Abstract
Background
It is unclear whether near-infrared (NIR) light-induced indocyanine green (ICG) fluorescence can effectively identify, and thus permit the preservation of, parathyroid glands in bilateral axillo-breast approach (BABA) robotic thyroidectomy. This case–control study with a prospectively recruited consecutive series and a retrospectively selected control group assessed the usefulness of ICG with Firefly(R) technology to identify the parathyroid glands intraoperatively during BABA robotic thyroidectomy.
Methods
All consecutive patients (N = 22) who were scheduled to undergo BABA robotic thyroidectomy for papillary thyroid carcinoma in December 2013–August 2015 and met the study eligibility criteria were recruited prospectively. ICG fluorescence was used with the Firefly system (NIR illuminator: 805 nm; filter: 825 nm) integrated in the da Vinci Si robot system to identify the lower parathyroid glands. Parathyroid hormone levels were recorded on postoperative days 0, 1, 2, and 14. Propensity score matching was used to identify an age-, gender-, tumor size-, and operation type-matched group of control patients who underwent BABA robotic thyroidectomy without the Firefly system. The two groups were compared in terms of parathyroid-related outcomes.
Results
ICG fluorescence-mediated identification of the parathyroid and thyroid glands required on average (range) 203 ± 89 (125–331) and 207 ± 112 (130–356) s, respectively. The mean (range) fluorescence duration in these glands was 20.8 ± 6.0 (16.6–35.8) and 20.1 ± 7.3 (15.5–33.8) min, respectively. The ICG group had a significantly lower rate of incidental parathyroidectomy than the control group (0 vs. 15.9%, P = 0.048).
Conclusions
ICG with NIR light may feasibly and safely identify the parathyroid glands in BABA robotic thyroidectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The rate of thyroid cancer has risen more rapidly in the last decade than other endocrine malignancies [1]. Many centers now use radical operations such as total thyroidectomy and lobectomy to treat it [2]. Recently, many studies showed that new endoscopic and laparoscopic techniques of thyroid surgery are safe and feasible [3]. One of these new techniques is bilateral axillo-breast approach (BABA) endoscopic thyroid surgery. This technique was first described by Youn’s group in 2004 and was shown to have favorable oncological and esthetic outcomes when used to treat benign and malignant thyroid disease [4]. In 2008, Youn’s group reported BABA robotic thyroidectomy (BABA RoT), in which BABA thyroidectomy is combined with the da Vinci robotic system (Intuitive Surgical Inc., Sunnyvale, CA, USA) [5]. Their 2013 review of their 1026 consecutive cases of BABA RoT showed that, when compared to open thyroidectomy, this technique associated with similar surgical completeness rates and acceptable rates of complications [6].
Although thyroid malignancy is generally indolent, and thus associates with high overall survival and low recurrence rates after thyroidectomy, thyroidectomy associates with complications such as hypoparathyroidism and recurrent laryngeal nerve (RLN) palsy, which can seriously impair patient quality of life [7]. Many studies show that intraoperative nerve monitoring can markedly improve RLN preservation rates [8]. However, there remains substantial debate on how to prevent hypoparathyroidism after thyroidectomy. Several studies show that approximately 50% of patients who undergo total thyroidectomy develop transient hypoparathyroidism; this results in many symptoms and the need for medications and calcium and vitamin D supplementation [9]. Moreover, 1–5% of patients develop permanent hypoparathyroidism after total thyroidectomy, which greatly impairs their long-term quality of life [10]. In the past, surgeons sought to avoid these problems by carefully identifying the parathyroid glands via anatomical dissection using landmarks such as the inferior thyroidal artery and RLN; this approach naturally requires considerable surgical experience [11]. More recently, various agents such as methylene blue, indocyanine green (ICG), and 99 m Technetium sestamibi (MIBI) are being used to detect the parathyroid glands [12–14].
