Abstract
Background
Surgical management of left colonic cancer presenting as an acute obstruction remains controversial and still is associated with high mortality and morbidity rates. Recently, self-expandable metallic stents (SEMS) have been used as a bridge to surgery in an attempt to decompress the colon and then allow elective one-stage surgical resection without stoma placement. This study aimed to compare the outcomes of emergency surgery alone with emergency placement of colonic SEMS as a bridge to surgery in terms of efficiency and reduction of the stoma placement rate.
Methods
A multicenter prospective, randomized, controlled trial was conducted according to the consolidated standards of reporting trials (CONSORT) Statement criteria. Patients eligible for the study were randomized to either emergency surgery or emergency SEMS as a bridge to surgery. The primary outcome was the need for a stoma (temporary or permanent) for any reason. The secondary end points were mortality, morbidity, and length of hospital stay.
Results
Nine centers participated in the trial. Among the 70 patients eligible for the study, 60 were randomized and included for the final analysis, 30 patients in each group. Seven patients were randomized but did not fulfill the entry requirements, whereas three further eligible patients were not randomized for various reasons. Concerning the primary outcome, 17 patients in the surgery group sustained a stoma placement versus 13 patients in the SEMS group (p = 0.30). No statistically significant difference was noted concerning the secondary outcomes. A total of 16 attempts at SEMS placement (53.3%) were technical failures. Two colonic perforations directly related to the stent placement procedure occurred among the 30 randomized patients and 1 perforation occurred among the nonrandomized patients, leading to premature closure of inclusions in the study before the expected number of 80 patients was reached.
Conclusion
This randomized trial failed to demonstrate that emergency preoperative SEMS for patients presenting with acute left-sided malignant colonic obstruction could significantly decrease the need for stoma placement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Surgical management of malignant large bowel obstruction remains controversial, particularly for left-sided tumors. In an emergency setting, several strategies are offered to the surgeon including the so-called two-stage surgery involving primary resection with colostomy (i.e., Hartmann’s procedure) or proximal colostomy followed by resection and one-stage surgery involving primary resection with anastomosis.
A Cochrane systematic review [1] failed to determine the best evidence-based approach due to the limited number of surgical trials in this field and the weakness of their methodology. Another systematic review including both randomized and nonrandomized studies [2] suggested that one-stage surgery could be superior.
Whatever the strategy chosen, the postoperative course remains poor for many patients undergoing emergency surgery. Findings have shown emergency surgery to be an independent factor of mortality and morbidity [3, 4], and about two-thirds of patients end up with a permanent stoma [5, 6].
Recently, experience accumulated with colonic endolumenal stenting for large bowel obstruction [7–10] (treating the obstruction and then allowing subsequent elective single-stage surgery) has led to consideration of self-expanding metal stents (SEMS) used as a potential bridge to surgery. According to a pooled review of 54 uncontrolled series of SEMS placement, including palliative indications, SEMS might be a useful option that avoids colostomy and facilitates safer single-stage surgery [11] with a clinical success rate of 84% to 94%, a perforation rate of 4%, a stent migration rate of 12%, and a re-obstruction rate of 7%.
More recently, a systematic review including 88 studies (15 of which were comparative) [12] and another smaller systematic review [13] have suggested that SEMS insertion is safe and effective, but the validity of these findings is limited by the small sample sizes. Currently, little high-level evidence is available, with only one published randomized trial comparing emergency surgery and SEMS insertion followed by elective surgery [14]. Two protocols of randomized trials also have been published [15, 16], but no results have been reported to date.
The main objective of the current trial was to determine whether the strategy of inserting a SEMS as a bridge to surgery could decrease the need for a stoma compared with emergency surgery. Secondary objectives involved analyzing the effectiveness of the two respective strategies in terms of mortality and morbidity.
