high because there is currently only one manufacturer of commercially available systems. Recently, a new Korean robotic surgical system, Revo-i, has been developed. The aim of this study was to evaluate the feasibility and safety of Revo-i by performing robotic cholecystectomy in a porcine model.
Methods
After approval by the Institutional Animal Care and Use Committee of Yonsei University Health System, cholecystectomy was performed in four pigs using the Revo-i robotic surgical system. Operative time and perioperative complications were recorded, and all animals were observed for postoperative complications for 2 weeks after surgery
Results
Robotic cholecystectomy was completed successfully and without gallbladder perforation in all cases. The mean operative time was 78 ± 12 min, the mean docking time was 4.5 ± 2.52 min, and the mean console time was 49.8 ± 14.17 min. There were no perioperative complications, and none of the animal used for the in vivo models exhibited abnormal behavior during the postoperative observation period.
Conclusions
These preliminary results verify the safety and efficacy of robotic cholecystectomy using the Revo-i robotic surgical system. Human trials are slated to begin accordingly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Since its introduction, many studies have confirmed the superiority of laparoscopic surgery compared to conventional open surgery in terms of cosmesis, postoperative pain control, length of hospital stay, and postoperative return to normal activities of daily living [1–4]. Although laparoscopic surgery has become the standard approach for many types of gastrointestinal surgery, laparoscopic procedures do have technical limitations that include two-dimensional imaging and the fulcrum effect, limited mobility of rigid instruments, and amplification of involuntary tremor by long instruments [5, 6]. Laparoscopic procedures require extended training periods. In addition, less experienced surgeons are more likely to make mistakes and to take a long time to complete surgery.
Many of these limitations can be overcome by using robotic surgical systems. Although several robotic surgical systems have been developed, including the Zeus system [7], the da Vinci system [8], the Telelap ALF-X platform [9], and the Micro Hand S robot system [10], only the da Vinci system is commercially available. More than 3000 da Vinci surgical systems have been adopted at medical centers worldwide, but the persistence of any monopoly can result in inflated prices of the system and higher medical costs for patients, and can become a hindrance to the continued development and evolution of technology.
The Meere Company in Korea has been invested in the development of new robotic surgical systems since 2006. During this time, robot hardware and software have been updated and stabilized. Finally, a new robotic surgical system, the Revo-i Model MSR-5000, has been produced by Meere. In this study, we have evaluated the feasibility and safety of this new system by performing cholecystectomy in a porcine model.
Materials and methods
Porcine model
This study was approved by the Institutional Animal Care and Use Committee of Yonsei University Health System (IACUC number 2015-0358). Four female Yorkshire pigs with a weight of 40 kg and an age of 90 days were used. Each pig was fasted for 12 h before surgery. All pigs received an injection of alfaxalone 1 mg/kg, xylazine 2 mg/kg, and azaperone 2 mg/kg for anesthesia, intravenous ketorolac 1 mg/kg for analgesia, cefazolin 30 mg/kg as an antibiotic, and a pre-anesthetic intramuscular injection of atropine 0.04 mg/kg. The pigs were intubated, and anesthesia was maintained with isoflurane 2%. The pigs were monitored using a three-lead electrocardiogram (ECG) and pulse oximetry.
Robotic surgical system
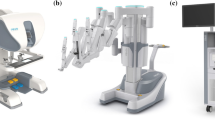
The Revo-i robotic surgical system is a master–slave robot system that consists of a surgeon control console (MSRC-5000), an operation cart (MSRO-5000), and vision system (MSRV-5000) (Fig. 1). The surgeon control console has a three-dimensional (3D) image viewer, a handle, and a foot pedal unit. The handle transmits the surgeon’s hand movements to the robot arm of the operation cart. To ensure patient safety of and to prevent uncontrollable errors, an emergency stop button is on the right side of the master console and on the back of the operation cart. The operation cart has four arms (A–D), including a camera (C) arm. All arms have three joints for ease of movement toward the operation field. The instrument is attached to the distal parts of the A, B, and D arms. These arms are used to manipulate the instruments. Instrument arms (A, B, and D) have seven degrees of motion freedom. The location of the D arms can be changed to the A arm side or B arm side. A comparison of the specifications of the da Vinci and Revo-i systems is described in Table 1. The characteristics and specification of Revo-i were confirmed by the testing laboratory of the Korean Ministry of Commerce, Industry and Energy.