ICG has long been used for liver excretory function testing [15]. Currently, it is also being used in a variety of other fields, such as to evaluate vessel patency after anastomosis or for fluorescence navigation to detect sentinel lymph nodes [16]. ICG provides information about deeper lying blood veins because it fluoresces under near-infrared (NIR) light, which penetrates deeply with a high signal-to-background ratio. This makes tissues seem more translucent, thus yielding good intraoperative images of well-vascularized structures [17]. Our recent canine model study showed that ICG under NIR is useful for identifying and preserving the parathyroid glands [18]. Furthermore, the Firefly system (Novadaq Technologies Inc., Mississauga, ON, CAN) (NIR illuminator: 805 nm/filter: 825 nm) integrated to the da Vinci Si robot system (Intuitive Surgical Inc., Sunnyvale, CA, USA) was introduced recently; this system makes it easier to use ICG fluorescence in robotic surgery [19].
At present, however, the usefulness of ICG fluorescence for identifying and preserving the parathyroid glands during thyroid surgery in humans remains poorly studied. To address this, the present case–control study evaluated the feasibility and safety with which ICG preserves the parathyroid glands in thyroid cancer patients who undergo BABA RoT.
Materials and methods
This case–control study of a prospective series and a retrospectively selected control group was approved by the Institutional Review Board of Seoul National University Bundang Hospital (no. B-1309/217-001) and was conducted in accordance with the principles of the Declaration of Helsinki and its revisions. All patients provided informed consent to undergo the procedure before surgery.
Patients
All consecutive patients who were diagnosed with papillary thyroid carcinoma (PTC) and were candidates for BABA RoT in Seoul National University Bundang Hospital between December 2013 and August 2015 were recruited prospectively. All operations were performed by a single endocrine surgeon. Patients who had a medical history of severe cardiovascular and respiratory disease, chronic kidney disease, cerebrovascular infarction, uncontrollable hypertension, diabetes mellitus, and/or drug allergy were excluded. In addition, patients younger than 20 years or older than 70 years and those who were pregnant were excluded.
A control group that matched the ICG group in terms of age, sex, tumor size, and surgery type (total thyroidectomy vs. thyroid lobectomy) was identified retrospectively using propensity score matching. There were two controls for every ICG group patient. All controls had undergone BABA RoT without ICG fluorescence and all satisfied the inclusion criteria listed above for the ICG group. All operations in the control group were conducted by the same surgeon who performed the operations in the ICG group.
The demographic and clinical characteristics of the patients in the two groups were recorded.
BABA RoT procedure
The surgical techniques in BABA RoT have been described in detail previously [20]. Briefly, the patient is placed in a supine position, and the neck is hyperextended by placing a pillow under the shoulders. To prevent flap bleeding and to dissect the soft tissues, hydro-dissection is performed by injecting saline-diluted epinephrine solution (1:200,000) into the subplatysmal layer of the neck and subcutaneously into the anterior chest using a long spinal tapping syringe. Thereafter, four skin incisions are made on the superomedial margins of both areolar plates and both axillary folds. After meticulous dissection with a subcutaneous tunneler, four trocars are placed at each port site (Fig. 1). The working space for robotic surgery is maintained by insufflating 5–6 mm Hg CO2. Once the da Vinci Si robot system is docked to the patient, the operator divides the isthmus using a harmonic scalpel. After ligating the middle thyroidal vein, the surgeon proceeds to dissecting the thyroid gland in the upward direction with ligation of the inferior thyroidal vessels and preservation of the RLNs and the superior and inferior parathyroid glands. Ipsilateral central lymph node dissection is performed after resection of the thyroid lobe in which the thyroid cancer is located. In the case of total thyroidectomy, contralateral lobectomy is performed in the same manner.
Skin flap for bilateral axillo-breast approach (BABA) robotic thyroidectomy. “v” indicates the thyroid cartilage, “+” the cricoid cartilage, and “u” the sternal notch. The black dotted line indicates the midline. The 12-mm trocar for the camera is placed at the right breast, while 8-mm trocars are placed at the left breast and both axillae
ICG injection
In the present study, the Firefly system was used to preserve the lower parathyroid gland in the ICG group. First, before surgery, ICG powder (25 mg) was dissolved by adding 10 cc of saline to the bottle (Fig. 2A). Thereafter, 4 cc of the ICG solution, which corresponds to 10 mg of ICG for 60 kg adult (0.17 mg/kg), was removed from the bottle using a syringe. As indicated by the use of ICG in the liver excretory function test, ICG doses of up to 30 mg are safe for a 60 kg adult. After dissecting the strap muscle from the thyroid, the ICG solution was administered via the patient’s intravenous line. After the injection, the Firefly system was turned on. The time to fluorescence onset in the parathyroid and thyroid glands, and the duration of fluorescence in each gland were recorded. The fluorescence pattern in the RLN was also assessed.