Methods
Study design and participants
All consecutive patients presenting with confirmed acute left-sided malignant large bowel obstruction in nine French academic hospitals were asked for informed consent to be included in this trial. After consent was obtained, the patients were randomized to undergo either emergency surgery (standard treatment) or SEMS insertion as a bridge to surgery.
Inclusion criteria
The inclusion criteria required that patients be older than 18 years, fit for both emergency surgery and colonic stenting, and presenting with obstructive symptoms, dilation of the colon, and typical abnormalities confirmed by water-soluble contrast enema, computed tomography (CT) scan, or findings at colonoscopy suggesting left-sided malignant obstruction. Eligibility for the study required that the primary tumor be located between (including) the splenic flexure and the rectosigmoid junction.
Exclusion criteria
The exclusion criteria ruled out patients presenting with obstruction located proximal to the splenic flexure or distal to the rectosigmoid junction who had symptoms suggesting bowel perforation (particularly a cecal diameter exceeding 12 cm), other septic symptoms, abdominal tenderness, spontaneous pneumoperitoneum, adjacent small bowel involvement, or stage 4 tumors. Patients younger than 18 years, pregnant, unfit for either strategy, or lacking informed consent also were not eligible for the study.
Patient demographics and the p-POSSUM score (Physiologic and Operative Severity Score for the enUmeration of Mortality and Morbidity) [17] were recorded for all patients preoperatively, but the p-POSSUM score was not considered as an exclusion criterion. Morbidity events were defined as complications leading to an extended hospital stay or rehospitalization. After randomization, all patients were followed by the surgical team, and data were collected even in the case of protocol violation or secondary exclusion for whatever reason (i.e., nonmalignant obstruction, secondary contraindication for the allocated strategy).
Randomization
Due to the two obvious strategies under evaluation, it was not possible to ensure any blinding. Randomization was accomplished through a specific secured Web site allowing for online control of inclusion criteria as well as randomization (during the first day of admission) using computer-generated lists.
Surgical techniques and stenting procedures
Emergency surgery
Emergency surgery was performed through laparotomy. Because there is no formal consensus about the gold standard treatment in this setting, the choice of the procedure performed was left to the discretion of the surgeon.
The current options included one-stage procedure (i.e., colectomy, segmental or subtotal) with primary anastomosis (with or without intraoperative colonic washout); two-stage procedure (i.e., colectomy with terminal colostomy [Hartmann’s procedure] or colectomy with primary anastomosis and ileostomy or loop colostomy followed by colectomy and stoma closure); and three-sage procedure (i.e., loop colostomy followed by colectomy maintaining the protective colostomy, to be closed at an later date).
When the patient’s physical status and disease staging allowed further restoration of bowel continuity, this could be undertaken after discharge. Colostomy could be considered a permanent solution in case of high reoperative risk or patient choice.
Colonic stenting
All the stents used in this trial were Bard nitinol uncovered self-expanding stents (Voisins le Bretonneux, France). All physicians, radiologists, or endoscopists had to attest that they felt competent with stent placement before being selected in a participating center.
After the level of obstruction had been confirmed with a water-soluble contrast enema, the SEMS was placed along a guidewire through the lesion under radiologic or endoscopic guidance, as available at each center. Dilation of the obstructive lesion before the stent placement was forbidden. When the SEMS did not cover the entire length of the lesion, a second overlapping stent was placed. A further water-soluble contrast enema was performed to authenticate the accurate positioning of the stent and its efficacy in decompressing the colon.
After SEMS insertion, clinical examination was repeated to assess successful bowel decompression. Clinical success was defined as the resolution of obstructive symptoms and the production of flatus or stool within the first 3 postprocedure days. In case of failure (no resolution of obstruction symptoms), no further attempt was made, and the patient underwent surgery.
Candidates for elective surgery, after clinical success of the procedure, had to undergo surgery within the same hospitalization period. In this group, urgent unplanned surgery was indicated in case of technical failure of stenting, iatrogenic morbidity of SEMS (bowel perforation), or clinical failure, defined as a lack of bowel decompression within the first 3 postprocedure days. Regardless of the procedure outcome, patients were analyzed based on their initial randomization arm according to the intention-to-treat principle.
