Abstract
This study evaluated the safety and efficacy of the newly developed Revo-i (Meerecompany, Yongin, Republic of Korea) robotic surgical system during robot-assisted cholecystectomy. This prospective, phase I clinical study involved 15 patients with gallbladder-related disease. The primary outcome evaluated was the intraoperative safety of the Revo-i; the secondary outcomes measured were the 30-day postoperative complications and patient satisfaction with the Revo-i’s performance. Between August 17 and December 23, 2016, we performed 15 robot-assisted cholecystectomies. The operations were successfully completed, without any conversions to open or laparoscopic approaches. The mean patient age (53.07 years), mean operative time (115.3 ± 17.31 min [± standard deviation]), docking time (10.6 ± 3.16 min), console time (49.7 ± 15.41 min), actual dissection time (33.1 ± 10.53 min), and estimated blood loss (3.33 ± 6.17 mL) were determined. There were no intra- or postoperative complications, including gallbladder perforations. The mean hospital stay was 2.0 ± 1.00 days. Most patients reported satisfaction with the Revo-i’s performance. Performing robot-assisted cholecystectomies using the Revo-i is feasible and safe. This report describes the first clinical study of the Revo-i robotic surgical system in human patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the da Vinci™ surgical system (Intuitive Surgical, Mountain View, CA, USA) was created in the late 1990s [1, 2], robot-assisted operations have been widely accepted, proven to be safe for patients, and associated with favorable patient outcomes [3,4,5,6,7]. The three-dimensional operative view, seven degrees of motion freedom, motion scaling, and tremor filtration enable surgeons to perform more delicate operations. However, although the da Vinci™ surgical system has been the sole leader in this area, debate continues regarding whether robot-assisted operations are necessary due to their high cost [8,9,10].
In Korea, Meerecompany, Inc. has invested in new robotic surgical systems since 2006, producing a new robotic surgical system called the Revo-i. Within the last 2 years, the Revo-i robotic surgical system has been used in in vivo porcine models. Preclinical studies of various operations, such as partial nephrectomy, cholecystectomy, and fallopian tube anastomosis, were successfully performed [11,12,13,14]. Following the results of these preclinical studies, the clinical testing of the system’s feasibility and safety was approved by the Korean Ministry of Food and Drug Safety.
The present study evaluated the safety and feasibility of the Revo-I during robot-assisted cholecystectomy (RAC) in humans.
Methods
The Korean Food and Drug Administration (FDA) approved (no. 14, 2016–04-26) clinical testing of the Revo-I robotic surgical system, based on porcine model results of cholecystectomies and nephrectomies using the system [13, 14]. The Yonsei University Health System, Severance Hospital Institutional Review Board approved the current human study protocol (no. 1–2016-0019), after FDA approval. The committee requested countermeasures suitable for risk level III (moderate risk). We fully informed and discussed all possible complications with the involved patients and their families. Only the patients who agreed to participate in this study were enrolled.
A surgeon with experience of performing more than 2000 laparoscopic cholecystectomies and 400 RACs, using the da Vinci™ surgical system, performed RACs using the Revo-i. Further, training (12 h) was provided to medical teams regarding use of the Revo-i robotic surgical system; only teams completing the training participated in this study.
The primary study outcome was an evaluation of the intraoperative safety of the Revo-i, i.e., successful completion of planned Revo-i operations without conversion to open or laparoscopic approaches because of robotic system malfunctions. The secondary outcomes included 30-day postoperative complication assessment. Postoperative complications due to the Revo-i procedure were defined as grade 3 or greater complications, according to the Clavien–Dindo classification [15]. More than two cases of surgical procedure failure were defined as study failure. A minimum of 15 patients were planned for study enrolment to provide adequate statistical power.
Robotic surgical system
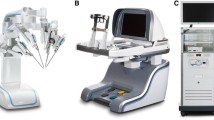
The Revo-i robotic surgical system consists of a master surgeon control console (MSRC-5000), a slave four-arm robotic operation cart (MSRO-5000), and a high-definition vision cart (MSRV-5000) (Fig. 1) [16]. All instruments are designed to be reusable, and the number of uses per instrument is counted. The Revo-i has smaller instruments than the da Vinci-Xi™ surgical system. Details of the Revo-i robotic surgical system are shown in Table 1 and are described in previous reports [12, 13].