Photograph of the bottle containing the powdered form of indocyanine green (ICG) (A) and the intraoperative tissue parathyroid hormone (PTH) assay (B). A Before surgery, a vial of ICG (25 mg) is mixed with 10 cc of normal saline, after which 10 mg is delivered via the intravenous line. B The intraoperative tissue PTH assay is performed after parathyroid fluorescence is detected by aspirating the surface of the assumed parathyroid gland (white circle) with a spinal tapping needle engaged with 3 cc of normal saline
To ensure that the visualization of the parathyroid glands is not hampered by ICG leakage into the operative field due to vessel damage, the strap muscle dissection should be performed carefully to prevent bleeding from the surrounding structures, such as the strap muscles, thyroid capsule, and central lymph nodes. In addition, the ICG should be injected slowly to allow the parathyroid glands to be discriminated from the thyroid gland; if the injection is too fast, both glands will light up simultaneously and strongly.
To confirm that the structures being illuminated by the ICG injection really are parathyroid glands, nine patients underwent an intraoperative tissue parathyroid hormone (PTH) assay. Thus, using the robot arms, the surgeon aspirated the surface of the assumed parathyroid gland with a long spinal tapping needle attached to a syringe containing 3 cc of saline (Fig. 2B). The tissue fluid from the needle tip was then placed in a test bottle along with the saline and sent to the laboratory.
Definitions
PTH levels were recorded on postoperative days 0, 1, 2, and 14. Transient hypoparathyroidism after total thyroidectomy was defined as the drop in serum PTH levels below 15 pg/mL (normal range 15–65 pg/mL) and/or the need for oral calcium and vitamin D supplementation to maintain normocalcemia. Permanent hypoparathyroidism was defined as the drop in serum PTH levels below 15 pg/mL and the need for ongoing oral calcium supplementation beyond 1 year. Incidental parathyroidectomy was defined as the presence of parathyroid gland material in the resected specimens on histology.
Data and statistical analysis
The two groups were compared in terms of demographic and clinical variables using Student’s t test and Chi-square tests. The statistical package used for all analyses was SPSS ver. 18.0 software (SPSS Inc., Chicago, IL, USA). P values of <0.05 were considered to indicate statistical significance.
Results
Before the study commenced, a pilot study was performed to identify the ICG dose that would allow the best visualization of the parathyroid glands. Thus, 14 patients were recruited and assigned to seven groups of two. The seven groups received 1, 2.5, 5, 7.5, 10, 15, or 20 mg of ICG. The fluorescence patterns in the lower parathyroid gland were assessed by the operator. This study showed that 10 mg of ICG generated the best parathyroid gland visualization intensity and identification time (around 3 min after the ICG injection) for 60 kg adult (0.17 mg/kg). No operation-related complications were observed.
The demographic and clinical characteristics of the ICG and control groups are listed in Table 1. In total, 22 (1 male and 21 females) and 44 (3 males and 41 females) patients underwent BABA RoT with and without ICG, respectively. The mean (range) age of the two groups was 38.6 ± 7.8 (22–54) and 39.4 ± 8.0 (22–60) years, respectively, and their mean (range) body weights were 59.2 ± 9.6 (39.2–76.8) and 57.7 ± 9.2 (47.2–90.2) kg, respectively. In the ICG group, 11 patients underwent total thyroidectomy and the remaining 11 patients underwent thyroid lobectomy. In the control group, 20 patients underwent total thyroidectomy and 24 underwent lobectomy. There were no cases of conversion to open thyroidectomy. All ICG group patients and all but one of the control group patients had papillary thyroid carcinoma. The mean (range) tumor sizes were 0.95 ± 0.72 (0.3–3.6) and 0.99 ± 0.61 (0.2–3.5) cm, respectively.
