Abstract
Background
The purpose of this study was to evaluate the associations between patient characteristics or surgical site classifications and the histologic remodeling scores of synthetic meshes biopsied from their abdominal wall repair sites in the first attempt to generate a multivariable risk prediction model of non-constructive remodeling.
Methods
Biopsies of the synthetic meshes were obtained from the abdominal wall repair sites of 51 patients during a subsequent abdominal re-exploration. Biopsies were stained with hematoxylin and eosin, and evaluated according to a semi-quantitative scoring system for remodeling characteristics (cell infiltration, cell types, extracellular matrix deposition, inflammation, fibrous encapsulation, and neovascularization) and a mean composite score (CR). Biopsies were also stained with Sirius Red and Fast Green, and analyzed to determine the collagen I:III ratio. Based on univariate analyses between subject clinical characteristics or surgical site classification and the histologic remodeling scores, cohort variables were selected for multivariable regression models using a threshold p value of ≤0.200.
Results
The model selection process for the extracellular matrix score yielded two variables: subject age at time of mesh implantation, and mesh classification (c-statistic = 0.842). For CR score, the model selection process yielded two variables: subject age at time of mesh implantation and mesh classification (r 2 = 0.464). The model selection process for the collagen III area yielded a model with two variables: subject body mass index at time of mesh explantation and pack-year history (r 2 = 0.244).
Conclusion
Host characteristics and surgical site assessments may predict degree of remodeling for synthetic meshes used to reinforce abdominal wall repair sites. These preliminary results constitute the first steps in generating a risk prediction model that predicts the patients and clinical circumstances for which non-constructive remodeling of an abdominal wall repair site with synthetic mesh reinforcement is most likely to occur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Analyses from national administrative databases estimate that 365,400 ventral hernia repairs were performed in the US in 2006 [1], reaffirming ventral hernia repair as one of the most common procedures in general surgery. The vast majority of these ventral hernias were repaired with reinforcement materials, given level A/B evidence supporting reinforcement with synthetic or biologic repair materials for all incisional ventral hernias to reduce recurrence [2, 3]. Of these available reinforcement materials, synthetic mesh was used for the majority of tension-free ventral hernia repairs, thus driving a $159.5 million market in the US in 2006 [4].
Although synthetic materials provide strong tissue repairs, these materials have been found to induce polymer-dependent inflammatory responses [5, 6]. The robustness of the inflammatory response to synthetic materials may vary widely, dependent not only on the polymer type and the foreign body burden, but also on the innate and acquired characteristics of the host [5, 7]. Furthermore, bacterial pathogens in the tissue milieu avidly adhere to synthetic polymers and synthesize biofilms. Chronic contamination or infection of the tissue repair site may result as these biofilms resist host immunologic defenses and avert hematogenous delivery of antibiotics to the tissue [8]. Furthermore, not all synthetic scaffolds are composed of inert materials. When exposed to oxidizing agents such as hydrogen peroxide and hypochlorous acid during neutrophil-mediated pathogen clearance, polypropylene fibers may degrade and become brittle [9]. Thus, the mesh composition and configuration, the tissue microenvironment of the surgical site, and the clinical characteristics of the host may each significantly influence host tissue incorporation of synthetic meshes and ultimately the long-term success rate of the abdominal wall repair.
The host tissue response to these materials is essential to the constructive tissue remodeling of soft tissue repair sites reinforced with synthetic mesh. While both proinflammatory (M1) and immunomodulatory and remodeling (M2) macrophages play pivotal roles in the host response to synthetic mesh, a high ratio of M2:M1 macrophages has been shown to favor constructive tissue remodeling over chronic inflammation [10]. Simultaneous tissue remodeling through cellular infiltration, host deposition of collagen and other extracellular matrix components, and neoangiogenesis gradually occur. Host fibroblasts that successfully infiltrate the scaffold will proliferate and secrete structural proteins of the extracellular matrix. Among these extracellular matrix proteins, the predominant collagen type gradually transitions from collagen III to collagen I during the process of constructive soft tissue remodeling. Collagen I confers greater mechanical strength to tissue than collagen III, and low ratios of collagen I:collagen III in soft tissue repair sites have been associated with failure of both initial and repeat soft tissue repairs [11, 12]. Host endothelial cells migrate from nearby existing blood vessels and organize to form new blood vessels in the soft tissue repair site. When a high ratio of M1:M2 macrophages occurs in soft tissue repair sites, the elicitation of a strong M1 macrophage inflammatory response can instead lead to fibrous encapsulation of the scaffolds and restricted cellular infiltration and neovascularization [10].
In addition to the tissue milieu and the innate immune response of the host, acquired characteristics of the host such as age, body mass index (BMI), diabetes mellitus, and smoking status are well-described risk factors for poor wound healing and may play a significant role in this perturbation of host tissue incorporation of synthetic meshes and soft tissue repair failure. Furthermore, pathogen burden of the surgical site can further inhibit constructive tissue remodeling and contribute to high rates of repair failure. Contaminated or infected hernia repair sites reinforced with synthetic meshes have demonstrated recurrence rates as high as 40 % [13]. Given the relatively high morbidity for complex abdominal wall reconstruction, repeat surgical repair of the abdominal wall presents the opportunity to procure biopsies of the previously-implanted synthetic mesh reinforcement materials. Histologic analysis of the synthetic meshes biopsied from the abdominal wall reconstruction sites of human subjects at the time of a clinically-indicated abdominal re-exploration can provide a wealth of information.
Despite the well-described role of the host tissue response to the constructive remodeling of soft tissue repair sites reinforced with synthetic meshes [14–28], statistical modeling of the influence of host comorbidities and wound characteristics on the remodeling has not previously been described. A risk prediction model that reliably predicts the patients and clinical circumstances for which non-constructive remodeling of an abdominal wall repair site with synthetic mesh reinforcement is most likely to occur would be a useful aid in clinical decision making. Thus, the purpose of this study was to evaluate the multivariable associations of patient characteristics and surgical site classifications to the histologic remodeling scores of synthetic meshes biopsied from the abdominal soft tissue repair sites of patients in the first attempt to generate a risk prediction model of non-constructive tissue remodeling. We hypothesized that higher collagen type I:III ratios and more favorable histologic remodeling scores of explanted meshes would directly correlate with indwelling duration of mesh, and inversely correlate with subject age at the time of mesh implantation, diabetes status, tobacco use, corticosteroid use, BMI, and Centers for Disease Control and Prevention (CDC) wound classification assessed at the time of both scaffold implantation (T1) and explantation (T2).
Materials and methods
Patient selection and specimen collection
The study protocol was approved by the Human Research Protection Office (HRPO) at Washington University, St. Louis [Institutional Review Board (IRB) Number 201012735], and meets the corresponding ethical guidelines for human research conduct. The study was also registered with clinicaltrials.gov (registration number NCT01880021). Fifty-one subjects with synthetic mesh implanted during a previous abdominal wall reconstruction and subsequently scheduled for a clinically-indicated abdominal re-exploration were identified and consented for the study between March 2008 and December 2012. Specimens of the synthetic meshes were harvested during the clinically-indicated abdominal re-exploration. Specimens were preserved in 10 % formalin, embedded in paraffin, sectioned to 5 μm, and stained with hematoxylin and eosin and Sirius Red/Fast Green.