End points
Because of our hypothesis that SEMS could decrease the need for stoma creation, the main end point was a stoma constructed for any reason, whether temporary or definitive. The secondary end points of the study were in-hospital mortality, stent-related morbidity (i.e., bowel perforation), surgical morbidity including both wound complications (hematoma, infections, dehiscence) and intraabdominal complications (peritonitis, abscess, hemoperitoneum, anastomotic leak), extraabdominal morbidity (pulmonary infection, urinary infection, venous thromboembolism, cardiovascular or neurologic complications), and need for reoperation for whatever reason.
Data collection
An electronic Case Record Form based on the source paper form completed by the treating physician was built and systematically monitored by the main investigator. The data then were verified and collected in a Microsoft-Excel file and analyzed using the SEM software [18].
The current study was under the control of the Agence Française de Sécurité Sanitaire des Produits de Sante (143/147, Boulevard Anatole France 93285 Saint-Denis Cedex) together with the Délégation de la Recherche Clinique du Centre Hospitalier Universitaire de Montpellier (Professor Bertrand Millat, main investigator). As defined in the protocol, the trial had to be discontinued in case of major side effect events related to the device used for stenting according to reports made to the trial monitor.
Statistical analysis
Data analyses were accomplished on an intention-to-treat basis and analyzed using the SEM software [18]. The chi-square test was used to compare stoma and other qualitative variables (including the center effect) between groups. For quantitative variables, intergroup comparisons used the Student t-test or the Kruskal-Wallis H test depending on normality of distributions, equality of variances, or both. All p values less than or equal to 0.05 were considered statistically significant.
According to the nationwide French experience [4] and published literature [2], we assumed that approximately 50% of patients presenting with a left-sided malignant colonic obstruction would have a stoma created at first intention and that the other 50% would undergo one-stage emergency surgery, with 10% of these patients possibly having a diverting stoma created because of complications. Thus, 55% of the patients who underwent emergency surgery would potentially end up with a stoma.
On the other hand, at the beginning of the current trial, according our own nationwide French experience and published literature [9], we assumed that 20% of the patients who underwent SEMS insertion would eventually have a stoma created subsequent to technical or clinical failure or due to complications. We calculated that the study should enroll 80 patients to have 90% power to detect a 5% effect size using one-tailed analysis.
Ethical issues
The trial protocol was submitted to the Ethical Committee of the University Hospital of Montpellier and approved (acceptance on 11 December 2001). Informed consent had to be signed by the patients before their inclusion in the study. The trial was conducted according the principles of the Declaration of Helsinki [19].
Results
Flow chart
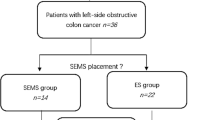
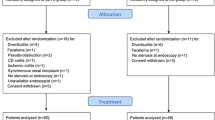
From December 2002 to October 2006, 70 consecutive patients presenting with left-sided malignant colonic obstruction were considered eligible for the trial. Three of these patients were not included because their treatment occurred before the randomization could take place (one primary surgery and two stent placements as bridges to surgery). Two randomized patients were excluded because of protocol violation. They did not have surgery after stent placement.
Of the remaining 65 randomized patients, 5 were not considered for further analysis: 3 because of a benign obstructive lesions and 2 because of a mistaken level of bowel obstruction (1 proximal to the splenic flexure and 1 distal to the rectosigmoid junction). In the final analysis, 60 patients were included in the trial, 30 in each group (Fig. 1).
Of the nine centers participating in this trial, one included 31 patients, two included 7 patients each, two included 4 patients each, two included 3 patients each, and one included 1 patient. Because the centers entered the study at different times, their durations of participation in the study were unequal.