Table 1 also shows the ICG injection variables of the ICG group. The mean (range) times between the injection and visualization of the inferior parathyroid and thyroid gland by ICG fluorescence were 203 ± 89 (125–331) and 207 ± 112 (130–356) s, respectively. The mean (range) durations with which ICG fluorescence was maintained in the inferior parathyroid and thyroid gland were 20.8 ± 6.0 (16.6–35.8) and 20.1 ± 7.3 (15.5–33.8) min, respectively. The average (range) operation time for each lobectomy was 27.3 ± 6.6 (20.2–37.0) min. In total, 32 parathyroid glands were targeted in the 22 ICG group patients for identification by ICG fluorescence under NIR light: All 32 were successfully visualized. One parathyroid gland was not identified during surgery and was not found in the specimen tissue. There is the possibility that parathyroid glands may be present in the thymus or other places.
In terms of postoperative complications, there were no cases of transient RLN palsy in the ICG group. However, four patients in the control group (9.1%) had transient RLN palsy. This difference did not achieve statistical significance (P = 0.145). None of the patients developed an allergic or anaphylactic reaction to the ICG intravenous injection. Table 2 shows the postoperative outcomes of the parathyroid glands. The ICG and control groups had similar rates of transient hypoparathyroidism (36.4 vs. 40%, P = 0.842) and permanent hypoparathyroidism (9.1 vs. 5%, P = 0.657). However, the ICG group had significantly lower rates of incidental parathyroidectomy (0 vs. 15.9%, P = 0.048). All incidental parathyroid glands were removed from lower parathyroid glands.
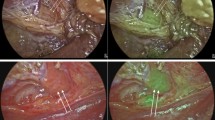
Figure 3 shows a photo of the inferior parathyroid gland and its subsequent fluorescence under NIR light after ICG injection. Around 3 min after the ICG injection, the lower parathyroid gland and the middle thyroidal vessel exhibited ICG uptake and thus could be seen in contrast to the surrounding tissues.
Photograph of the right inferior parathyroid gland (A) and its fluorescence after intravenous indocyanine green (ICG) administration (B). A The photograph shows the right inferior parathyroid gland (white circle) and the carotid sheath (white arrow). B After ICG administration, the right inferior parathyroid gland (white circle) and the middle thyroidal vein (white arrow) were visualized by focal and intense fluorescence 3 min after the injection. The surrounding tissues exhibited significantly less fluorescence
PTH assay
In the last nine patients of the ICG group, the intraoperative tissue PTH assay was performed on 13 assumed parathyroid glands. The assay showed that all 13 tissues were indeed parathyroid glands. Thus, there was 100% biochemical confirmation of the ICG-detected parathyroid glands. For reference, the structures surrounding the parathyroid glands, namely the subcutaneous fat, sternocleidomastoid muscle, and central lymph nodes, were also assessed by the tissue PTH assay. However, PTH was never detected in these samples.
Discussion
Up until 1850, the mortality rate after thyroid surgery was approximately 40% due to the high intraoperative bleeding and postoperative infection rates [21]. Subsequently, the innovations introduced by Theodor Kocher, who is considered to be the pioneer of modern thyroid surgery, made thyroid surgery a safe operation [22]. In 1996, Gagner reported the first case of endoscopic parathyroidectomy. This increased the demand for scarless surgery, leading to the emergence of many kinds of minimally invasive surgery techniques [23]. These include BABA RoT, which is now widely considered to be a safe and effective surgical method [3]. However, thyroid surgery continues to associate with various complications, particularly hypocalcemia due to parathyroid gland damage and voice change caused by nerve injury [7]. As a result, surgeons in recent years have aimed to avoid inducing hypocalcemia and RLN injury when performing total thyroidectomy [24]. The study by Youn’s group of their large consecutive series (n = 1026) shows that BABA RoT associates with good preservation of the RLN and the parathyroid glands [20]; in particular, the rates of temporary and permanent hypocalcemia after total thyroidectomy were 36 and 2%, respectively. This degree of surgical complication is acceptable compared to that seen with other methods [6].