Remodeling characteristics
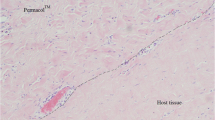
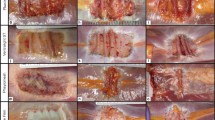
Hematoxylin and eosin-stained slides were evaluated for the degree of six remodeling characteristics, including cellular infiltration, cell types, host extracellular matrix deposition, inflammation, fibrous encapsulation, and neovascularization. A single slide of each specimen was evaluated under light microscopy at 100× magnification by a pathologist using a semi-quantitative scoring system adapted from Valentin et al. [29] for synthetic mesh implants and presented previously [30, 31]. Assigned to each specimen was a single score for each remodeling characteristic, ranging in value from 0 to 3, with higher scores representing more favorable remodeling characteristics (Table 5). A composite remodeling score was then calculated as the mean of the six component remodeling scores for each specimen.
Collagen distribution
Sirius Red/Fast Green-stained slides were prepared and evaluated according to methods presented previously [32]. In brief, Sirius Red stains collagen fibers, whereas Fast Green stains non-collagenous proteins for contrast. Under polarized light, Sirius Red-stained collagen I fibers appear bright red, while Sirius Red-stained collagen III fibers appear pale green. Each slide was photographed under cross-polarized light using an Axioskop 40® microscope (Carl Zeiss®, Thornwood, NY, USA) equipped with a Zeiss Axiocam® at a magnification of 400× (n = 10 photographs per specimen). Axiovision 4.7® (Zeiss®) software was utilized to semi-quantitatively evaluate both areas (μm2) that appeared red under cross-polarized light (for collagen I) and the areas that appeared green under cross-polarized light (for collagen III) on each slide. A collagen I:III ratio was then calculated.
Variables
The dependent variables selected for this study are histologic scores for the assessment of constructive synthetic mesh remodeling. The composite histologic remodeling score and its six component scores describe the degree of cellular infiltration, cell types, extracellular matrix deposition, inflammation, fibrous encapsulation, and neovascularization of the specimens [30, 31]. The Sirius Red-stained area evaluated under cross-polarized light further quantifies the collagen I surface area, collagen III surface area, and collagen I:III ratio of the specimens [32].
The independent variables selected for investigation were as follows, with some data ascribed at the time of scaffold implantation (T1) to assess the potential contribution of baseline host and surgical site characteristics, and at the time of scaffold explantation (T2) to assess the potential contribution of host and surgical site characteristics acquired during the period of scaffold indwelling: mesh classification (uncoated permanent synthetic mesh, permanent synthetic mesh with an absorbable adhesion barrier, composite or non-composite permanent synthetic mesh with a permanent adhesion barrier, or uncoated absorbable synthetic mesh); gender (male or female); race (Caucasian or non-Caucasian); mean age at T1 (years); median duration of in vivo scaffold dwelling (days); diabetes mellitus diagnosis status (diabetic or non-diabetic); smoking history (positive or negative history of ever being a tobacco smoker); smoking status (never smoked, quit 30 days before T1 without resumption, quit 30 days before T2 without resumption, or current smoker); pack-year history (median pack-years); corticosteroid use (positive or negative history of ever using corticosteroids); mean BMI (kg/m2) at T1 and T2; and CDC wound class at T1 and T2 (clean or clean-contaminated/contaminated/infected) [33, 34]. Note that the race variable was dichotomized to Caucasian or non-Caucasian because the small sample size and the racial homogeneity of the subject population did not allow for further distinction. Similarly, the CDC wound class variable was dichotomized to clean or ‘not clean’ (clean-contaminated/contaminated/infected) because the small sample size and data distribution for the wound class variable did not allow for further distinction.
Independent variable data for the following variables were abstracted from the medical record by several trained co-investigators (JAC, JO, JC, SB): mesh type, gender, race, age at T1, duration of in vivo scaffold dwelling, diabetes mellitus diagnosis status, smoking history, smoking status, pack-year history, corticosteroid use, and BMI at T1 and T2. Subjects were presumed to be non-diabetic if the medical record did not assign a diagnosis of diabetes mellitus type 1 or type 2. Similarly, subjects were presumed to have never smoked tobacco or never used corticosteroids if the medical record reported neither previous nor current use of tobacco or corticosteroids, respectively. If the medical record reported a previous history of tobacco smoking or corticosteroid use but did not report discontinuation, the subject was assumed to be a current tobacco smoker or user of corticosteroids, respectively. Using surgical site descriptions and data abstracted from the medical record, two trained co-investigators (JAC and BDM) independently assessed the surgical site environment according to previously established definitions [33, 34], then reached consensus by discussion of any discrepant assessments before assigning the final CDC wound class at T1 and T2 for each subject.
Study data were managed through a customized electronic database using Research Electronic Data Capture® (REDCap®) tools hosted at Washington University, St. Louis [35]. REDCap® is a secure, web-based application designed to support electronic data capture for research studies. REDCap® provided an intuitive interface for validated data entry, audit trails for tracking data manipulation, and automated export procedures for data downloads to statistical packages.
Statistical analysis
Data from the REDCap® study database were securely exported to Statistical Analysis System® version 9.3 (SAS Institute, Inc.®; Cary, NC, USA) to perform all statistical analyses. The following dependent variables were analyzed as continuous variables: composite remodeling score, collagen I area, collagen III area, and collagen I:III ratio. Given the low sample size and sparseness of the data, the following dependent variables with greater than two ordinal categories were collapsed based on the data distribution, and analyzed as dichotomous variables: cell infiltration score (≤2 or >2), cell type score (≤1 or >1), extracellular matrix deposition score (≤2 or >2), inflammation score (≤0.5 or >0.5), fibrous encapsulation score (≤2 or >2), and neovascularization score (≤2 or >2) (Table 5). As for the independent variables, gender, race, diabetes mellitus diagnosis status, smoking history, corticosteroid use, and CDC wound class at both T1 and T2 were treated as dichotomous variables; mesh classification and smoking status were treated as categorical variables; and host age at T1, BMI at T1 and T2, pack-year smoking history, and duration of in vivo scaffold dwelling were treated as continuous variables.
Data for all continuous variables were assessed for approximate symmetry of distribution before univariate analyses ensued. Continuous cohort characteristics were summarized as means [standard deviation (SD)] or medians [25th percentile (q1), 75th percentile (q3)] in the case of skewed distributions. In the univariate analyses, the continuous characteristics were compared by the dichotomous outcomes with t tests or Wilcoxon rank-sum tests. For continuous outcomes, Pearson (r) or Spearman (ρ) correlations were utilized to assess the relationship between the cohort characteristics. Categorical cohort characteristics were summarized as column percent n (%) and compared by the dichotomous outcomes with Chi square tests or Fisher’s exact tests in cases with small cell sizes. The relationship between categorical characteristics with more than two categories and an ordinal structure, and dichotomous outcomes was assessed via exact Cochran–Armitage trend tests. The relationship between categorical characteristics and continuous outcomes was determined with t tests or Wilcoxon rank-sum tests, while for characteristics with more than two categories, Kruskal–Wallis tests were used.