Surgical procedure
Robot-assisted cholecystectomy
Each patient was placed, supine, on the operating table (Supplemental Digital Content, Video 1). After inducing general anesthesia, the abdomen was draped in a usual, sterile manner, and a 1.5-cm supraumbilical vertical skin incision was made. The fascia and peritoneum were opened, and a camera trocar was inserted. Carbon dioxide gas was infused to create a pneumoperitoneum with a pressure of 12–14 mmHg. The table was tilted to a partial reverse Trendelenburg position (20°) and rotated on its right axis by about 30°. Three working ports for the robotic arms were created under laparoscopic vision, including two in the left and right upper quadrant areas and a third near the right anterior axillary line (used for exposure and retraction). The slave robot was situated near the operating table, and each robotic arm was docked to its respective port. After cholecystectomy preparations were completed, the surgeon began the cholecystectomy using the master console. The operative procedure was similar to a general laparoscopic cholecystectomy. Cadiere forceps were inserted through the third port, and the gallbladder was retracted for visualization of the Calot triangle. After performing dissection of the tissue surrounding of the cystic artery and duct, the cystic artery was ligated using a medium–large-sized clip and divided. The cystic duct was ligated using Vicryl 3–0 suture material and a medium–large-sized clip, and divided. The gallbladder was detached from the liver bed using monopolar hook cautery, and removed from the abdominal cavity in an endopouch. Postoperative data, including the docking time, console time, total operative time, blood loss, and intraoperative complications, were recorded.
Patient satisfaction survey
Patient satisfaction with the robot-assisted operation was determined using postoperatively administered questionnaires. The questionnaires assessed the degree of pain at discharge (using a numeric rating scale), inconvenience associated with the preoperative preparation and procedure, and willingness to undergo future operations involving the Revo-i (using a Likert scale) [17].
Results
Between August and December 2016, 15 patients (8 males) underwent RAC using the Revo-i robotic surgical system (patient demographics and outcomes are shown in Table 2). The mean patient age was 53.07 ± 12.05 years and the mean body mass index was 25.94 ± 2.56 kg/m2. The preoperative diagnoses included gallbladder stones with chronic cholecystitis (9 patients, 60%), gallbladder polyps (4 patients, 26.67%), and gallbladder polyps and stones (2 patients, 13.33%). All operations were completed using the Revo-i robotic surgical system, without any laparoscopic or open conversions. The mean total operative time (115.3 ± 17.31 min), docking time (10.6 ± 3.16 min), and console time (49.7 ± 15.41 min) were determined. The mean actual dissection time (time from exposure of Calot’s triangle to gallbladder detachment from the liver bed) was 33.1 ± 10.53 min. The mean estimated blood loss was 3.3 ± 6.17 mL. No intra- or postoperative complications, including gallbladder perforations, occurred. All patients were prescribed a general soft diet, after recovering from anesthesia, and were discharged 2.0 ± 1.00 days after surgery. No wound infections were noted at the initial postoperative visit at 2 weeks (Table 2).
Patient satisfaction with Revo-i.
Patients were postoperatively surveyed about their satisfaction with the Revo-i robot-assisted surgery. Most patients did not complain of inconvenience due to the preoperative preparation or the procedure. All patients, except one, were willing to undergo another operation involving Revo-i, if necessary; the one patient did not agree to a future operation if additional costs were incurred for the robot-assisted surgery (Fig. 2).
Discussion
This is the first clinical trial to evaluate the safety and feasibility of the Revo-i robotic surgical system in humans undergoing minimally invasive operations. Cholecystectomies were carefully chosen as the first procedures to test the Revo-i because, historically, they were the first surgeries in which laparoscopic techniques were popularized [18]. Thus, cholecystectomies have standardized laparoscopic procedures, making them appropriate procedures for evaluating the basic performance of a new robotic surgical system [19]. Additionally, the close proximity of important anatomic structures, such as the cystic duct, hepatic artery, and common bile duct, makes this procedure one of the best for testing the safety and feasibility of new robotic surgical systems.