ICG is the first fluorophore that was approved for clinical use by the US Food and Drug Administration. It is particularly useful because it has a short half-life of about 3–5 min and is excreted into the bile [18]. Moreover, under NIR light, ICG fluoresces, thus allowing the visualization of deep-lying vessels or tissues. This largely reflects the fact that NIR penetrates more deeply than visible wave lengths, thus causing the tissues to become more translucent [17]. ICG fluorescence has long been used for liver function testing and has since been adapted for other assays in humans such as oncology imaging [16]. We recently used a canine model to evaluate the safety of ICG and to identify the optimal ICG dose for NIR fluorescent imaging of the parathyroid gland [18]. We found that the time to peak fluorescent intensity after ICG injection was 50.2 s and that the estimated optimal ICG dose in dogs was 18.75 µg/kg [18]. This optimal ICG dose is equivalent to 1 mg of ICG for human patients who weigh 60 kg. However, in our initial tests, we found that, when a dose of 1 mg was used in human patients, it took more than 15 min after the injection to see the fluorescence in the parathyroid glands. Moreover, the fluorescence intensity was not strong enough to allow the parathyroid glands to be distinguished from the thyroid. These observations initially cast doubt on the usefulness of ICG in thyroidectomy, especially because a fluorescence onset time of 15 min could delay the operation. However, we speculated that the weak intensity of ICG and the delay in fluorescence were probably due to the fact that humans have a more complex vessel anatomy and different cardiac function and blood circulation times compared to dogs. Therefore, prior to our case–control study, we performed a pilot study to determine the optimal ICG dose in humans for detecting parathyroid glands. Thus, 14 patients were placed in seven groups of two that received ICG doses ranging from 1 to 20 mg. The 10-mg dose was found to be optimal for patients whose weight was around 60 kg (0.17 mg/kg): At this dose, ICG fluorescence of the parathyroid glands was observed on average 203 s after injection. This was on average 4 s earlier than when the thyroid glands started fluorescing. This was long enough to allow the parathyroid gland to be distinguished from the thyroid.
In the present study, ICG was mainly used to identify the lower parathyroid gland. The anatomical location of the lower parathyroid glands varies because they move downward with the thymus during embryogenesis. This anatomical variability often makes it difficult to identify the inferior parathyroid glands, which in turn renders these glands particularly vulnerable to injury during thyroid surgery. Thus, the Firefly technique is particularly suitable for identifying the lower parathyroid glands. While it can also be used to find the upper parathyroid glands, it may not be necessary because the upper parathyroid glands are commonly fixed in the upper part of Berry’s ligament. Another advantage of the Firefly technique is that the short clearance time of ICG means that the injection can be rapidly repeated for identifying the parathyroid glands in the contralateral side. In the present study, the mean duration of parathyroid fluorescence was around 20 min, which is shorter than the mean operation time needed for lobectomy (around 27 min). Thus, after the first lobectomy has been completed, 10 mg of ICG can be reinjected, after which the contralateral lobectomy can be performed. Thus, the use of the Firefly technique does not extend the operative time of the second lobectomy. Notably, we found that, even when patients received a total of 20 mg of ICG during surgery (i.e., in the patients who underwent total thyroidectomy), dye-related adverse effects were not observed, similar to the patients who underwent a single lobectomy.
This study showed that, when the Firefly technology was combined with BABA RoT, it improved the identification of the parathyroid glands: There were significantly fewer cases of incidental parathyroidectomy in the ICG group than in the control group (0 vs. 15.9%, P = 0.048). Notably, the Firefly technique also helps the surgeon to observe the vascular structure of the parathyroid glands after thyroid surgery since ICG fluorescence only appears and disappears in the orderly expected fashion when the vascular structures are well preserved. If the blood flow in the parathyroid gland has been disrupted by injury, the ICG fluorescence in the gland can be prolonged. Bleeding during surgery can also lead to spillage of ICG into the operative field. Thus, dissection should be performed carefully and gently using energy devices such as the harmonic scalpel, as this will reduce bleeding around the operative field. BABA RoT is particularly suitable for reducing bleeding because it involves precise and fine movement. It should be noted that the risk of bleeding is high when there is inflammation. Preoperative ultrasonography and blood tests such as those detecting microsomal antibodies can be used to predict whether inflammation is absent or present. We have confirmed that the rate of RLN palsy of control group was slightly higher than the study group (9.1 vs. 0%, P = 0.145). It may be concerned that the control group may have some other confounding factors such as surgeon skills. However, the study was conducted by a single surgeon who was expert, and all studies have been conducted after the learning curve. Further large sized study may be needed for confirm the rate of RLN palsy.