Multivariable associations with dichotomous outcomes were evaluated via logistic regression models using Firth’s penalized likelihood approach [36] to address issues of small sample sizes and data sparseness. Based on the univariate analyses, cohort characteristics were selected for multivariable models using a liberal cut-off of p ≤ 0.200. The multivariable models were then reduced via backward elimination using a cut-off of p ≤ 0.100. p values were based on penalized likelihood ratio tests [37, 38]. Multivariable associations with continuous outcomes were assessed via linear regression models. As with dichotomous outcomes, cohort characteristics were selected for multivariable models using a liberal cut-off of p ≤ 0.200. Based on the selected characteristics, all possible combinations of models were fit and discriminated between using corrected Akaike Information Criterion (AIC) [39]. Residual analyses were performed for the final linear models selected, and where the normality assumption was questionable, standard errors and p values were computed using 10,000 paired bootstrap replications. Categorical cohort characteristics with more than two categories were treated as a single entity in the model selection processes for both dichotomous and continuous outcomes and tested with an overall F-test or penalized likelihood ratio test.
Results
Patient characteristics
Under an IRB-approved protocol, synthetic mesh specimens were biopsied from 51 subjects at the time of a subsequent clinically-indicated abdominal surgery. As shown in Table 1, subjects (16 males, 35 females) had a mean age at T1 of 55.2 ± 11.0 years (range 33.4–80.6 years), and a racial distribution of 44 Caucasian, 4 Black (three not reported). Median duration of synthetic mesh implantation was 769 days (range 50–5,091 days), and the onlay, inlay, sublay locations of mesh were 4, 6, 33, respectively (seven not reported). Indications for synthetic mesh implantation were ventral hernia repair (n = 48), inguinal hernia repair (n = 2), hiatal hernia repair (n = 1). Of the initial ventral hernia repairs, the surgical sites were clean (n = 39), clean-contaminated (n = 2), contaminated (n = 1), or infected (n = 1) [seven not reported]. Indications for abdominal re-exploration at the time of specimen collection were repeat ventral hernia repair (n = 45), repeat inguinal hernia repair (n = 2), repeat hiatal hernia repair (n = 1), small bowel resection (n = 1), excision of mesh (n = 2). Of the abdominal re-explorations, the surgical sites were clean (n = 33), clean-contaminated (n = 3), contaminated (n = 13), or infected (n = 0) [two not reported]. At the time of abdominal re-exploration, clinical evidence of persistent surgical site contamination was present in none of the four subjects for whom surgical site contamination or infection was present at the time of original synthetic mesh implantation.
Remodeling characteristics
Cell type score
The cohort variables whose associations with cell type score were evaluated in multivariable models were mesh classification (univariate p = 0.045), wound class at T1 (univariate p = 0.016), wound class at T2 (univariate p = 0.187), BMI at T1 (univariate p = 0.099), smoking history (univariate p = 0.099), pack-year history (univariate p = 0.027) and corticosteroid use during study period (univariate p = 0.042). Wound class at T1 and corticosteroid use were not considered in the backward elimination process because their inclusion caused model instability, likely due to the inclusion of three covariates with zero cell counts. Of the remaining five variables, mesh classification remained in the model after the backward elimination process. Compared with subjects with permanent synthetic mesh with an absorbable adhesion barrier, subjects with uncoated absorbable synthetic mesh were estimated to have 29.400 times greater odds of having a cell type score >1 (p = 0.012). Compared with subjects with composite or non-composite permanent synthetic mesh with a permanent adhesion barrier, subjects with uncoated absorbable synthetic mesh were estimated to have 20.999 times greater odds of having a cell type score >1 (p = 0.028) (3 df penalized likelihood ratio test, p = 0.044; n = 29).
Cell infiltration score
The cohort variables whose associations with cell infiltration score were evaluated in multivariable models were gender (univariate p = 0.074), race (univariate p = 0.072), mesh classification (univariate p = 0.075), and wound class at T2 (univariate p = 0.012). Of these four variables, subject gender remained in the model after the backward elimination process. Female gender was associated with 0.153 times the odds of having a cell infiltration score >2 relative to male gender (p = 0.022; n = 40).
Extracellular matrix deposition score
The cohort variables whose associations with extracellular matrix deposition score were evaluated in multivariable models were gender (univariate p = 0.175), race (univariate p = 0.115), age at T1 (univariate p = 0.141), mesh classification (univariate p = 0.097), and wound class at T2 (univariate p = 0.008). Of these five characteristics, mesh classification and age at T1 remained in the model after the backward elimination process (n = 40) (Table 2). Compared with subjects with uncoated permanent synthetic mesh, subjects with permanent synthetic mesh with an absorbable adhesion barrier were estimated to have 0.021 times the odds of having an extracellular matrix deposition score >2 (p = 0.003), subjects with composite or non-composite permanent synthetic mesh with a permanent adhesion barrier were estimated to have 0.076 times the odds of having an extracellular matrix deposition score >2 (p = 0.041) [3 df penalized likelihood ratio test, p = 0.035]. A 1-year increase in subject age at T1 was associated with 1.096 times higher odds of having an extracellular matrix deposition score >2 (p = 0.011). The multivariable model’s area under the receiver operating curve (ROC) was 0.842, suggesting good predictive capacity in this sample.
Inflammation score
The cohort variables whose associations with inflammation score were evaluated in multivariable models were race (univariate p = 0.146), age at T1 (univariate p = 0.074), mesh classification (univariate p = 0.105), and BMI at T2 (univariate p = 0.117). Of these four variables, none remained in the model after the backward elimination process.
Fibrous encapsulation score
The cohort variables whose associations with fibrous encapsulation score were evaluated in the multivariable models were race (univariate p = 0.076), mesh classification (univariate p = 0.169) and wound class at T2 (univariate p < 0.001). Wound class at T2 remained in the model after the backward elimination process. A wound class of ‘not clean’ (clean-contaminated/contaminated/infected) at T2 was associated with an 8.581 times higher odds of having a fibrous encapsulation score >2 (p = 0.003; n = 44).
Neovascularization score
The only cohort variable whose association with neovascularization score demonstrated a p value of ≤0.200 was wound class at T2. A wound class of ‘not clean’ (clean-contaminated/contaminated/infected) at T2 was associated with a 0.238 times lower odds of having a neovascularization score >2 (p = 0.018; n = 50).