Comparisons of the main outcome criteria between the center that included the largest number of patients (n = 31) (Rouen University Hospital) and the seven other centers did not show any center effect except a stoma rate (11/31 vs 17/29) with marginal significance (p = 0.073, Fisher’s exact test).
Characteristics of patients
The preoperative population demographics were not found to differ statistically in terms of age, body mass index (BMI), sex, tumor location, or p-POSSUM score (Table 1). The median time between the onset of obstructive symptoms and hospital admission was not statistically different between the two groups: 2 days (range, 0–6 days) in the surgery group versus 2 days (range, 0–5 days) in the SEMS group. On the other hand, the median times between the hospital admission and the initial procedure also were statistically equal: 1 day (range, 0–6 days) in the surgery group versus 2 days (range, 0–10 days) in the SEMS group (p = 0.18).
Main end point
Stoma placement was performed for 17 patients (57%) in the surgery group versus 13 patients (43%) in the SEMS group (p = 0.30). Of the 17 stomas placed in the surgery group, 16 were constructed at the initial operation, whereas 1 was indicated because of a colonic anastomotic leakage. Two of these stomas placed at first intention were closed during the same hospital stay.
In the SEMS group, 12 stoma were placed at first intention because of failure or complication of the procedure, whereas 1 was fashioned secondary to an ileorectal anastomotic leakage after subtotal colectomy.
Restoration of bowel continuity was obtained for nine patients (30%) in the surgery group and four patients (13%) in the SEMS group (p = 0.12). The median duration of stoma placement did not differ statistically between the surgery group (84 days; range, 5–638 days) and the SEMS group (96 days; range, 22–118 days) (p = 0.68).
SEMS placement outcomes
Radiologists performed 13 attempts and gastroenterologists 17 attempts at SEMS placement. The mean duration of the procedure was 61 ± 31 min (median, 52 min; range, 20–150 min).
For 14 patients (47%), SEMS insertion was technically successful. Subsequent bowel transit recovery was experienced by 12 patients (40%), whereas clinical failure of colonic decompression occurred for 2 patients (14%). One of these two patients had persisting obstruction symptoms, and one experienced immediate postprocedure symptoms of peritonitis due to cecal perforation after an endoscopic procedure.
The result for 16 attempts (53%) at SEMS placement was technical failure. The reason for the technical failure was inability to pass the stricture with the guidewire for 13 patients, malfunction of the stent delivery system for 1 patient, and observation of direct colonic perforation during the procedure for 2 patients (7%). Among the unsuccessful procedures, seven had been performed radiologically, whereas nine had been done endoscopically.
In the inclusion period, two bowel perforations occurred during the stenting procedures, in addition to one perforation in a nonrandomized patient. These major side effects, associated with the unexpected high rate of technical failures, led the steering committee to interrupt the trial after 65 patient inclusions.
Surgical outcomes
Surgery group
Various surgical strategies were carried out. One-stage procedures were achieved for 14 patients (47%). Two-stage surgery was completed for eight patients, whereas two patients underwent three-stage surgery. Bowel continuity was not restored in six patients (four proximal loop colostomies and two Hartmann procedures). The mean duration of surgery was 122.5 ± 48.4 min.
SEMS group
All 12 patients who underwent SEMS placement with both technical and clinical success had elective colonic resection and primary anastomosis, with a median interval time of 7 days (range, 5–19 days) between the two procedures. The postoperative course was uneventful for all 12 patients.
Among the remaining 18 patients in the SEMS group, the surgical strategy consisted of one-stage surgery for 6 patients, Hartmann procedure for 6 patients, colonic resection with protective stoma for 4 patients, and loop stoma placement for 3 patients (1 followed by colonic resection). All these surgical procedures took place within 24 h after failure of SEMS placement.
Overall, the colonic resection rate was similar in the surgery (87%) and SEMS (93%) groups (p = 0.7). No statistical difference was found between the two groups regarding the overall rate of primary anastomosis without leakage (43% in the surgery group vs 53% in the SEMS group; p = 0.45).