The first limitation of the study is that the patients had a small mean size of cancer (0.95 cm). This many not be generalizable to those with large multinodular goiter or those with larger tumors or gross lymph node metastasis. In addition, the small sample size of this study and the fact that the control patients were selected retrospectively were limitations of this study. However, the control group patients were selected by propensity score matching analysis, which may reduce selection bias. Further prospective randomized controlled trials are needed to confirm the study observations. Nevertheless, to the best of our knowledge, this is the first clinical study to assess the usefulness of ICG fluorescence imaging under NIR light for identifying and preserving the parathyroid glands in humans undergoing BABA RoT. The study results may be helpful when establishing protocols for the identification and preservation of the parathyroid glands in BABA RoT.
In conclusion, the integration of the Firefly technique, namely intraoperative NIR imaging of ICG, into the da Vinci Si robot system allowed the successful and safe identification and preservation of the inferior parathyroid glands. While many studies have assessed the ability of various methods to reduce the rate of parathyroid injury-related complications in thyroid surgery, standard techniques have still not been established in this field. The present study suggests that ICG fluorescence imaging under NIR light may effectively and safely help to preserve parathyroid glands in BABA RoT.
References
Fallahi P, Giannini R, Miccoli P, Antonelli A, Basolo F (2014) Molecular diagnostics of fine needle aspiration for the presurgical screening of thyroid nodules. Curr Genom 15:171–177. doi:10.2174/1389202915999140404100347
Schneider DF (2012) New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin 29:997–1003. doi:10.1016/j.biotechadv.2011.08.021.Secreted
Lee KE, Choi JY, Youn Y-K (2011) Bilateral axillo-breast approach robotic thyroidectomy. Surg Laparosc Endosc Percutan Tech 21:230–236. doi:10.1097/SLE.0b013e31822d0455
Choe JH, Kim SW, Chung KW, Park KS, Han W, Noh DY, Oh SK, Youn YK (2007) Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg 31:601–606. doi:10.1007/s00268-006-0481-y
Lee KE, Rao J, Youn Y-K (2009) Endoscopic thyroidectomy with the da Vinci robot system using the bilateral axillary breast approach (BABA) technique: our initial experience. Surg Laparosc Endosc Percutan Tech 19:e71–e75. doi:10.1097/SLE.0b013e3181a4ccae
Lee KE, Kim E, Koo DH, Choi JY, Kim KH, Youn YK (2013) Robotic thyroidectomy by bilateral axillo-breast approach: review of 1026 cases and surgical completeness. Surg Endosc Other Interv Tech 27:2955–2962. doi:10.1007/s00464-013-2863-1
Christou N, Mathonnet M (2013) Complications after total thyroidectomy. J Visc Surg 150:249–256. doi:10.1016/j.jviscsurg.2013.04.003
Dionigi G, Lombardi D, Lombardi CP, Carcoforo P, Boniardi M, Innaro N, Chiofalo MG, Cavicchi O, Biondi A, Basile F, Zaccaroni A, Mangano A, Leotta A, Lavazza M, Calò PG, Nicolosi A, Castelnuovo P, Nicolai P, Pezzullo L, De Toma G, Bellantone R, Sacco R (2014) Intraoperative neuromonitoring in thyroid surgery: a point prevalence survey on utilization, management, and documentation in Italy. Updates Surg 66:269–276. doi:10.1007/s13304-014-0275-y
Reeve T, Thompson NW (2000) Complications of thyroid surgery: how to avoid them, how to manage them, and observations on their possible effect on the whole patient. World J Surg 24:971–975. doi:10.1007/s002680010160