Composite remodeling score
The cohort variables whose associations with composite remodeling score were evaluated in multivariable models were age at T1 (univariate p = 0.101), BMI at T1 (univariate p = 0.091), pack-year history (univariate p = 0.097), mesh classification (univariate p = 0.149), and wound class at T2 (univariate p = 0.004). The model selection process yielded a model with mesh classification and age at T1 (n = 28) (Table 3). Compared with subjects with uncoated permanent synthetic mesh, subjects with permanent synthetic mesh with an absorbable adhesion barrier were estimated to have mean composite remodeling scores 0.568 units lower; compared with subjects with permanent synthetic mesh with an absorbable adhesion barrier, subjects with composite or non-composite permanent synthetic mesh with a permanent adhesion barrier were estimated to have mean composite remodeling scores 0.481 units higher; and compared with subjects with permanent synthetic mesh with an absorbable adhesion barrier, subjects with uncoated absorbable synthetic mesh were estimated to have mean composite remodeling scores 0.670 units higher (p = 0.006, p = 0.005, p = 0.003, respectively; 3 df F-test, p = 0.004). A 1-year increase in age at T1 was associated with a 0.015-point increase in mean composite remodeling scores (p = 0.013). As indicated by the r2 value, the model explained 46.4 % of the variability in the composite remodeling scores.
Mean collagen I area
The cohort variables whose associations with mean collagen I area were evaluated in multivariable models were age at T1 (univariate p = 0.092), mesh classification (univariate p = 0.119), and diabetes status (univariate p = 0.109). The model selection process yielded a model with only diabetes status. Subjects with diabetes had an estimated mean collagen I area 3,719.2 units higher relative to subjects without diabetes (p = 0.067; n = 24).
Mean collagen III area
The cohort variables whose associations with mean collagen III area were evaluated in multivariable models were BMI at T2 (univariate p = 0.023), and pack-year history (univariate p = 0.161). Both of these variables were selected in the model selection process (n = 30) (Table 4). An increase of 1 kg/m2 in the BMI at T2 was associated with a 43.969-unit increase in the mean collagen III area. An increase of 1 pack-year was associated with a 13.744-point decrease in the mean collagen III area. As indicated by the r2 value, the model explained 24.4 % of the variability in the mean collagen III area.
Mean collagen I:III ratio
The cohort variables whose associations with mean collagen I:III area were evaluated in multivariable models were age at T1 (univariate p = 0.193), and diabetes status (univariate p = 0.103). The model selection process resulted in a model with only diabetes status. Subjects with diabetes had an estimated mean collagen I:III ratio 1,523.9 units higher relative to subjects without diabetes (p = 0.150; n = 25).
Discussion
Over the past decade, the physiomechanical properties and histologic remodeling of synthetic mesh materials following reinforcement of abdominal wall repair sites have been evaluated in a variety of animal models [14–24]. Performance reports of these materials in the human body have thus far been limited to case reports [25, 26] and small case series [27, 28] documenting physiomechanical characterization and histologic analyses of synthetic meshes explanted from human subjects following abdominal wall reconstruction. The literature is currently lacking a risk prediction model that reliably predicts the patients and clinical circumstances for which non-constructive remodeling of an abdominal wall repair site with synthetic mesh reinforcement is most likely to occur. Thus, the purpose of this study was to evaluate the multivariable associations of patient characteristics and surgical site classifications to the histologic remodeling scores of synthetic meshes biopsied from the abdominal soft tissue repair sites of patients in the first attempt to generate a risk prediction model of non-constructive remodeling. Fifty-one subjects with synthetic mesh implanted during a previous abdominal soft tissue repair were identified and consented for the study, and specimens of the synthetic meshes were procured during a subsequent clinically-indicated abdominal surgery.
Cell type scores were dichotomized to scores ≤1 and scores >1. The distinguishing histologic feature therefore became the prevalence of inflammatory cells versus the prevalence of fibroblasts in the specimens (Table 5). Permanent synthetic mesh with an absorbable adhesion barrier, and composite or non-composite mesh with a permanent adhesion barrier, were more highly associated with the prevalence of inflammatory cells in the mesh specimens, whereas uncoated permanent synthetic mesh and uncoated absorbable synthetic mesh were more highly associated with the prevalence of fibroblasts in the mesh specimens. These data are consistent with the lack of a persistent foreign body inflammatory response to absorbable synthetic materials, and a possible inhibitory effect of adhesion barriers on the infiltration of fibroblasts into the scaffold for constructive remodeling. Surgical sites that were ‘not clean’ (clean-contaminated, contaminated, or infected) at the time of mesh implantation were more highly associated with the prevalence of fibroblasts in the mesh specimens, indicating that the inflammatory response associated with the initial contamination or infection had transitioned to a constructive remodeling response by the time of mesh specimen explantation. As expected, surgical sites that were ‘not clean’ at the time of mesh explantation were more highly associated with the prevalence of inflammatory cells in the mesh specimens. A greater mean BMI at the time of mesh implantation was associated with the prevalence of inflammatory cells in the mesh specimens. However, corticosteroid use during the interval between mesh implantation and mesh explantation, a positive history of ever having smoked tobacco, and a greater median pack-year history were all associated with the prevalence of fibroblasts in the mesh specimens, perhaps as a result of immunosuppression.
Mesh classification remained in the multivariable model for cell type score after the backward elimination process. Compared with subjects with permanent synthetic mesh with an absorbable adhesion barrier, subjects with uncoated absorbable synthetic mesh were estimated to have significantly increased odds of having predominantly fibroblasts on histologic analysis [odds ratio (OR) 29.400; p = 0.012]. Compared with subjects with composite or non-composite permanent synthetic mesh with a permanent adhesion barrier, subjects with uncoated absorbable synthetic mesh were estimated to have significantly increased odds of having predominantly fibroblasts on histologic analysis (OR 20.999; p = 0.028). This indicates a presence of cell types significantly more favorable for constructive remodeling for absorbable synthetic mesh types compared with permanent synthetic mesh types, and for uncoated meshes compared with meshes with an adhesion barrier.
Cellular infiltration scores were dichotomized to scores ≤2 and >2. The distinguishing histologic feature therefore became the lack of cellular penetrance to the center of the mesh specimens versus cellular penetrance to the center of the mesh specimens (Table 5). Note that references to the center pertain to the center of the mesh biopsy specimen, and not necessarily the center of the entire piece of mesh implanted into the subject. Specimens from male subjects and subjects of Caucasian race were more highly associated with cellular penetrance to the center of the mesh specimen; however, these findings are likely an artifact of the sparse gender and race distributions. Uncoated permanent synthetic mesh, and composite or non-composite permanent synthetic mesh with a permanent adhesion barrier were more highly associated with cellular penetrance to the center of the mesh specimen, whereas permanent synthetic mesh with an absorbable adhesion barrier and uncoated absorbable synthetic mesh were more highly associated with lack of cellular penetrance to the center of the mesh specimen. These data may suggest that the biodegradation of absorbable barriers and absorbable fibers negatively affect uniform cellular migration to the center of the scaffold during remodeling. Surgical sites that were ‘not clean’ at the time of mesh explantation were associated with a lack of cellular penetrance to the center of the mesh specimen, indicating less constructive remodeling. Subject gender remained in the multivariable model for cell infiltration score after the backward elimination process. Compared with male subjects, female subjects had significantly reduced odds of having cellular penetrance to the center of the mesh specimen (OR 0.153; p = 0.022).