All of the Revo-i cholecystectomies performed in this study were successful, and did not involve any major intraoperative complications, organ injuries, or conversions to open or laparoscopic approaches. Docking time and console time were so long than general robotic cholecystectomy. We spent a lot of time checking whether we could safely perform the surgery rather than reducing the time because this study is the first attempt to apply the robot system to humans. Early period report about robotic cholecystectomy using da Vinci™ system also spent long docking time and console time [20, 21]. Moreover, there were no postoperative complications defined as Clavien–Dindo grade ≥ 3. According to the postoperative patient satisfaction survey, the patients were generally satisfied with the operations using the new system, and patient satisfaction is an important component of minimally invasive surgeries. Further, patient satisfaction is closely associated with short hospitalization periods, extent of postoperative pain, and resultant wound sizes. These results reflect the effectiveness of RACs, using Revo-i, despite the study not involving a comparison with currently available robotic systems.
Hospital stay after cholecystectomy in Korea was longer than other country. That was related with Korean culture and almost complete national health insurance system. Many studies from Korea also reported one or two hospital stays after cholecystectomy [22, 23].
After the introduction of the da Vinci™ system, robot-assisted operations have become a major treatment option in the fields of gastrointestinal, gynecologic, and urologic operations [24,25,26,27]. The da Vinci™ system has had a great impact on the initiation of robotic abdominal operations and has led to the development of this surgical field. This system has been used to conduct more than 3,000,000 procedures in 64 countries, and there are more than 10,000 peer-reviewed publications about the use of the da Vinci™ system in various surgical fields [28]. As a result, robot-assisted operations, in the abdominal field, are considered to involve use of the da Vinci™ system. This monopoly of the da Vinci™ system has influenced the high operative expenses associated with the procedure, and da Vinci™ system has expensive annual service costs. Thus, this situation has hindered the development and evolution of robotic surgical technologies.
The Meerecompany has been developing its robotic surgical system since 2006, with a prototype being introduced in 2007. Accordingly, the Korea Ministry of Knowledge Economy selected Meerecompany to further develop its surgical robot system. This resulted in many prototypes being developed that had improved function and performance. During this period, several preliminary animal studies were also performed, and the incremental system models became more stable and showed good function. A preclinical study was successfully performed, and KFDA approval for the clinical use of the system followed in 2016 [13, 14].
Recently, a new robotic surgical system, Telelap Alf-x (now, Senhance™, TransEnterix, Morrisville, NC, USA), was developed and its first clinical study was published [29]. This system has haptic feedback and an eye tracking endoscope, but is limited by non-articulated instrumentation. In contrast, the Revo-i system has three arms with seven degrees of freedom. The three articulating arms allow the surgeon to perform delicate procedures and meticulous dissections. An articulating arm that functions similarly to a human hand is an important element that distinguishes robot-assisted operations from conventional laparoscopic operations.
Based on the current results, the KFDA approved the clinical use of Revo-i in minimally invasive operations in early August 2017. Hence, the Revo-i is expected to be used in minimally invasive operations beginning in early 2018, making this system an alternative to the da Vinci™ system. This advancement is expected to somewhat resolve the cost–benefit issue associated with current robot-assisted operations. The clinical efficacy and safety of the Revo-i require further validation in various surgical procedures in the near future.
Conclusion
In our studies, RAC was successfully performed in 15 patients. There were no complications and no open or laparoscopic conversion. Most patients were satisfied with the surgical results. Revo-i show good performance during RAC. Performing RACs using the Revo-i is feasible and safe. This report describes the first clinical study of the Revo-i robotic surgical system in human patients.
Availability of data and material
All data was included in the paper.