Surgical procedure
After induction of general anesthesia, the pig was placed in the supine position. The surgical field was shaved and prepared with an antiseptic agent, and a 15-mm infraumbilical incision was made. The peritoneum was opened, and a camera port was inserted. Pneumoperitoneum was created with a maximum pressure of 12 mmHg, and the pig was then repositioned into a 20° reverse Trendelenburg position with a 20° left decubitus. The three working ports (Fig. 2A) were inserted under laparoscopic vision to ensure that no intra-abdominal organs were injured during port placement. The operation cart was moved toward the operating table, and each robotic arm was docked in its respective port (Fig. 2B). After all the preparations were completed for robotic cholecystectomy, console operation was started under 3D vision (Fig. 2C, D). Cadiere forceps were inserted through the D arm, and the gallbladder was retracted for visualization of the Calot’s triangle. The cystic artery and cystic duct were carefully dissected from the surrounding tissues using forceps and a monopolar hook (Fig. 3A). Medium–large-sized clips were applied with a robotic clip applier to ligate the cystic artery (Fig. 3B). The cystic duct was ligated with a knot-tying (Fig. 3C). Robotic curved scissors were used to divide the cystic artery and duct (Fig. 3D), which were then carefully divided again to permit dissection of the gallbladder from the liver bed using monopolar hook cautery. The detached gallbladder was retrieved from the abdominal cavity in an endopouch, the instruments were removed, and the robotic arms were detached from the ports. The wounds were sutured with 3-0 nylon. Data including docking time, console time, total operative time, blood loss, and intraoperative complications were recorded (supplementary video).
Procedure of robotic cholecystectomy using a porcine model. A The cystic artery and cystic duct were carefully dissected from the surrounding tissues using forceps and a monopolar hook. B Medium–large-sized clips were applied with a robotic clip applier to ligate the cystic artery. C The cystic duct was ligated with knot-tying. D The cystic artery was divided with robotic curved scissors
Postoperative care
All the pigs resumed their regular diets at 6 h after surgery. During the first 7 days after surgery, all pigs received prophylactic antibiotics (amoxicillin–clavulanate, 14 mg/kg orally, twice daily) along with meloxicam 0.2 mg/kg three times daily with food for analgesia. Pigs were closely monitored and examined three times daily by a veterinarian. Any behavioral changes, including distress and loss of appetite, were noted. All animals were weighed and euthanized 2 weeks after surgery.
Statistics
Results are expressed as the mean value and standard deviation. Statistical analyses were performed using the paired Student’s t test and using SPSS 21 for Microsoft Windows (SPSS Inc., Chicago, IL USA). P values <0.05 were considered statistically significant.
Results
All surgical procedures were completed successfully, and all animals maintained normal vital signs throughout the procedures. The mean operative time was 78 ± 12 min, the mean docking time was 4.5 ± 2.52 min, and the mean console time was 49.8 ± 14.18 min. In one case (case 3), the console time was longer because of minor bleeding in the liver bed, which was ultimately coagulated successfully. Overall, there was no significant blood loss for any case, and no intraoperative complications were recorded. There were no cases of gallbladder perforation or bile leakage.
All pigs survived for 2 weeks after surgery before they were euthanized according to the study protocol. All pigs had good appetites for food, and all of them gained weight. The mean weight at 2 weeks was 41.8 ± 0.76 kg (P = 0.018). There were no postoperative complications or adverse effects recorded during the postoperative observation period. The detailed results are summarized in Table 2.