Secondary end points
Four patients died during the initial hospital stay. One patient in the surgery group died on the day of surgery (Hartmann procedure) of multivisceral failure. His preoperative p-POSSUM was calculated at 46. Three patients died in the SEMS group (1 from rapid progression of his neoplastic illness, 1 from mesenteric infarction, and 1 from septic shock and multivisceral failure after anastomotic leakage). These three patients had undergone initially unsuccessful SEMS placement due to impassable stricture along the guidewire. Their p-POSSUM scores were respectively 18, 22, and 17.
The overall postoperative complications are listed in Table 2. No statistically significant difference involving these items was observed.
Five patients had to undergo reoperation (2 in the surgical group and 3 in the SEMS group). The reasons were four anastomotic leaks with diffuse peritonitis (2 in each group) and one intraperitoneal abscess evacuation (for a patient allocated to the surgery group). None of these anastomotic leaks occurred after one-stage anastomosis subsequent to effective SEMS placement.
In addition to the aforementioned direct colonic perforations reported during SEMS placement procedures, eight pathology reports of the colonic resection specimen demonstrated clinically silent bowel perforation by the prosthesis. Most were described as perforations located at one or both extremities of the stent.
The median cumulative global duration of hospital stay was not statistically different between the two groups: 17 days; range, 7–126 days in the surgery group vs 23 days; range, 9–67 days in the SEMS group (p = 0.13).
Discussion
The main finding of the current trial was that no change occurred in the stoma rate whether a SEMS was placed or not. Our findings failed to confirm the findings to date from nonrandomized or retrospective studies or from studies with palliative intent suggesting a significant reduction in morbidity, mortality, and need for stoma placement when SEMS are inserted before surgery [11, 12, 20, 21].
More importantly, our findings are in contrast to those of the randomized trial published recently [14]. This trial from China was quite similar to our trial but included fewer patients (n = 48), in the stent group. The patients underwent subsequent laparoscopic resection, and significantly more one-stage surgery was performed in this group (compared with emergency open surgery). None of the patients in this group had a permanent stoma.
These favorable and impressive results are clearly in contrast to the results of the current trial. Thus, contradicting the Chinese experience [14], SEMS did not meet its goal as a bridge to surgery within a multicenter French experience.
The conflicting results from China and France (obtained during the same period, 2002–2006) highlights the need for further evidence-based evaluation of stenting as a bridge to surgery in the setting of further randomized trials or at least a further systematic quantitative review.
It is notable that all 12 patients who underwent the SEMS procedure with both technical and clinical success had elective colonic resection and primary anastomosis afterward, with an uneventful postoperative course. This finding could suggest that inserting the stent permitted an uncomplicated surgery. Another possible reading could be that the technical possibility of the SEMS placement itself reflects initial better conditions, thus followed by an easy surgical management.
Of ethical concern is the decision to discontinue the trial early because of the high number of major adverse events occurring in the SEMS group, namely, bowel perforation during the stent placement procedure. Similar findings were reported as reasons for the recent premature closure of a randomized study due to an unexpectedly high rate of stent-related morbidity [14, 22], although under different circumstances because that trial was conducted in a palliative setting, and perforations occurred later.
The number of technical failures (50%) in our trial also deserves comment. We intentionally selected patients with complete colonic obstruction proven on clinical and radiologic grounds, thereby restricting the inclusions to absolute acute situations. The acute condition probably is the key point of our trial. It might explain, despite the expertise of the participating physicians, the high rate of technical failure, particularly the recurrent difficulty passing the constrictive bowel lesion.
Although the reported time between the hospital admission and the initial procedure was equivalent between the two groups, it probably would have been interesting to record the time of the patient’s randomization. Indeed, this would have ignored a possible period preceding the definitive diagnosis and complete evaluation of the patient. Comparing the time between randomization of the patient and achievement of the procedure could obtain information about the practicality of these tactics for patients requiring a true urgent colonic decompression.