References
Falk V, Diegeler A, Walther T, Banusch J, Brucerius J, Raumans J, Autschbach R, Mohr FW (2000) Total endoscopic computer enhanced coronary artery bypass grafting. Eur J Cardiothorac Surg 17(1):38–45
Himpens J, Leman G, Cadiere GB (1998) Telesurgical laparoscopic cholecystectomy. Surg Endosc 12(8):1091
Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH (2012) A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol 19(8):2485–2493. https://doi.org/10.1245/s10434-012-2262-1
Hagen ME, Pugin F, Chassot G, Huber O, Buchs N, Iranmanesh P, Morel P (2012) Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg 22(1):52–61. https://doi.org/10.1007/s11695-011-0422-1
Lee J, Chung WY (2013) Robotic thyroidectomy and neck dissection: past, present, and future. Cancer J 19(2):151–161. https://doi.org/10.1097/PPO.0b013e31828aab61
Novara G, Ficarra V, Rosen RC, Artibani W, Costello A, Eastham JA, Graefen M, Guazzoni G, Shariat SF, Stolzenburg JU, Van Poppel H, Zattoni F, Montorsi F, Mottrie A, Wilson TG (2012) Systematic review and meta-analysis of perioperative outcomes and complications after robot-assisted radical prostatectomy. Eur Urol 62(3):431–452. https://doi.org/10.1016/j.eururo.2012.05.044
Weinberg L, Rao S, Escobar PF (2011) Robotic surgery in gynecology: an updated systematic review. Obstet Gynecol Int 2011:852061. https://doi.org/10.1155/2011/852061
deSouza AL, Prasad LM, Park JJ, Marecik SJ, Blumetti J, Abcarian H (2010) Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum 53(7):1000–1006. https://doi.org/10.1007/DCR.0b013e3181d32096
Barbash GI, Glied SA (2010) New technology and health care costs–the case of robot-assisted surgery. N Engl J Med 363(8):701–704. https://doi.org/10.1056/NEJMp1006602
Wright JD, Ananth CV, Lewin SN, Burke WM, Lu YS, Neugut AI, Herzog TJ, Hershman DL (2013) Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA 309(7):689–698. https://doi.org/10.1001/jama.2013.186
Kim DK, Park DW, Rha KH (2016) Robot-assisted Partial Nephrectomy with the REVO-I Robot Platform in Porcine Models. Eur Urol 69(3):541–542. https://doi.org/10.1016/j.eururo.2015.11.024
Kang CM, Chong JU, Lim JH, Park DW, Park SJ, Gim S, Ye HJ, Kim SH, Lee WJ (2017) Robotic cholecystectomy using the newly developed Korean robotic surgical system, Revo-i: a preclinical experiment in a porcine model. Yonsei Med J 58(5):1075–1077. https://doi.org/10.3349/ymj.2017.58.5.1075
Lim JH, Lee WJ, Park DW, Yea HJ, Kim SH, Kang CM (2017) Robotic cholecystectomy using Revo-i Model MSR-5000, the newly developed Korean robotic surgical system: a preclinical study. Surg Endosc 31(8):3391–3397. https://doi.org/10.1007/s00464-016-5357-0
Abdel Raheem A, Troya IS, Kim DK, Kim SH, Won PD, Joon PS, Hyun GS, Rha KH (2016) Robot-assisted fallopian tube transection and anastomosis using the new REVO-I robotic surgical system: feasibility in a chronic porcine model. BJU Int 118(4):604–609. https://doi.org/10.1111/bju.13517
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Revo-i surgical solution website. https://revosurgical.com/#/main.asp Accessed April 2018
Jamieson S (2004) Likert scales: how to (ab)use them. Med Educ 38(12):1217–1218. https://doi.org/10.1111/j.1365-2929.2004.02012.x
Cuschier A, Terblanche J (1990) Laparoscopic cholecystectomy: evolution, not revolution. Surg Endosc 4(3):125–126
Marescaux J, Smith MK, Folscher D, Jamali F, Malassagne B, Leroy J (2001) Telerobotic laparoscopic cholecystectomy: initial clinical experience with 25 patients. Ann Surg 234(1):1–7
Wakiya T, Kudo D, Toyoki Y, Ishido K, Kimura N, Narumi S, Kijima H, Hakamada K (2014) Evaluation of the usefulness of the indocyanine green clearance test for chemotherapy-associated liver injury in patients with colorectal cancer liver metastasis. Ann Surg Oncol 21(1):167–172. https://doi.org/10.1245/s10434-013-3203-3
Vidovszky TJ, Smith W, Ghosh J, Ali MR (2006) Robotic cholecystectomy: learning curve, advantages, and limitations. J Surg Res 136(2):172–178. https://doi.org/10.1016/j.jss.2006.03.021