Extracellular matrix deposition scores were dichotomized to scores ≤2 and >2. The distinguishing histologic feature therefore became the absence of extracellular matrix protein deposition across mesh interstices versus the presence of extracellular matrix protein deposition across mesh interstices and between synthetic fibers of the mesh specimens (Table 5). Specimens from male subjects and subjects of Caucasian race were more highly associated with extracellular matrix protein deposition across mesh interstices and between synthetic fibers of the mesh specimens. Note, however, that the sparse gender and race distributions may have produced these findings as an artifact. Specimens from subjects of greater age at the time of mesh implantation were also more highly associated with extracellular matrix protein deposition across mesh interstices and between synthetic fibers of the mesh specimens. Surgical sites that were ‘not clean’ at the time of mesh explantation were associated with an absence of extracellular matrix protein deposition across the interstices of the mesh specimens, indicating less constructive remodeling. Permanent synthetic mesh with an absorbable adhesion barrier, and composite or non-composite mesh with a permanent adhesion barrier, were more highly associated with the absence of extracellular matrix protein deposition across the interstices of the mesh specimens, whereas uncoated permanent synthetic mesh and uncoated absorbable synthetic mesh were more highly associated with the presence of extracellular matrix protein deposition across the interstices and between synthetic fibers of the mesh specimens. These data are consistent with a possible inhibitory effect of adhesion barriers on the infiltration of fibroblasts and the secretion of a network of extracellular matrix proteins across the interstices of the scaffold for constructive remodeling.
Mesh classification and subject age at the time of mesh implantation remained in the multivariable model for extracellular matrix deposition score after the backward elimination process. Compared with subjects with uncoated permanent synthetic mesh, subjects with permanent synthetic mesh with an absorbable adhesion barrier, and subjects with composite or non-composite permanent synthetic mesh with a permanent adhesion barrier were estimated to have reduced odds of having extracellular matrix deposition across mesh interstices and between mesh fibers on histologic analysis while controlling for subject age at the time of mesh implantation (OR 0.021, p = 0.003; and OR 0.076, p = 0.041, respectively; 3 df penalized likelihood ratio test, p = 0.035). This indicates significantly more favorable extracellular matrix deposition for uncoated permanent synthetic mesh compared with permanent synthetic mesh with an absorbable adhesion barrier, and permanent synthetic mesh with a permanent adhesion barrier. Furthermore, controlling for mesh classification, a 1-year increase in subject age at the time of mesh implantation was associated with 1.096 times significantly higher odds of having extracellular matrix deposition across mesh interstices and between mesh fibers on histologic analysis (OR 1.096; 95 % CI 1.020–1.204; p = 0.011). Otherwise stated, for each 1-year increase in subject age at the time of abdominal wall repair with synthetic mesh reinforcement, there is a 10 % increase in the odds of having extracellular matrix deposition across mesh interstices for constructive remodeling while controlling for mesh classification. The c-statistic or area under the ROC for this multivariable model for extracellular matrix deposition score was 0.842, suggesting good predictive capability in this sample.
Inflammation scores were dichotomized to scores ≤0.5 and >0.5. The distinguishing histologic feature therefore became the presence of foreign body giant cells versus the absence of foreign body giant cells in the mesh specimens (Table 5). Specimens from subjects of Caucasian race were more highly associated with the presence of foreign body giant cells during histologic analysis. Note, however, that this result may be an artifact of the sparse distribution of the race variable. Greater age at the time of mesh implantation was more highly associated with the absence of foreign body giant cells. As expected, greater subject BMI at the time of mesh explantation was associated with the presence of foreign body giant cells during histologic evaluation. Uncoated permanent synthetic mesh was more highly associated with the presence of foreign body giant cells, whereas permanent synthetic mesh with an absorbable adhesion barrier, composite or non-composite permanent synthetic mesh with a permanent adhesion barrier, and uncoated absorbable synthetic mesh were more highly associated with the absence of foreign body giant cells in the mesh specimens. None of these variables remained in the multivariable model for inflammation score after the backward elimination process.
Fibrous encapsulation scores were dichotomized to scores ≤2 and >2. The distinguishing histologic feature therefore became the presence of fibrosis versus the absence of fibrosis in the mesh specimens (Table 5). Specimens from female subjects and subjects of non-Caucasian race were more highly associated with the absence of fibrosis during histologic analysis; however, these findings are likely an artifact of the sparse gender and race distributions. Uncoated permanent synthetic mesh was more highly associated with the presence of fibrosis, whereas permanent synthetic mesh with an absorbable adhesion barrier, composite or non-composite permanent synthetic mesh with a permanent adhesion barrier, and uncoated absorbable synthetic mesh were more highly associated with the absence of fibrosis in the mesh specimens. Surgical sites that were ‘not clean’ at the time of mesh explantation were associated with the absence of fibrosis during histologic evaluation. Of these variables, surgical site classification at the time of explantation remained in the multivariable model for inflammation score after the backward elimination process. Surgical sites that were ‘not clean’ at the time of mesh explantation were associated with 8.581 times significantly higher odds of having the absence of fibrosis during histologic evaluation of the mesh specimens (p = 0.003).
Neovascularization scores were dichotomized to scores ≤2 and >2. The distinguishing histologic feature therefore became the absence of blood vessels bridging across mesh interstices and microvascular networks versus the presence of blood vessels bridging across mesh interstices and microvascular networks in the mesh specimens (Table 5). Only wound classification at the time of mesh explantation was found to significantly correlate with the neovascularization scores in the univariate analysis. Surgical sites that were ‘not clean’ at the time of mesh explantation were associated with the absence of blood vessels bridging across mesh interstices and microvascular networks in the mesh specimens. That is, surgical sites that were ‘not clean’ at the time of mesh explantation were associated with a 0.238 times significantly lower odds of having blood vessels bridge across the mesh interstices and form microvascular networks (OR 0.238; p = 0.018).
The composite remodeling score was calculated as the mean of the six component remodeling scores. As one might expect, more favorable composite scores (CR) for constructive remodeling inversely correlated with subject BMI at the time of mesh implantation (ρ = −0.320). However, as the antithesis of what one might expect, more favorable CRs for constructive remodeling were directly correlated with subject age at the time of mesh implantation (ρ = 0.260), and pack-year history (ρ = 0.240). Furthermore, the mean composite remodeling score was 1.4 ± 0.5 SD for specimens from surgical sites that were ‘not clean’ at the time of mesh explantation and 1.9 ± 0.3 SD for specimens from surgical sites that were clean at the time of mesh explantation, indicating significantly more favorable scores for constructive remodeling for the latter cohort (p = 0.004). Interestingly, the mean composite remodeling score was 1.5 ± 0.4 SD for permanent synthetic mesh with an absorbable adhesion barrier, 1.7 ± 0.5 SD for composite or non-composite permanent synthetic mesh with a permanent adhesion barrier, 1.9 ± 0.2 SD for uncoated permanent synthetic mesh, and 2.0 ± 0.4 SD for uncoated absorbable synthetic mesh. These data are consistent with the lack of a persistent foreign body inflammatory response to absorbable synthetic materials, and a possible inhibitory effect of adhesion barriers on the infiltration of fibroblasts and the deposition of extracellular matrix proteins across mesh interstices for constructive remodeling noted earlier.