Besides the clinical perforations after stenting in our trial, another finding deserves comment, namely, the numerous (n = 8) histologic findings in our study of silent bowel perforations in the resected specimens related to the design of the stents. This matter of concern from an oncologic aspect raises the question of possible tumor dissemination after stenting [23].
Furthermore, care for the cost effectiveness of the SEMS procedure should constitute an additional concern, particularly if the method is proposed as a bridge to laparoscopic surgery. Our approach has in some way indirectly appraised the feasibility of both strategies and practical access to both, presuming a 24-h available skilled specialist in either domain and assuming the possible greater difficulty of stent placement compared with diverting stoma construction in this particular challenging condition. Obviously, further safety and efficacy evaluation of SEMS placement as a bridge to surgery is needed to assess its feasibility and expected benefits compared with surgical treatments.
The upcoming results of randomized controlled trials currently being conducted should hopefully continue to highlight the problem and assist the physician in selecting the best strategy when faced with this somewhat complex situation. In the future, particular attention needs to be paid to the clinical severity of malignant colonic obstruction and the time needed to obtain relief from this condition.
In conclusion, our study has shown that colonic stenting for acute malignant obstruction as a bridge to surgery is neither safer nor more effective for relief of obstruction than traditional surgery in the emergency setting. Further studies are necessary compare the carcinologic outcomes of the two reported strategies.
References
De Salvo GL, Gava C, Pucciarelli S, Lise M (2004) Curative surgery for obstruction from primary left colorectal carcinoma: primary or staged resection? Cochrane Database Syst Rev 2:CD002101
Breitenstein S, Rickenbacher A, Berdajs D, Puhan M, Clavien PA, Demartines N (2007) Systematic evaluation of surgical strategies for acute left-sided malignant colonic obstruction. Br J Surg 94:1451–1460
Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD, Association of Coloproctology of Great Britain, Ireland (2004) The Association of Coloprotology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg 240:76–81
Alves A, Panis Y, Mathieu P, Mantion G, Kwiatkowski F, Slim K, Association Française de Chirurgie (2005) Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg 140:278–283
Deans GT, Krukowski ZH, Irwin ST (1994) Malignant obstruction of the left colon. Br J Surg 81:1270–1276
Mealy K, O’Brian E, Donohue J, Tanner A, Keane FB (1996) Reversible colostomy: what is the outcome? Dis Colon Rectum 39:1227–1231
Dohmoto M (1991) New method: endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig 3:1507–1512
Tejero E, Fernandez Lobato R, Mainar A, Montes C, Pinto I, Fernández L et al (1997) Initial results of a new procedure for treatment of malignant obstruction of the left colon. Dis Colon Rectum 40:432–436
Baron TH, Dean PA, Yates MR III, Canon C, Koehler RE (1998) Expandable metal stents for the treatment of colonic obstruction: techniques and outcomes. Gastrointest Endosc 47:277–286
Camunez F, Eschenagusia A, Simo G, Turégano F, Vázquez J, Barreiro-Meiro I (2000) Malignant colorectal obstruction treated by means of self-expanding metallic stents: effectiveness before surgery and in palliation. Radiology 216:492–497
Sebastian S, Johnston S, Geoghegan S, Torreggiani W, Buckley M (2004) Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol 99:2051–2057
Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ (2007) Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg 246:24–30
Tilney HS, Lovegrove RE, Purkayastha S, Sains PS, Weston-Petrides GK, Darzi AW, Tekkis PP, Heriot AG (2007) Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc 21:225–233