Discussion
This is one of the first preclinical studies to demonstrate the availability and feasibility of the Korean-made Revo-i Model MSR-5000 robotic surgical system for robot-assisted laparoscopic cholecystectomy. There was no surgical morbidity or mortality during the study period, and there were no perioperative complications. Operative times were acceptable for every case. These results demonstrate that the new Revo-i robotic surgical system is safe for routine use, and could potentially create a more competitive and cost-efficient market for such systems.
After the first robotic cholecystectomy was performed in Korea in 2005 [11], the Meere Company began developing new robotic surgical systems for minimally invasive surgery. The first prototype (MSR-1000) was developed in 2007 and introduced in 2009. Because of the results, Meere was selected by the Korea Ministry of Knowledge Economy to develop surgical robotic systems for minimally invasive surgery. After development of the first prototype, many prototypes (MSR-HI, MSR-2000, MSR-3000, MSR-Ceiling type, MSR-MAC, MSR-4000, MSR-BSP, and MSR-MAS) were developed during this period and approximately 20 different animal studies (cholecystectomy, bowel anastomosis, and choledochojejunostomy) were performed. Since then, the robotic surgical systems have been updated and stabilized. In 2015, the latest version, Revo-i (MSR-5000), was introduced and a preclinical study was performed, with successful results.
Several robotic systems have recently been developed, and several clinical studies have been performed to evaluate these systems. Bo et al. [10] have reported success with appendectomy and primary repair of gastric perforation using the Micro Hand S robotic surgical system, and Fanfani et al. [9] reported success with gynecologic procedures using the new Telelap ALF-X platform. The Micro Hand S system has only two arms; it dose not have a third arm for tissue retraction. The Telelap ALF-X system has the limitation of non-wristed instrumentation, as do non-robotic laparoscopic instruments. In contrast, the Revo-i robotic surgical system has three arms with seven degrees of freedom. It offers motion scaling, tremor filtration, and 3D visualization of the surgical field, all of which can overcome the limitations of non-robotic laparoscopic surgery.
Robotic surgery became widely accepted after Intuitive Surgical introduced the da Vinci system in 1999. It is estimated that approximately 1.5 million robotic surgical procedures have now been performed worldwide. However, the need for cost-effective healthcare is a pressing issue in many countries, and the costs associated with this seminal robotic surgical system can be prohibitive. We hope that the introduction of the Revo-i robotic surgical system will open the market and help to resolve this situation.
Currently, the Revo-i robotic surgical system commonly uses only monopolar- and bipolar-type instruments to achieve adequate dissection and hemostasis. Therefore, this is one of the potential obstacles that new robotic surgery systems will need to overcome. The next-generation of robotic surgical systems should be able to be used with energy devices such as vessel sealers and harmonic scalpels. These devices allow rapid coagulation and easy tissue dissection. The availability of robot-mountable energy devices will contribute to the growth of robotic surgery. Therefore, Meere is currently planning the development of robot-mountable energy devices. Another challenge is the lack of haptic sensing during robotic surgeries. Surgeons gain much information from tactile sensations during tissue manipulation [12]; thus far, only the Telelap ALF-X platform provides a haptic feedback system. The Meere has almost completed the integration of a haptic feedback system with the Revo-i robotic surgical system.
In conclusion, the aim of this study was to prove that the Revo-i robotic surgical system is safe and meets the necessary specifications for human surgery. Cholecystectomy is standardized thorough the worldwide, and it is a fundamental laparoscopic procedure. It is straightforward and allows direct comparisons between robotic and conventional laparoscopic techniques. Therefore, cholecystectomy was chosen as the first procedure to be used as a functional test of Revo-i robotic surgical system. After the successful results of this preclinical study, the Korean Ministry of Food and Drug Safety confirmed good manufacturing practice of the robot and instruments used with this surgical system. With this confirmation, we are able to begin clinical trials involving humans in an attempt to achieve approval for clinical usage. We will soon report the results of that clinical study.