The multivariable model selection process for composite remodeling score yielded a model with subject age at the time of mesh implantation and mesh classification. Controlling for mesh classification, a 1-year increase in subject age at the time of mesh implantation was associated with a 0.015-point significant increase in the mean composite remodeling score (p = 0.013). Compared with subjects with uncoated permanent synthetic mesh, subjects with permanent synthetic mesh with an absorbable adhesion barrier were estimated to have mean composite remodeling scores 0.568 units significantly lower, controlling for subject age at the time of mesh implantation (p = 0.006). Compared with subjects with permanent synthetic mesh with an absorbable adhesion barrier, subjects with composite or non-composite permanent synthetic mesh with a permanent adhesion barrier were estimated to have mean composite remodeling scores 0.481 units significantly higher, controlling for subject age at the time of mesh implantation (p = 0.005). Finally, compared with subjects with permanent synthetic mesh with an absorbable adhesion barrier, subjects with uncoated absorbable synthetic mesh were estimated to have mean composite remodeling scores 0.670 units significantly higher, controlling for subject age at the time of mesh implantation (p = 0.003; 3 df F test, p = 0.004). These data indicate significantly more favorable CRs for constructive remodeling for absorbable synthetic mesh compared with permanent synthetic mesh, and mesh without adhesion barriers compared with mesh with adhesion barriers. Furthermore, the data indicate that among adhesion barriers, permanent adhesion barriers are associated with more favorable CRs for constructive remodeling compared with absorbable adhesion barriers. This model with two independent variables explained 46.4 % of the variability in the composite remodeling scores of this subject population. With greater sample sizes, this multivariable model may be expanded to predict more of the variability in composite remodeling scores.
As one might expect, the mean collagen I area was inversely correlated with subject age at the time of mesh implantation. That is, the stronger type I collagen fibers decrease with advanced subject age. Interestingly, the mean quantity of type I collagen was 1,738.9 ± 1,802.1 SD for permanent synthetic mesh with an absorbable adhesion barrier, 3,908.2 ± 3,442.0 SD for composite or non-composite permanent synthetic mesh with a permanent adhesion barrier, 4,953.8 ± 2,975.6 SD for uncoated permanent synthetic mesh, and 9,202.6 ± 8,119.1 SD for uncoated absorbable synthetic mesh. These data indicate significantly more favorable quantities of type I collagen for absorbable synthetic mesh compared with permanent synthetic mesh, and mesh without adhesion barriers compared with mesh with adhesion barriers. As the antithesis of what one might expect, the mean quantity of type I collagen was greater for subjects with a diagnosis of diabetes mellitus compared with subjects without a diagnosis of diabetes mellitus (6,836.9 ± 5,600.5 SD vs. 3,590.9 ± 3,214.8 SD, respectively; p = 0.109). The multivariable model selection process for type I collagen quantity yielded a model with only subject diabetes status. Subjects with diabetes had an estimated mean quantity of type I collagen 3,719.2 units higher relative to subjects without diabetes, although a significant difference was not detected (p = 0.067).
As one might expect, the mean collagen III area was directly correlated with subject BMI at the time of mesh explantation. That is, the type III collagen fibers with less tensile strength compared with type I collagen increase in quantity with increases in subject BMI. As the antithesis of what one might expect, the mean quantity of type III collagen was inversely correlated with the pack-year history of the subject. That is, the greater the product of the number of cigarette packs and the number of years smoked, the less the quantity of type III collagen in the specimens. Both the subject BMI at the time of mesh explantation and the pack-year history variables were selected in the multivariable model selection process for type III collagen quantity. An increase of 1 kg/m2 in the subject BMI at the time of mesh explantation was associated with a 43.969-unit significant increase in the mean quantity of type III collagen, controlling for pack-year history (p = 0.009). Controlling for subject BMI at the time of mesh explantation, an increase of 1 pack-year was associated with a 13.744-point decrease in the mean quantity of type III collagen, although a significant difference was not detected (p = 0.091). This model with two independent variables explained 24.4 % of the variability in the mean quantity of type III collagen. With greater sample sizes, this multivariable model may be expanded to predict more of the variability in the mean quantity of type III collagen.
As expected, the mean collagen I:III ratio was inversely correlated with subject age at the time of mesh implantation, and is likely the result of the inverse correlation between collagen type I quantity and subject age at the time of mesh implantation. As the antithesis of what one might expect, the mean ratio of collagen type I:III was greater for subjects with a diagnosis of diabetes mellitus compared with subjects without a diagnosis of diabetes mellitus (3,793 ± 2,803.7 SD and 2,288 ± 1,781.8 SD, respectively; p = 0.103). The multivariable model selection process for mean collagen I:III ratio resulted in a model with only diabetes status. Subjects with diabetes had an estimated mean collagen I:III ratio 1,523.9 units higher relative to subjects without diabetes, although a significant difference was not detected (p = 0.150).
The combination of Sirius Red staining and visualization under polarized light microscopy is considered highly sensitive and specific for collagen types I, II, and III [40–42]. However, Sirius Red staining does enhance the birefringency of other oriented basic proteins. These proteins include keratin, fibrin, component C1q of the compliment cascade, and amyloid [41, 42]. Keratin is not likely to have been present in the tissues studied. Amyloid can easily be distinguished from collagen proteins because it does not have the fibrous configuration characteristic of collagen [41]. Fibrin and C1q are both fibrous in configuration and appear pale green under polarized light [41, 42]. Therefore, it is possible that the presence of fibrin and C1q may have led to an overestimation of collagen III and an underestimation of the collagen I:III ratio in the tissues evaluated. Furthermore, Sirius Red staining will cause collagen type II to appear yellow or blue in hue when visualized under polarized light microscopy; however, collagen type II exists in cartilage and chondrosarcoma [40], neither of which is likely to have been present in the tissues studied. Therefore, it is reasonable to conclude that the collagen that appeared red and green in the tissues studied were collagen I and III, respectively. Future studies will attempt to verify these findings using additional methodologies, including immunohistochemistry. Furthermore, it is not possible to visualize all of the collagen present under linearly-polarized light microscopy [42]. Collagen fibers will appear dark when aligned parallel to the transmission axes of the crossed polarizing filters. The use of circular polarized light microscopy, in which a rotating microscope stage changes the orientation of the tissue section with respect to the transmission axes to minimize underestimations of the collagen types [42], will be explored in future studies. However, even with circular polarized light microscopy, collagen fibers that enter or exit the two-dimensional plane of the tissue section may appear in various hues of the spectrum and lead to inaccurate estimations of the collagen types present [42].