Kim JH, Baek NH, Li G, Choi SH, Jeong IH, Hwang JC, Kim JH, Yoo BM, Kim WH (2013) Robotic cholecystectomy with new port sites. World J Gastroenterol 19(20):3077–3082. https://doi.org/10.3748/wjg.v19.i20.3077
Lee EK, Park E, Oh WO, Shin NM (2017) Comparison of the outcomes of robotic cholecystectomy and laparoscopic cholecystectomy. Ann Surg Treat Res 93(1):27–34. https://doi.org/10.4174/astr.2017.93.1.27
Giulianotti PC, Quadri P, Durgam S, Bianco FM (2017) Reconstruction/repair of iatrogenic biliary injuries: is the robot offering a new option? Ann Surg Short Clin Rep. https://doi.org/10.1097/sla.0000000000002343
Zakhari A, Czuzoj-Shulman N, Spence AR, Gotlieb WH, Abenhaim HA (2015) Laparoscopic and robot-assisted hysterectomy for uterine cancer: a comparison of costs and complications. Am J Obstet Gynecol 213(5):665.e661–667. https://doi.org/10.1016/j.ajog.2015.07.004
Pokorny M, Novara G, Geurts N, Dovey Z, De Groote R, Ploumidis A, Schatteman P, de Naeyer G, Mottrie A (2015) Robot-assisted simple prostatectomy for treatment of lower urinary tract symptoms secondary to benign prostatic enlargement: surgical technique and outcomes in a high-volume robotic centre. Eur Urol 68(3):451–457. https://doi.org/10.1016/j.eururo.2015.03.003
Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam BH, Sohn DK, Oh JH (2018) Robot-assisted versus laparoscopic surgery for rectal cancer: a phase II open label prospective randomized controlled trial. Ann Surg 267(2):243–251. https://doi.org/10.1097/sla.0000000000002321
Intuitive Surgical company website. https://www.intuitivesurgical.com/company/faqs.html. Accessed October 2017
Gueli Alletti S, Rossitto C, Fanfani F, Fagotti A, Costantini B, Gidaro S, Monterossi G, Selvaggi L, Scambia G (2015) Telelap Alf-X-assisted laparoscopy for ovarian cyst enucleation: report of the first 10 cases. J Min Invasive Gynecol 22(6):1079–1083. https://doi.org/10.1016/j.jmig.2015.05.007
Acknowledgements
This work was supported by a National Research Foundation of Korea grant funded by the Korea Government (NRF-2015R1A2A2A04003460). The funding agency had no role in the data collection, study design, or writing of the manuscript. Dr. W.J. Lee serves as a consultant for Meerecompany (Yongin, Republic of Korea). The other authors have no conflicts of interest or financial ties to disclose.
Funding
This work was supported by a National Research Foundation of Korea Grant, funded by the Korea Government (NRF-2015R1A2A2A04003460). The funding body did not have a role in the data collection, study design, or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JHL, SHC and CMK. WJL provided advice about robotic systems and the setting up of a clinical trial. The first draft of the manuscript was written by JHL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
This work was supported by a National Research Foundation of Korea Grant funded by the Korea Government (NRF-2015R1A2A2A04003460). The funding agency had no role in the data collection, study design, or writing of the manuscript. Dr. W. J. Lee serves as a consultant for Meerecompany (Yongin, Republic of Korea). He was not involved in the surgeries, data collection, or analyses. He provided advice about robotic systems and the setting up of a clinical trial. None of the other authors have conflicts of interest or financial ties to disclose.
Ethics approval
Severance Hospital Institutional Review Board approved the current human study protocol (No. 1–2016-0019).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (WMV 89538 kb)
Rights and permissions
About this article
Cite this article
Lim, J.H., Lee, W.J., Choi, S.H. et al. Cholecystectomy using the Revo-i robotic surgical system from Korea: the first clinical study. Updates Surg 73, 1029–1035 (2021). https://doi.org/10.1007/s13304-020-00877-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-020-00877-5