Cheung HYS, Chung CC, Tsang WWC, Wong JCH, Yau KKK, Li MKW (2009) Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer. Arch Surg 144:1127–1132
van Hooft JE, Bemelman WA, Breumelhof R, Siersema PD, Kruyt PM, van der Linde K et al (2007) Colonic stenting as bridge to surgery versus emergency surgery for management of acute left-sided malignant colonic obstruction: a multicenter randomized trial (stent-in 2 study). BMC Surg 7:7–12
Anthony T, Baron T, Mercadante S, Green S, Chi D, Cunningham J et al (2007) Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manag 34:S49–S59
Tekkis PP, Kocher HM, Bentley AJ, Cullen PT, South LM, Trotter GA, Ellul JP (2000) Operative mortality rates among surgeons: comparison of POSSUM and p-POSSUM scoring systems in gastrointestinal surgery. Dis Colon Rectum 43:1528–1532
Kwiatkowski F, Girard M, Hacene K, Berlie J (2000) SEM: a suitable statistical software adapted for research in oncology. Bull Cancer 87:715–721
World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. http://www.wma.net/en/30publications/10policies/b3/17c.pdf. Accessed 30 March 2010
Khot UP, Wenk Lang A, Murali K, Parker MC (2002) Systematic review of the efficacy and safety of colorectal stents. Br J Surg 89:1096–1102
Martinez-Santos C, Lobato RF, Fradejas JM, Pinto I, Ortega-Deballon P, Moreno-Azcoita M (2002) Self-expandable stent before elective surgery vs emergency surgery for the treatment of malignant colorectal obstructions: comparison of primary anastomosis and morbidity rates. Dis Colon Rectum 45:401–406
Van Hooft JE, Fockens P, Marinelli A, Bossuyt PM, Bemelman WA (2006) Premature closure of the Dutch Stent-in I study. Lancet 368:1573–1574
Maruthachalam K, Lash GE, Shenton BK, Horgan AF (2007) Tumour cell dissemination following endoscopic stent insertion. Br J Surg 94:1151–1154
Acknowledgments
The metallic stent devices were provided free of charge by BARD France SAS. Financial support was provided by the hospital clinical research program (PHRC), Montpellier University Hospital and by the A.F.S.S.A.P.S. (Agence Française de Sécurité sanitaire des produits de santé). The study was performed by the cooperation of the following investigators: D. Collet (Digestive Surgery Unit), J. Drouillard (Medical Imaging Unit), Bordeaux University Hospital, France; F. Michot, O. Foulatier (Digestive Surgery Unit), E. Lerebours, P. Ducrotte, M. Antonietti, E. Bensoussan (Gastroenterology Unit), Rouen University Hospital, France; J. P. Triboulet, C. Mariette (Digestive Surgery Unit), O. Ernst (Digestive and Endocrine Imaging Unit), Lille University Hospital, France; B. Suc, F. Muscari (Digestive Surgery Unit), P. Otal (Medical Imaging Unit), Toulouse University Hospital, France; B. Meunier (Digestive Surgery Unit), S. Manfredi (Gastroenterology Unit) Rennes University Hospital, France; J. P. Arnaud (Digestive Surgery Unit), C. Aubé, C. Ridereau (Medical Imaging Unit), Angers University Hospital, France; C. Letoublon, E. Desroche (Digestive Surgery Unit), C. Sengel (Medical Imaging Unit), Grenoble University Hospital, France; J. M. Hay, S. Msika (Digestive Surgery Unit), M. Levesque (Medical Imaging Unit), University Hospital Louis Mourrier, Colombes, France; P. Puche, P. M. Blanc, H. Bouyabrine (Digestive Surgery Unit), B. Gallix (Medical Imaging Unit), A. Mouraret, C. Lacombe (Clinical Research Associates), Montpellier University Hospital. The protocol has been registered in ClinicalTrials.gov with the identifier number NCT00514332.
Disclosures
Isabelle A. Pirlet, Karem Slim, Fabrice Kwiatkowski, Francis Michot, and Bertrand L. Millat have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pirlet, I.A., Slim, K., Kwiatkowski, F. et al. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc 25, 1814–1821 (2011). https://doi.org/10.1007/s00464-010-1471-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-010-1471-6