Selection bias may have been inherent to the study design. To introduce minimal health risk to the consenting subjects, biopsies of the synthetic mesh were procured at the time of a clinically-indicated abdominal re-exploration. Since all but three specimens were procured during the repair of a hernia recurrence, note that these specimens were biased toward selection of synthetic mesh explants following soft tissue repair failure. Therefore, the data contributed by these specimens may have been biased toward non-constructive remodeling. As four specimens were procured from surgical sites that were clean-contaminated, contaminated, or infected at the time of mesh implantation, and 16 specimens were procured from surgical sites that were clean-contaminated or contaminated at the time of mesh explantation, these specimens contributed data that may have been additionally biased toward non-constructive remodeling by local inflammatory response to the pathogens present during the time of synthetic mesh indwelling. Of the four specimens for which surgical site contamination or infection was present at the time of mesh implantation, none were procured from surgical sites with evidence of persistent contamination or infection on clinical examination. Several significant findings were discovered in the univariate analyses and multivariable model estimations. However, it should be noted that the absence of other significant univariate associations does not necessarily imply that these associations do not exist; rather, the study may not have been sufficiently powered to detect them and include them in the multivariable models. Furthermore, the limited sample size and sparseness of the data necessitated the dichotomization of the remodeling characteristic outcome variables and the exclusion of subjects with incomplete independent variable data during the statistical analyses. The histologic scoring system of remodeling characteristics would also benefit from studies validating the expected distribution of the component scores. With greater sample sizes, these multivariable models may then be expanded to predict more of the variability in the histologic remodeling scores and the relative quantities of collagen types I and III for synthetic meshes used to reinforce abdominal wall repair sites.
Conclusions
These preliminary results are the first steps in generating a risk prediction model that reliably predicts the patients and clinical circumstances for which non-constructive remodeling of an abdominal wall repair site with synthetic mesh reinforcement is most likely to occur. Ultimately, this risk prediction model will be further developed, and validated with internal and external prospectively collected patient datasets. Future studies will examine whether these scores reliability predict soft tissue repair integrity. In this era of individualized healthcare, the resulting model may serve in several ways as a useful clinical decision-making tool. The risk prediction model may be used to identify modifiable risk factors to preoperatively address with each patient for improvement in the likelihood of abdominal wall reconstruction success. Moreover, with larger sample sizes and diversity of mesh-type data, the risk prediction model can be further developed to aid surgeon selection of the most appropriate reinforcement material for an individual patient given the clinical characteristics of the patient and the classification of the surgical site. Such clinical decision-making tools will be critical to improvements in the quality of surgical care for patients with complex abdominal wall defects.
References
Poulose BK, Shelton J, Phillips S, Moore D, Nealon W, Penson D et al (2012) Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 16(2):179–183
Millennium Research Group (2006) US markets for soft tissue repair devices 2006. Millennium Research Group Inc., Toronto
Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J (2004) Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 240(4):578–583
Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, Ijzermans JN et al (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343(6):393–398
Klinge U, Klosterhalfen B, Muller M, Schumpelick V (1999) Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg 165(7):665–673
Klosterhalfen B, Klinge U, Hermanns B, Schumpelick V (2000) Pathology of traditional surgical nets for hernia repair after long-term implantation in humans. Chirurg 71(1):43–51
Schachtrupp A, Klinge U, Junge K, Rosch R, Bhardwaj RS, Schumpelick V (2003) Individual inflammatory response of human blood monocytes to mesh biomaterials. Br J Surg 90(1):114–120
Bellows CF, Alder A, Helton WS (2006) Abdominal wall reconstruction using biological tissue grafts: present status and future opportunities. Expert Rev Med Devices 3(5):657–675
Ratner B, Hoffman AS, Shoen FJ, Lemons JE (1996) Biomaterials science. Academic Press, San Diego, pp 243–254
Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM (2008) Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14(11):1835–1842
Klinge U, Si ZY, Zheng H, Schumpelick V, Bhardwaj RS, Klosterhalfen B (2000) Abnormal collagen I to III distribution in the skin of patients with incisional hernia. Eur Surg Res 32(1):43–48
Klinge U, Si ZY, Zheng H, Schumpelick V, Bhardwaj RS, Klosterhalfen B (2001) Collagen I/III and matrix metalloproteinases (MMP) 1 and 13 in the fascia of patients with incisional hernias. J Invest Surg 14(1):47–54
El-Gazzaz GH, Farag SH, El-Sayd MA, Mohamed HH (2012) The use of synthetic mesh in patients undergoing ventral hernia repair during colorectal resection: risk of infection and recurrence. Asian J Surg 35(4):149–153
Matthews BD, Mostafa G, Carbonell AM, Joels CS, Kercher KW, Austin C et al (2005) Evaluation of adhesion formation and host tissue response to intra-abdominal polytetrafluoroethylene mesh and composite prosthetic mesh. J Surg Res 123(2):227–234
Harrell AG, Novitsky YW, Cristiano JA, Gersin KS, Norton HJ, Kercher KW et al (2007) Prospective histologic evaluation of intra-abdominal prosthetics four months after implantation in a rabbit model. Surg Endosc 21(7):1170–1174
Novitsky YW, Harrell AG, Cristiano JA, Paton BL, Norton HJ, Peindl RD et al (2007) Comparative evaluation of adhesion formation, strength of ingrowth, and textile properties of prosthetic meshes after long-term intra-abdominal implantation in a rabbit. J Surg Res 140(1):6–11
Novitsky YW, Cristiano JA, Harrell AG, Newcomb W, Norton JH, Kercher KW et al (2008) Immunohistochemical analysis of host reaction to heavyweight-, reduced-weight-, and expanded polytetrafluoroethylene (ePTFE)-based meshes after short-term and long-term intraabdominal implantations. Surg Endosc 22(4):1070–1076
Pascual G, Rodriguez M, Gomez-Gil V, Garcia-Honduvilla N, Bujan J, Bellon JM (2008) Early tissue incorporation and collagen deposition in lightweight polypropylene meshes: bioassay in an experimental model of ventral hernia. Surgery 144(3):427–435
Bellon JM, Rodriguez M, Garcia-Honduvilla N, Gomez-Gil V, Pascual G, Bujan J (2008) Postimplant behavior of lightweight polypropylene meshes in an experimental model of abdominal hernia. J Invest Surg 21(5):280–287
Bellon JM, Rodriguez M, Garcia-Honduvilla N, Gomez-Gil V, Pascual G, Bujan J (2009) Comparing the behavior of different polypropylene meshes (heavy and lightweight) in an experimental model of ventral hernia repair. J Biomed Mater Res B 89(2):448–455