References
Belli G, Fantini C, D’Agostino A, Cioffi L, Langella S, Russolillo N, Belli A (2007) Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc 21(11):2004–2011
Mochiki E, Nakabayashi T, Kamimura H, Haga N, Asao T, Kuwano H (2002) Gastrointestinal recovery and outcome after laparoscopy-assisted versus conventional open distal gastrectomy for early gastric cancer. World J Surg 26(9):1145–1149
Zhang RC, Zhou YC, Mou YP, Huang CJ, Jin WW, Yan JF, Wang YX, Liao Y (2015) Laparoscopic versus open enucleation for pancreatic neoplasms: clinical outcomes and pancreatic function analysis. Surg Endosc. doi:10.1007/s00464-015-4538-6
Zhou ZG, Hu M, Li Y, Lei WZ, Yu YY, Cheng Z, Li L, Shu Y, Wang TC (2004) Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc 18(8):1211–1215
Hawasli A, Lloyd LR (1991) Laparoscopic cholecystectomy. The learning curve: report of 50 patients. Am Surg 57(8):542–544 (discussion 545)
Jordan JA, Gallagher AG, McGuigan J, McClure N (2000) Randomly alternating image presentation during laparoscopic training leads to faster automation to the “fulcrum effect”. Endoscopy 32(4):317–321
Nio D, Bemelman WA, Busch OR, Vrouenraets BC, Gouma DJ (2004) Robot-assisted laparoscopic cholecystectomy versus conventional laparoscopic cholecystectomy: a comparative study. Surg Endosc 18(3):379–382
Cadiere GB, Himpens J, Germay O, Izizaw R, Degueldre M, Vandromme J, Capelluto E, Bruyns J (2001) Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg 25(11):1467–1477
Fanfani F, Monterossi G, Fagotti A, Rossitto C, Alletti SG, Costantini B, Gallotta V, Selvaggi L, Restaino S, Scambia G (2015) The new robotic TELELAP ALF-X in gynecological surgery: single-center experience. Surg Endosc. doi:10.1007/s00464-015-4187-9
Yi B, Wang G, Li J, Jiang J, Son Z, Su H, Zhu S (2015) The first clinical use of domestically produced Chinese minimally invasive surgical robot system “Micro Hand S”. Surg Endosc. doi:10.1007/s00464-015-4506-1
Kang CM, Chi HS, Hyeung WJ, Kim KS, Choi JS, Lee WJ, Kim BR (2007) The first korean experience of telemanipulative robot-assisted laparoscopic cholecystectomy using the da vinci system. Yonsei Med J 48(3):540–545
Schostek S, Binser MJ, Rieber F, Ho CN, Schurr MO, Buess GF (2010) Artificial tactile feedback can significantly improve tissue examination through remote palpation. Surg Endosc 24(9):2299–2307
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean government (MEST; NRF-2015R1A2A2A04003460).
Authors’ contribution
JHL Authors make substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data; WJL Authors make substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data; DWP Authors participate in drafting the article or revising it critically for important intellectual content; HJY Authors participate in drafting the article or revising it critically for important intellectual content; SHK Authors participate in drafting the article or revising it critically for important intellectual content; CMK Authors give final approval of the version to be submitted and any revised version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Woo Jung Lee serves as consultant for Meere Company Inc, Pangyo Techno Valley, Seongnam, Republic of Korea. Dong Won Park participates in development of robotic system, Meere Company Inc, Pangyo Techno Valley, Seongnam, Republic of Korea. Jin Hong Lim, Hye Jin Yea, Se Hoon Kim, Chang Moo Kang have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (WMV 138160 kb)
Rights and permissions
About this article
Cite this article
Lim, J.H., Lee, W.J., Park, D.W. et al. Robotic cholecystectomy using Revo-i Model MSR-5000, the newly developed Korean robotic surgical system: a preclinical study. Surg Endosc 31, 3391–3397 (2017). https://doi.org/10.1007/s00464-016-5357-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5357-0