Adler JT, Sippel RS, Schaefer S, Chen H (2008) Preserving function and quality of life after thyroid and parathyroid surgery. Lancet Oncol 9:1069–1075. doi:10.1016/S1470-2045(08)70276-6
Delbridge L (2003) Total thyroidectomy: the evolution of surgical technique. ANZ J Surg 73:761–768. doi:10.1046/j.1445-2197.2003.02756.x
Patel HP, Chadwick DR, Harrison BJ, Balasubramanian SP (2012) Systematic review of intravenous methylene blue in parathyroid surgery. Br J Surg 99:1345–1351. doi:10.1002/bjs.8814
Zaidi N, Bucak E, Yazici P, Soundararajan S, Okoh A, Yigitbas H, Dural C, Berber E (2016) The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 113:775–778. doi:10.1002/jso.24237
Nichols KJ, Tronco GG, Palestro CJ (2015) Effect of reconstruction algorithms on the accuracy of 99 m Tc sestamibi SPECT/CT parathyroid imaging. Am J Nucl Med Mol Imaging 5:195–203
Halle BM, Poulsen TD, Pedersen HP (2014) Indocyanine green plasma disappearance rate as dynamic liver function test in critically ill patients. Acta Anaesthesiol Scand 58:1214–1219. doi:10.1111/aas.12406
Marshall MV, Rasmussen JC, Tan I, Aldrich MB, Kristen E, Wang X, Fife CE, Maus EA, Smith LA, Eva M (2012) Near-infrared fluorescence imaging in humans with indocyanine green: a review and update. Open Surg Oncol J 2:12–25. doi:10.2174/1876504101002010012.Near-Infrared
Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo P (2012) A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. doi:10.1155/2012/940585
Suh YJ, Choi JY, Chai YJ, Kwon H, Woo JW, Kim SJ, Kim KH, Lee KE, Lim YT, Youn YK (2015) Indocyanine green as a near-infrared fluorescent agent for identifying parathyroid glands during thyroid surgery in dogs. Surg Endosc Other Interv Tech 29:2811–2817. doi:10.1007/s00464-014-3971-2
Gudeloglu A, Brahmbhatt JV, Parekattil SJ (2014) Robotic-assisted microsurgery for an elective microsurgical practice. Semin Plast Surg 28:11–19. doi:10.1055/s-0034-1368162
Lee KE, Koo DH, Kim SJ, Lee J, Park KS, Oh SK, Youn YK (2010) Outcomes of 109 patients with papillary thyroid carcinoma who underwent robotic total thyroidectomy with central node dissection via the bilateral axillo-breast approach. Surgery 148:1207–1213. doi:10.1016/j.surg.2010.09.018
Mitreci MZ, Kaplan EL, Gaz RD, Slough CM, Bura M, Romanchishen AF, Martinac M GWR (2012) Surgery of the thyroid and parathyroid glands, 2nd edn
Chiesa F (2009) The 100 years anniversary of the Nobel Prize Award winner Emil Theodor Kocher, a brilliant far-sighted surgeon. Acta Otorhinolaryngol Ital 29:289
Gagner M (1996) Endoscopic subtotal parathyroidectomy in patients with primary hyperparathyroidism. Br J Surg 83:875
Hauch A, Al-Qurayshi Z, Randolph G, Kandil E (2014) Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann Surg Oncol. doi:10.1245/s10434-014-3846-8
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (Grant number: 2015R1C1A1A01055464).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Hyeong Won Yu, Joon Woo Chung, Jin Wook Yi, Ra-Yeong Song, Joon-Hyop Lee, Hyungju Kwon, Su-jin Kim, Young Jun Chai, June Young Choi, and Kyu Eun Lee have no conflicts of interest or financial ties to disclosure.
Additional information
Hyeong Won Yu and Joon Woo Chung have contributed equally to this article as co-first authors.
Rights and permissions
About this article
Cite this article
Yu, H.W., Chung, J.W., Yi, J.W. et al. Intraoperative localization of the parathyroid glands with indocyanine green and Firefly(R) technology during BABA robotic thyroidectomy. Surg Endosc 31, 3020–3027 (2017). https://doi.org/10.1007/s00464-016-5330-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5330-y