Orenstein SB, Saberski ER, Kreutzer DL, Novitsky YW (2012) Comparative analysis of histopathologic effects of synthetic meshes based on material, weight, and pore size in mice. J Surg Res 176(2):423–429
Pascual G, Rodriguez M, Sotomayor S, Perez-Kohler B, Bellon JM (2012) Inflammatory reaction and neotissue maturation in the early host tissue incorporation of polypropylene prostheses. Hernia 16(6):697–707
Pascual G, Hernandez-Gascon B, Rodriguez M, Sotomayor S, Pena E, Calvo B et al (2012) The long-term behavior of lightweight and heavyweight meshes used to repair abdominal wall defects is determined by the host tissue repair process provoked by the mesh. Surgery 152(5):886–895
Pascual G, Hernandez-Gascon B, Sotomayor S, Pena E, Calvo B, Bujan J et al (2013) Short-term behavior of different polymer structure lightweight meshes used to repair abdominal wall defects. Histol Histopathol 28(5):611–621
Costello CR, Bachman SL, Grant SA, Cleveland DS, Loy TS, Ramshaw BJ (2007) Characterization of heavyweight and lightweight polypropylene prosthetic mesh explants from a single patient. Surg Innov 14(3):168–176
Wood AJ, Cozad MJ, Grant DA, Ostdiek AM, Bachman SL, Grant SA (2013) Materials characterization and histological analysis of explanted polypropylene, PTFE, and PET hernia meshes from an individual patient. J Mater Sci Mater Med 24(4):1113–1122
Costello CR, Bachman SL, Ramshaw BJ, Grant SA (2007) Materials characterization of explanted polypropylene hernia meshes. J Biomed Mater Res B 83(1):44–49
Cozad MJ, Grant DA, Bachman SL, Grant DN, Ramshaw BJ, Grant SA (2010) Materials characterization of explanted polypropylene, polyethylene terephthalate, and expanded polytetrafluoroethylene composites: spectral and thermal analysis. J Biomed Mater Res B 94(2):455–462
Valentin JE, Badylak JS, McCabe GP, Badylak SF (2006) Extracellular matrix bioscaffolds for orthopaedic applications: a comparative histologic study. J Bone Joint Surg Am 88(12):2673–2686
Jenkins ED, Melman L, Desai S, Brown SR, Frisella MM, Deeken CR et al (2011) Evaluation of intraperitoneal placement of absorbable and nonabsorbable barrier coated mesh secured with fibrin sealant in a New Zealand white rabbit model. Surg Endosc 25(2):604–612
Jenkins ED, Melman L, Desai S, Deeken CR, Greco SC, Frisella MM et al (2011) Histologic evaluation of absorbable and non-absorbable barrier coated mesh secured to the peritoneum with fibrin sealant in a New Zealand white rabbit model. Hernia 15(6):677–684
Brown SR, Melman L, Jenkins ED, Deeken CR, Frisella MM, Brunt LM et al (2011) Collagen type I:III ratio of the gastroesophageal junction in patients with paraesophageal hernias. Surg Endosc 25(5):1390–1394
Berard F, Gandon J (1964) Postoperative wound infections: the influence of ultraviolet irradiation of the operating room and of various other factors. Ann Surg 160(Suppl 2):1–192
Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13(10):606–608
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
Firth D (1993) Bias reduction of maximum likelihood estimates. Biometrika 80(1):27–38
Heinze G, Schemper M (2002) A solution to the problem of separation in logistic regression. Stat Med 21(16):2409–2419
Heinze G, Ploner M (2003) Fixing the nonconvergence bug in logistic regression with SPLUS and SAS. Comput Methods Programs Biomed 71(2):181–187
Sugiura N (1978) Further analysis of the data by Akaike’s information and finite corrections. Comm Statist 7(1):13–26
Junqueira LC, Cossermelli W, Brentani R (1978) Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn 41(3):267–274
Junqueira LC, Bignolas G, Brentani RR (1979) Picosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11(4):447–455
Rich L, Whittaker P (2005) Collagen and picosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci 22(2):97–104
Acknowledgments
This study utilized the REDCap® application for data maintenance, which is supported at the Washington University, St. Louis, by a Clinical and Translational Science Award (CTSA; UL1TR000448), and a National Cancer Institute (NCI) Cancer Center Support Grant to the Siteman Comprehensive Cancer Center (P30CA091842). Jaime A. Cavallo is supported by a KM1 Comparative Effectiveness Research (CER) Career Development Award (KM1CA156708) through the NCI of the National Institutes of Health (NIH), and the Washington University, St. Louis, CTSA program (UL1TR000448) through the National Center for Advancing Translational Sciences (NCATS) of the NIH. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NCI, NCATS, or NIH.
Author contributions
Study conception and design: Jaime A. Cavallo, Brent D. Matthews, and Corey R. Deeken; acquisition of data: Jaime A. Cavallo, Andres A. Roma, Jenny Ousley, Jennifer Creamer, Matthew D. Pichert, Sara Baalman, and Margaret M. Frisella; analysis and interpretation of data: Jaime A. Cavallo, Mateusz S. Jasielec, Brent D. Matthews, and Corey R. Deeken; drafting of manuscript: Jaime A. Cavallo, Mateusz S. Jasielec, Brent D. Matthews, and Corey R. Deeken; critical revision: Jaime A. Cavallo, Andres A. Roma, Mateusz S. Jasielec, Jenny Ousley, Jennifer Creamer, Matthew D. Pichert, Sara Baalman, Margaret M. Frisella, Brent D. Matthews, and Corey R. Deeken.
Disclosure
Dr. Cavallo has received research grant funding for unrelated studies from the NIH, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), and the American Hernia Society in collaboration with Davol® Incorporated. Ms. Frisella has received funding from Atrium Medical Corporation® and W. L. Gore and Associates® Incorporated for unrelated service contracts, as well as research grant funding for unrelated studies from the Foundation for Barnes-Jewish Hospital. Dr. Matthews has served on advisory boards for the Musculoskeletal Transplant Foundation, Covidien® Incorporated, and Synthes® Incorporated; served as a consultant for Atrium Medical Corporation®; received speaking fees or honoraria from Atrium Medical Corporation®, Davol® Incorporated, Ethicon® Incorporated, and W. L. Gore and Associates® Incorporated; received payments for authorship of an unrelated publication from McMahon Group® Incorporated; received research grant funding for unrelated research studies from Covidien® Incorporated, Ethicon® Incorporated, Karl Storz Endoscopy America® Incorporated, Kensey Nash Corporation®, Musculoskeletal Transplant Foundation, Synovis Surgical Innovations®, SAGES, NIH, and the Foundation for Barnes-Jewish Hospital. Dr. Deeken has served as a consultant for Atrium Medical Corporation® and Davol® Incorporated; received speaking fees or honoraria from Covidien® Incorporated and the Musculoskeletal Transplant Foundation; received research grant funding for unrelated research studies from Atrium Medical Corporation®, Covidien® Incorporated, Ethicon® Incorporated, Kensey Nash Corporation®, Musculoskeletal Transplant Foundation, OBI Biologics Incorporated®, and SAGES. Dr. Roma, Mr. Jasielec, Ms. Ousley, Dr. Creamer, Mr. Pichert, and Ms. Baalman have no conflicts of interest or financial ties to disclose.
Funding
Funding for this project was provided by the Department of Surgery, Washington University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cavallo, J.A., Roma, A.A., Jasielec, M.S. et al. Remodeling characteristics and collagen distribution in synthetic mesh materials explanted from human subjects after abdominal wall reconstruction: an analysis of remodeling characteristics by patient risk factors and surgical site classifications. Surg Endosc 28, 1852–1865 (2014). https://doi.org/10.1007/s00464-013-3405-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-3405-6